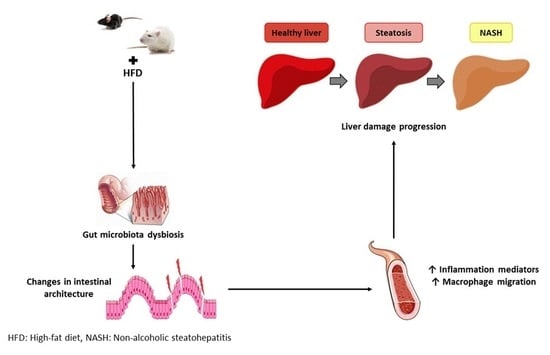
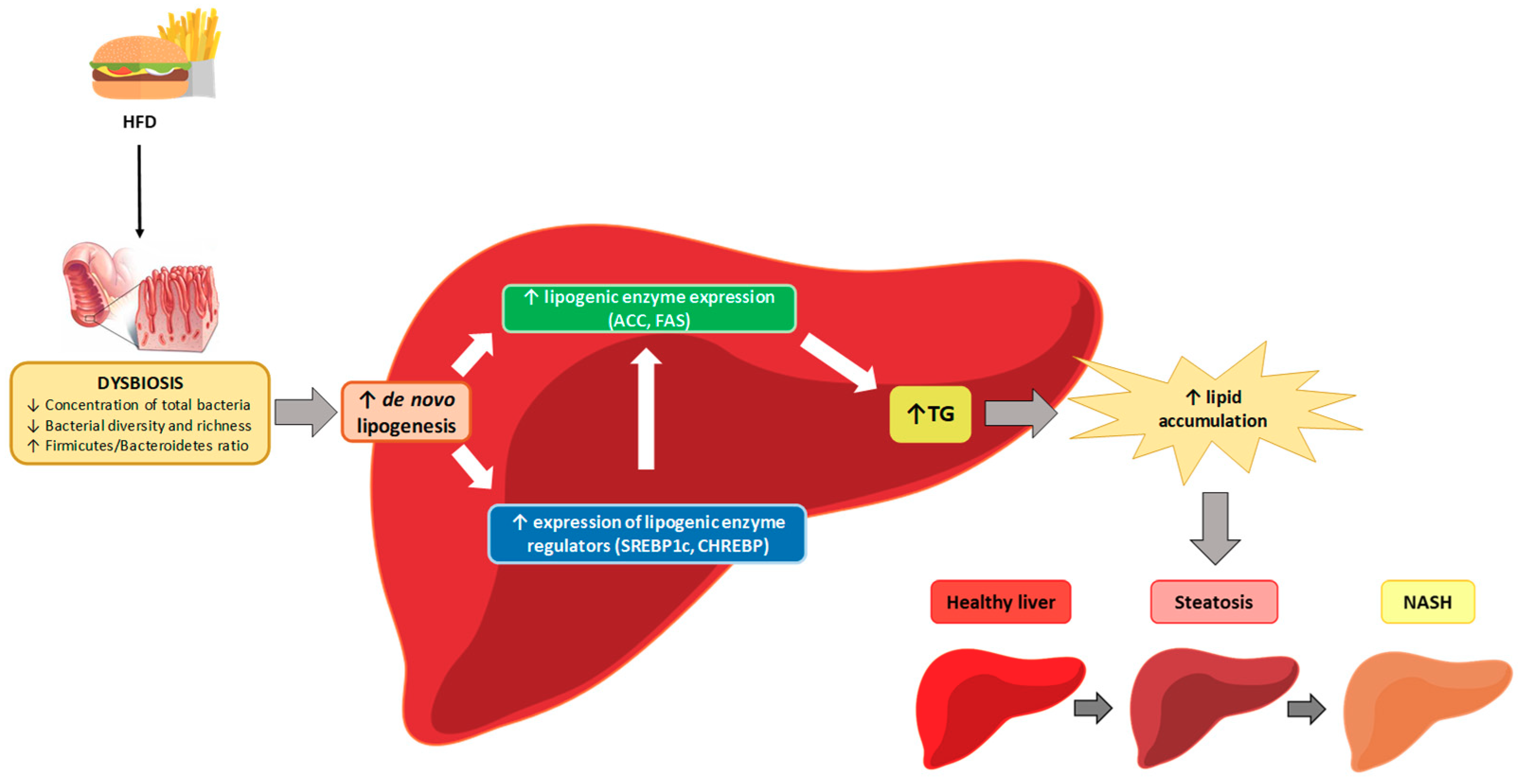
Relationship between Changes in Microbiota and Liver Steatosis Induced by High-Fat Feeding—A Review of Rodent Models
Abstract
:1. Introduction
2. Studies Carried Out by Using Lard as the Main Dietary Fat Source
3. Studies Carried Out by Using Other Lipid Sources
4. Studies with No Identified Dietary Lipid Source
5. Mechanisms of Action
5.1. Mechanisms Related to Gut Permeability
5.2. Intestinal Architecture Modification
5.3. Inflammation
5.4. Short-Chain Fatty Acids and Ethanol
5.5. Bile Acid Composition
5.6. Other Mechanisms
6. Concluding Remarks
Funding
Conflicts of Interest
References
- Levene, A.P.; Goldin, R.D. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology 2012, 61, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Petäjä, E.M.; Yki-Järvinen, H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD-A Systematic Review. Int. J. Mol. Sci. 2016, 17, 633. [Google Scholar] [CrossRef] [PubMed]
- Harjes, U. Dividing paths in fatty liver disease. Nat. Rev. Cancer 2019, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, P.S.; Cortez-Pinto, H.; Solá, S.; Castro, R.E.; Ramalho, R.M.; Baptista, A.; Moura, M.C.; Camilo, M.E.; Rodrigues, C.M. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am. J. Gastroenterol. 2004, 99, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, H.E.; Teterina, A.; Comelli, E.M.; Taibi, A.; Arendt, B.M.; Fischer, S.E.; Lou, W.; Allard, J.P. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci. Rep. 2018, 8, 1466. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.M.B.; Stefano, J.T.; Miele, L.; Ponziani, F.R.; Souza-Basqueira, M.; Okada, L.S.R.R.; de Barros Costa, F.G.; Toda, K.; Mazo, D.F.C.; Sabino, E.C.; et al. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: A prospective pilot study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: Mechanisms and implications for metabolic disorders. Curr. Opin. Lipidol. 2010, 21, 76–83. [Google Scholar] [CrossRef]
- Wieland, A.; Frank, D.N.; Harnke, B.; Bambha, K. Systematic review: Microbial dysbiosis and nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2015, 42, 1051–1063. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Sanduzzi Zamparelli, M.; Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; Marrone, G.; Gasbarrini, A.; Grieco, A.; Nardone, G.; Miele, L. The Metabolic Role of Gut Microbiota in the Development of Nonalcoholic Fatty Liver Disease and Cardiovascular Disease. Int. J. Mol. Sci. 2016, 17, 1225. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Gauffin Cano, P.; Santacruz, A.; Moya, Á.; Sanz, Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS ONE 2012, 7, e41079. [Google Scholar] [CrossRef]
- Solga, S.F.; Diehl, A.M. Non-alcoholic fatty liver disease: Lumen-liver interactions and possible role for probiotics. J. Hepatol. 2003, 38, 681–687. [Google Scholar] [CrossRef]
