All You Can Feed: Some Comments on Production of Mouse Diets Used in Biomedical Research with Special Emphasis on Non-Alcoholic Fatty Liver Disease Research
Abstract
:1. General Aspects of Mice in Biomedical Research
2. Producers of Mouse Diets