- Wang, C.C.; Yen, J.H.; Cheng, Y.C.; Lin, C.Y.; Hsieh, C.T.; Gau, R.J.; Chiou, S.J.; Chang, H.Y. Polygala tenuifolia extract inhibits lipid accumulation in 3T3-L1 adipocytes and high-fat diet-induced obese mouse model and affects hepatic transcriptome and gut microbiota profiles. Food Nutr. Res. 2017, 61, 1379861. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Hong, F.; Wang, J.; Zhao, X.; Wang, S.; Xue, T.; Xu, J.; Zheng, X.; Zhai, Y. DBZ is a putative PPARγ agonist that prevents high fat diet-induced obesity, insulin resistance and gut dysbiosis. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2690–2701. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Nie, Y.; Zhu, A.; Chen, Z.; Wu, P.; Zhang, L.; Luo, M.; Sun, Q.; Cai, L.; Lai, Y.; et al. Vitamin D Signaling through Induction of Paneth Cell Defensins Maintains Gut Microbiota and Improves Metabolic Disorders and Hepatic Steatosis in Animal Models. Front. Physiol. 2016, 7, 498. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Tang, Y.; Li, M.; Yang, P.; Liu, Z.; Yuan, J.; Zheng, P. Co-Administration of Cholesterol-Lowering Probiotics and Anthraquinone from Cassia obtusifolia L. Ameliorate Non-Alcoholic Fatty Liver. PLoS ONE 2015, 10, e0138078. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.B.; Jeong, H.W.; Cho, D.; Lee, B.J.; Lee, J.H.; Choi, J.Y.; Bae, I.H.; Lee, S.J. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J. Med. Food 2015, 18, 549–556. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Leal-Díaz, A.M.; Noriega, L.G.; Torre-Villalvazo, I.; Torres, N.; Alemán-Escondrillas, G.; López-Romero, P.; Sánchez-Tapia, M.; Aguilar-López, M.; Furuzawa-Carballeda, J.; Velázquez-Villegas, L.A.; et al. Aguamiel concentrate from Agave salmiana and its extracted saponins attenuated obesity and hepatic steatosis and increased Akkermansia muciniphila in C57BL6 mice. Sci. Rep. 2016, 6, 34242. [Google Scholar] [CrossRef]
- Liu, J.P.; Zou, W.L.; Chen, S.J.; Wei, H.Y.; Yin, Y.N.; Zou, Y.Y.; Lu, F.G. Effects of different diets on intestinal microbiota and nonalcoholic fatty liver disease development. World J. Gastroenterol. 2016, 22, 7353–7364. [Google Scholar] [CrossRef]
- Monteiro, N.E.S.; Roquetto, A.R.; de Pace, F.; Moura, C.S.; Santos, A.D.; Yamada, A.T.; Saad, M.J.A.; Amaya-Farfan, J. Dietary whey proteins shield murine cecal microbiota from extensive disarray caused by a high-fat diet. Food Res. Int. 2016, 85, 121–130. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.; Yuan, F.; Li, N.; Huang, Q.; He, L.; Wang, L.; Liu, Z. Perilla Oil Has Similar Protective Effects of Fish Oil on High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease and Gut Dysbiosis. Biomed. Res. Int. 2016, 2016, 9462571. [Google Scholar] [CrossRef]
- Tung, Y.C.; Lin, Y.H.; Chen, H.J.; Chou, S.C.; Cheng, A.C.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Piceatannol Exerts Anti-Obesity Effects in C57BL/6 Mice through Modulating Adipogenic Proteins and Gut Microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Ching, Y.H.; Li, Y.P.; Liu, J.Y.; Huang, Y.T.; Huang, Y.W.; Yang, S.S.; Huang, W.C.; Chuang, H.L. Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis. Nutrients 2017, 9, 1220. [Google Scholar] [CrossRef] [PubMed]
- Duparc, T.; Plovier, H.; Marrachelli, V.G.; Van Hul, M.; Essaghir, A.; Ståhlman, M.; Matamoros, S.; Geurts, L.; Pardo-Tendero, M.M.; Druart, C.; et al. Hepatocyte MyD88 affects bile acids, gut microbiota and metabolome contributing to regulate glucose and lipid metabolism. Gut 2017, 66, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Chen, Y.T.; Lin, Y.C.; Lin, J.S.; Yang, N.S.; Chen, M.J. Sugary Kefir Strain Lactobacillus mali APS1 Ameliorated Hepatic Steatosis by Regulation of SIRT-1/Nrf-2 and Gut Microbiota in Rats. Mol. Nutr. Food Res. 2018, 62, 1700903. [Google Scholar] [CrossRef]
- Jia, N.; Lin, X.; Ma, S.; Ge, S.; Mu, S.; Yang, C.; Shi, S.; Gao, L.; Xu, J.; Bo, T.; et al. Amelioration of hepatic steatosis is associated with modulation of gut microbiota and suppression of hepatic miR-34a in. Nutr. Metab. (Lond.) 2018, 15, 86. [Google Scholar] [CrossRef]
- Jing, C.; Wen, Z.; Zou, P.; Yuan, Y.; Jing, W.; Li, Y.; Zhang, C. Consumption of Black Legumes Glycine soja and Glycine max Lowers Serum Lipids and Alters the Gut Microbiome Profile in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2018, 66, 7367–7375. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Mao, G.; Wu, T.; Hu, Y.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. A fucoidan from sea cucumber Pearsonothuria graeffei with well-repeated structure alleviates gut microbiota dysbiosis and metabolic syndromes in HFD-fed mice. Food Funct. 2018, 9, 5371–5380. [Google Scholar] [CrossRef]
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef]
- Martins, L.M.S.; Perez, M.M.; Pereira, C.A.; Costa, F.R.C.; Dias, M.S.; Tostes, R.C.; Ramos, S.G.; de Zoete, M.R.; Ryffel, B.; Silva, J.S.; et al. Interleukin-23 promotes intestinal T helper type17 immunity and ameliorates obesity-associated metabolic syndrome in a murine high-fat diet model. Immunology 2018, 154, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Bose, S.; Shin, N.R.; Chin, Y.W.; Choi, Y.H.; Kim, H. Pharmaceutical Impact of Houttuynia Cordata and Metformin Combination on High-Fat-Diet-Induced Metabolic Disorders: Link to Intestinal Microbiota and Metabolic Endotoxemia. Front. Endocrinol. (Lausanne) 2018, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Niu, M.; Tang, W.; Hu, J.; Wei, G.; He, Z.; Chen, Y.; Jiang, Y.; Chen, P. L-Fucose ameliorates high-fat diet-induced obesity and hepatic steatosis in mice. J. Transl. Med. 2018, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, H.; Bai, Y.; Zhi, F. Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem. Biophys. Res. Commun. 2019, 509, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Sun, X.; Ma, N.; Liu, Y.; Luo, T.; Song, S.; Ai, C. Polysaccharides from Laminaria japonica alleviated metabolic syndrome in BALB/c mice by normalizing the gut microbiota. Int. J. Biol. Macromol. 2019, 121, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, J.; Li, J.; Lei, X.; Xu, D.; Wang, Y.; Li, C.; Li, X.; Mao, Y. Turnover of bile acids in liver, serum and caecal content by high-fat diet feeding affects hepatic steatosis in rats. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1293–1304. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Qi, G.; Liu, D.; Cao, X.; Yu, J.; Zhang, R.; Lin, W.; Guo, P. Asperlin Stimulates Energy Expenditure and Modulates Gut Microbiota in HFD-Fed Mice. Mar. Drugs 2019, 17, 38. [Google Scholar] [CrossRef]
- Fox, J.G.; Ge, Z.; Whary, M.T.; Erdman, S.E.; Horwitz, B.H. Helicobacter hepaticus infection in mice: Models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011, 4, 22–30. [Google Scholar] [CrossRef]
- Chen, M.; Hui, S.; Lang, H.; Zhou, M.; Zhang, Y.; Kang, C.; Zeng, X.; Zhang, Q.; Yi, L.; Mi, M. SIRT3 Deficiency Promotes High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Correlation with Impaired Intestinal Permeability through Gut Microbial Dysbiosis. Mol. Nutr. Food Res. 2019, 63, e1800612. [Google Scholar] [CrossRef]
- Zhuang, P.; Shou, Q.; Lu, Y.; Wang, G.; Qiu, J.; Wang, J.; He, L.; Chen, J.; Jiao, J.; Zhang, Y. Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2715–2726. [Google Scholar] [CrossRef]
- Baldwin, J.; Collins, B.; Wolf, P.G.; Martinez, K.; Shen, W.; Chuang, C.C.; Zhong, W.; Cooney, P.; Cockrell, C.; Chang, E.; et al. Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice. J. Nutr. Biochem. 2016, 27, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.; Hoffman, J.; Martinez, K.; Grace, M.; Lila, M.A.; Cockrell, C.; Nadimpalli, A.; Chang, E.; Chuang, C.C.; Zhong, W.; et al. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J. Nutr. Biochem. 2016, 31, 150–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, M.T.; Gentile, C.L.; Cox-York, K.; Wei, Y.; Wang, D.; Estrada, A.L.; Reese, L.; Miller, T.; Pagliassotti, M.J.; Weir, T.L. Fuzhuan tea consumption imparts hepatoprotective effects and alters intestinal microbiota in high saturated fat diet-fed rats. Mol. Nutr. Food Res. 2016, 60, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, M.; Miura, K.; Minami, S.; Shimura, Y.; Ohnishi, H. Altered Gut Microbiota Composition and Immune Response in Experimental Steatohepatitis Mouse Models. Dig. Dis. Sci. 2017, 62, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Hong, F.; Wang, J.; Cong, Y.; Dai, S.; Wang, S.; Jin, X.; Wang, F.; Liu, J.; Zhai, Y. Microbiome Remodeling via the Montmorillonite Adsorption-Excretion Axis Prevents Obesity-related Metabolic Disorders. EBioMedicine 2017, 16, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, S.; Kamada, N.; Amiya, T.; Nakamoto, N.; Nakaoka, T.; Kimura, M.; Saito, Y.; Ejima, C.; Kanai, T.; Saito, H. Gut microbiota-mediated generation of saturated fatty acids elicits inflammation in the liver in murine high-fat diet-induced steatohepatitis. BMC Gastroenterol. 2017, 17, 136. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Yan, C.; Xie, R.; Guo, Z.; Wang, S.; Zhang, Y.; Li, Z.; Wang, B.; Cao, H. Diammonium Glycyrrhizinate Protects against Nonalcoholic Fatty Liver Disease in Mice through Modulation of Gut Microbiota and Restoration of Intestinal Barrier. Mol. Pharm. 2018, 15, 3860–3870. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef]
- Milard, M.; Laugerette, F.; Durand, A.; Buisson, C.; Meugnier, E.; Loizon, E.; Louche-Pelissier, C.; Sauvinet, V.; Garnier, L.; Viel, S.; et al. Milk Polar Lipids in a High-Fat Diet Can Prevent Body Weight Gain: Modulated Abundance of Gut Bacteria in Relation with Fecal Loss of Specific Fatty Acids. Mol. Nutr. Food Res. 2019, 63, e1801078. [Google Scholar] [CrossRef] [Green Version]
- Bashiardes, S.; Zilberman-Schapira, G.; Elinav, E. Use of Metatranscriptomics in Microbiome Research. Bioinform. Biol. Insights 2016, 10, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Intestinal Microbiota Modulation in Obesity-Related Non-alcoholic Fatty Liver Disease. Front. Physiol. 2018, 9, 1813. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirera, I.; Bauer, T.M.; Navasa, M.; Vila, J.; Grande, L.; Taurá, P.; Fuster, J.; García-Valdecasas, J.C.; Lacy, A.; Suárez, M.J.; et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J. Hepatol. 2001, 34, 32–37. [Google Scholar] [CrossRef]
- Hollander, D. Intestinal permeability, leaky gut, and intestinal disorders. Curr. Gastroenterol. Rep. 1999, 1, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995, 269, G467–G475. [Google Scholar] [CrossRef] [PubMed]
- Lingaraju, A.; Long, T.M.; Wang, Y.; Austin, J.R.; Turner, J.R. Conceptual barriers to understanding physical barriers. Semin. Cell Dev. Biol. 2015, 42, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Marchiando, A.M.; Shen, L.; Graham, W.V.; Weber, C.R.; Schwarz, B.T.; Austin, J.R.; Raleigh, D.R.; Guan, Y.; Watson, A.J.; Montrose, M.H.; et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 2010, 189, 111–126. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Parnell, J.A.; Reimer, R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef]
- Ferreira, D.F.; Fiamoncini, J.; Prist, I.H.; Ariga, S.K.; de Souza, H.P.; de Lima, T.M. Novel role of TLR4 in NAFLD development: Modulation of metabolic enzymes expression. Biochim. Biophys. Acta 2015, 1851, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; De Minicis, S.; Osterreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Mendes, V.; Galvão, I.; Vieira, A.T. Mechanisms by Which the Gut Microbiota Influences Cytokine Production and Modulates Host Inflammatory Responses. J. Interferon Cytokine Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019, 1–21. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [Green Version]
- Ingerslev, A.K.; Theil, P.K.; Hedemann, M.S.; Lærke, H.N.; Bach Knudsen, K.E. Resistant starch and arabinoxylan augment SCFA absorption, but affect postprandial glucose and insulin responses differently. Br. J. Nutr. 2014, 111, 1564–1576. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.J.; Sellmann, C.; Engstler, A.J.; Ziegenhardt, D.; Bergheim, I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br. J. Nutr. 2015, 114, 1745–1755. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut microbiome and liver diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef]
- Singh, M.; Singh, A.; Kundu, S.; Bansal, S.; Bajaj, A. Deciphering the role of charge, hydration, and hydrophobicity for cytotoxic activities and membrane interactions of bile acid based facial amphiphiles. Biochim. Biophys. Acta 2013, 1828, 1926–1937. [Google Scholar] [CrossRef] [Green Version]
- Marin, J.J.; Macias, R.I.; Briz, O.; Banales, J.M.; Monte, M.J. Bile Acids in Physiology, Pathology and Pharmacology. Curr. Drug Metab. 2015, 17, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, K.M.; Albers, S.; Trautwein, C. Role of bile acids in the gut-liver axis. J. Hepatol. 2018, 68, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]

| Reference | Animal Model | % Fat | Main Fat Source | Changes in Gut Microbiota | Potential Mechanisms Involved in the Relationship between Changes in Microbiota and Liver Steatosis |
|---|---|---|---|---|---|
| Gauffin Cano et al. (2012) [19] | Male C57BL-6 mice (6–8-week-old) | 60% | Lard | ↓ Lactobacillus, Bifidobacterium, Clostridium coccoides and Clostridium leptum ↑ Enterobacteriaceae | ↑ Production of TNF-α by macrophages and dendritic cells (in vitro, stool-induced) |
| Mei et al. (2015) [24] | Male Sprague–Dawley rats | 45% | Lard | ↑ Firmicutes ↓ Bacteroidetes | Not detailed |
| Seo et al. (2015) [25] | Male C57BL/6 mice (6-week-old) | 45% | Lard | ↑ Firmicutes/Bacteroidetes ratio ↑ Bacteroides/Prevotella ratio | Not detailed |
| Wang et al. (2015) [26] | Male C57BL/6J mice (10-week-old) | 60% | Lard | ↑ Firmicutes/Bacteroidetes ratio ↑ Proteobacteria ↓ Actinobacteria | ↑ Hepatic Tnf-α mRNA induced by ↑ LPS levels in plasma |
| Leal-Díaz et al. (2016) [27] | Male C57BL/6 mice (5-week-old) | 45% | Lard | ↓ Bacteria diversity ↓ Prevotella, Mucispirillum and Oscillospira | Not detailed |
| Liu et al. (2016) [28] | Male Sprague–Dawley rats (5-week-old) | 60% | Lard | ↓ Bacteroidetes/Firmicutes ratio ↑ Roseburia and Oscillospira ↓ Bacteroides and Parabacterioides | ↑ LPS levels in plasma |
| Monteiro et al. (2016) [29] | Male C57BL/6 mice | 38% | Lard | ↑ Firmicutes, Verrucomicrobia and Akkermansia ↓ Bacteroidetes, Proteobacteria, Helicobacter and Bacteroides | ↑ LPS |
| Tian et al. (2016) [30] | Male Sprague–Dawley rats (8–9-week-old) | 45% | Lard | ↑ Prevotella and Bacteroides ↑ Blautia, Faecalibacterium, Lactobacillus, Escherichia and Sutterella ↓ Ruminococcus, Oscillospira and Clostridium | Not detailed |
| Tung et al. (2016) [31] | Male C57BL/6 mice (5-week-old) | 45% | Lard | ↓ Firmicutes ↑ Bacteroidetes ↓ Clostridiales ↑ Bacteroidales and Sphingobacteriales | Not detailed |
| Chiu et al. (2017) [32] | Male GF C57BL/6JNarl mice (3–4-week-old) | 60% | Lard | ↑ Firmicutes | Not detailed |
| Duparc et al. (2017) [33] | C57BL/6 WT mice (Hepatocyte-specific Myd88 KO mice) | 60% | Lard | ↑ Bacterial diversity ↑ Firmicutes ↓ Tenericutes ↓ Sutterella and Allobaculum ↑ Ruminococcus and Oscillospira | Not detailed |
| Feng et al. (2017) [34] | Male Sprague–Dawley rats (4-week-old) | 45% | Lard | ↑ Actinobacteria, Proteobacteria and Deferribacteres ↓ Spirochaetae ↑ Collinsella, Streptococcus, Gemella and Elusimicrobium ↓ Treponema and Quinella | ↑ LPS levels in plasma ↓ Intestinal tight-junction |
| Porras et al. (2017) [35] | Male C57BL/6J mice (7-week-old) | 60% | Lard | ↓ Concentration of total bacteria ↑ Helicobacter expansion ↑ Firmicutes/Bacteroidetes ratio ↑ Proteobacteria ↑ Clostridia, Bacilli and Deltaproteobacteria ↓ Bacteroidia, Erysipelotrichi and Betaproteobacteria | ↓ Intestinal tight-junction ↓ Acetate, butyrate and propionate SCFAs ↑ LPS and ethanol levels in plasma |
| Su et al. (2017) [23] | Male BALB/c mice (4–6-week-old) | 60% | Lard | ↑ Firmicutes ↓ Bacteroidetes ↑ Helicobacter hepaticus ↓ Akkermansia muciniphila | ↑ LPS levels in plasma ↓ Intestinal tight junctions |
| Wang et al. (2017) [21] | Male ICR mice (4-week-old) | 60% | Lard | ↓ Bacteroidetes/Firmicutes ratio ↓ Deferribacteres | Not detailed |
| Xu et al. (2017) [22] | Male C57BL/6J mice (10-week-old) | 60% | Lard | ↑ Firmicutes/Bacteroidetes ratio ↑Helicobacter marmotae, Odoribacter and Anaerotruncus | Not detailed |
| Chen et al. (2018) [36] | Male Sprague–Dawley rats (6-week-old) | 60% | Lard | ↑ Proteobacteria and Verrucomicrobia ↓ Bacterioidetes and Tenericutes relative abundances | Not detailed |
| Jia et al. (2018) [37] | C57BL/6 J mice (6-week-old) | 60% | Lard | ↑ Firmicutes, Eubacterium, Blautia, Clostridium, Lactobacillus and Escherichia ↓ Parasutterella | Not detailed |
| Jing et al. (2018) [38] | Male C57BL/6J mice | 60% | Lard | ↓ Firmicutes ↑ Verrucomicrobia | Not detailed |
| Li et al. (2018) [39] | Male C57BL/6J mice (8-week-old) | 60% | Lard | ↑ Firmicutes, Proteobacteria, Lachnoclostridium, Acetatifactor, Lactococcus, Romboutsia, Enterorhabdus and Dorea ↓ Actinobacteria, Bacteroidetes, Verrucomicrobia, Akkermansia, Olsenella, Barnesiella and Alloprevotella | Not detailed |
| Li et al. (2018) [40] | Male C57BL/6J mice | 30% | Lard | ↑ Candidatus, Saccharibacteria, Proteobacteria, Firmicutes, Bacilli, Lactobacillaceae, Helicobacteraceae, Coriobacteriacea and Ruminococcaceae ↓Bacteroidetes, Deltaproteobacteria and Porphyromonadaceae | Not detailed |
| Martins et al. (2018) [41] | Female C57BL/6 mice (deficient in IL-23) | 60% | Lard | ↑ Lactobacillaeceae and S24-7 ↓ Lachnospiraceae | ↑LPS levels in plasma |
| Wang et al. (2018) [42] | C57BL/6 J mice | 60% | Lard | ↑ Clostridium leptum ↓ Bacteriodetes fragilis | Not detailed |
| Wu et al. (2018) [43] | C57BL/6 J mice | 60% | Lard | ↑Deltaproteobacteria, Deferribacteres, Desulfovibrionaceae, Deferribacteraceae, Porphyromonadaceae | Not detailed |
| Chen et al. (2019) [44] | C57BL/6J WT mice | 60% | Lard | ↑ Firmicutes/Bacteroidetes ratio | ↑Mucosal damage ↑ Co-localization of HMGB1 and CD63, in gut ↑HMGB1 in serous exosomes |
| C57BL/6J ASC-KO mice | 60% | Lard | ↓ Bacterial diversity ↑ Firmicutes/Bacteroidetes ratio ↑ Streptococcaceae | ↑Mucosal damage ↑ Co-localization of HMGB1 and CD63, in gut ↑ HMGB1 in serous exosomes | |
| Duan et al. (2019) [45] | BALB/c mice | 45% | Lard | ↑ Firmicutes, Allobaculum spp, Pseudomonas and Lachnoclostridium. ↓ Bacteriodetes, Rikenellaceae, Prevotellaceae, Bacteroidales (S24-7) | Not detailed |
| Tang et al. (2019) [46] | Male Sprague–Dawley rats (6-week-old) | 60% | Lard | ↑ Firmicutes ↓ Bacteriodetes | Changes in bile acids (↑ taurocholic and ↓ taurohyodeoxycholic and urodeoxycholic acid contents) |
| Wu et al. (2019) [47] | Male C57BL/c mice | 60% | Lard | ↓ Bacteroidetes/Firmicutes ratio ↑ Anaerotruncus, Streptococcus and Bacteroides | Not detailed |
| Reference | Animal Model | % Fat | Main Fat Source | Changes in Gut Microbiota | Potential Mechanisms Involved in the Relationship between Changes in Microbiota and Liver Steatosis |
|---|---|---|---|---|---|
| Baldwin et al. (2016) [51] | Male C57BL/6J mice (4-week-old) | 34% | Butter | ↓ Firmicutes, Actinobacteria and Tenericutes | ↑ Mucosal damage |
| Collins et al. (2016) [52] | Male C57BL/6J mice | 44% | Soybean oil | ↓ Lachnospiraceae | ↓ Intestinal mucosa integrity ↑ Cd11c, Cd68 and SCFA receptors mRNA levels |
| Foster et al. (2016) [53] | Male Wistar rats | 45% | Cocoa butter | ↑ Bacteroidetes and Verrucomicrobia ↓ Firmicutes and Lactobacillus | Not detailed |
| Tian et al. (2016) [30] | Male Sprague–Dawley rats (8–9-week-old) | 45% | Lard (1/2) + FOH (1/2) | ↑ Prevotella relative abundance ↑ Akkermansia ↓ Ruminococcus, Oscillospira and Clostridium | Not detailed |
| Lard (3/4) + POH (1/4) | ↑ Prevotella relative abundande ↓ Ruminococcus, Oscillospira and Clostridium | Not detailed | |||
| Ishioka et al. (2017) [54] | C57BL/6 mice | 60% | Not specified | ↑ Firmicutes/ Bacteroidetes ratio ↑ Lactobacillales (lactate-producing bacteria) | Not detailed |
| Xu et al. (2017) [55] | Male C57BL/6J (10-week-old) | 60% | Not specified | ↓ Cyanobacteria ↑ Tenericutes and Actinobacteria | Intestinal morphology changes ↓ Villus length (proximal jejunum) ↓ Occludin mRNA expression |
| Yamada et al. (2017) [56] | SPF C57BL/6J mice | 72% | Not specified (high content of SFA and cholesterol) | ↓ Bifidobacterium, Enterococcus and Bacteroides ↑ Clostridium (XIVa and XIII subclusters) | Not detailed |
| Zhou et al. (2017) [57] | Male C57BL/6J mice | 20% | Rapeseed oil | ↓ Firmicutes to Bacteroidetes proportion ↓ Proteobacteria | ↑ Total SCFA content (feces) |
| Zhuang et al. (2017) [50] | C57BL/6J mice (4-week-old) | 45% | Milk-based fat | ↓ Microbial richness ↓ Firmicutes/Bacteroidetes ratio and Proteobacteria ↓ Firmicutes/Bacteroidetes ratio (females) | ↓ Villus and crypt lengths (cecum section) ↑ LPS levels in plasma |
| Li et al. (2018) [58] | Male mice C57BL/6J(4-week-old) | 60% | Not specified | ↓ Bacteroidetes ↑ Firmicutes | ↑ NF-κB activation and ↑ IL-6 and TNF-α production |
| Natividad et al. (2018) [59] | Male C57BL/6J | 38% | Milk fat | ↓ Ruminococcus, Bifidobacterium, Parabacteroides and Akkermansia ↑ Dorea and Ruminococcus gnavus ↑ Sutterella genera | Not detailed |
| Milard et al. (2019) [60] | Male C57Bl/6 mice | 46% | Palm oil | ↑ Bifidobacterium animalis | Not detailed |
| Palm oil + 1.1% MPL | ↑ Bifidobacterium animalis (vs. HF) | Not detailed | |||
| Palm oil + 1.6% MPL | ↓ Lactobacillus reuteri ↑ Bifidobacterium animalis (vs. C) | ↑ Crypt depth (vs. HF) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Zorita, S.; Aguirre, L.; Milton-Laskibar, I.; Fernández-Quintela, A.; Trepiana, J.; Kajarabille, N.; Mosqueda-Solís, A.; González, M.; Portillo, M.P. Relationship between Changes in Microbiota and Liver Steatosis Induced by High-Fat Feeding—A Review of Rodent Models. Nutrients 2019, 11, 2156. https://doi.org/10.3390/nu11092156
Gómez-Zorita S, Aguirre L, Milton-Laskibar I, Fernández-Quintela A, Trepiana J, Kajarabille N, Mosqueda-Solís A, González M, Portillo MP. Relationship between Changes in Microbiota and Liver Steatosis Induced by High-Fat Feeding—A Review of Rodent Models. Nutrients. 2019; 11(9):2156. https://doi.org/10.3390/nu11092156
Chicago/Turabian StyleGómez-Zorita, Saioa, Leixuri Aguirre, Iñaki Milton-Laskibar, Alfredo Fernández-Quintela, Jenifer Trepiana, Naroa Kajarabille, Andrea Mosqueda-Solís, Marcela González, and María P. Portillo. 2019. "Relationship between Changes in Microbiota and Liver Steatosis Induced by High-Fat Feeding—A Review of Rodent Models" Nutrients 11, no. 9: 2156. https://doi.org/10.3390/nu11092156
APA StyleGómez-Zorita, S., Aguirre, L., Milton-Laskibar, I., Fernández-Quintela, A., Trepiana, J., Kajarabille, N., Mosqueda-Solís, A., González, M., & Portillo, M. P. (2019). Relationship between Changes in Microbiota and Liver Steatosis Induced by High-Fat Feeding—A Review of Rodent Models. Nutrients, 11(9), 2156. https://doi.org/10.3390/nu11092156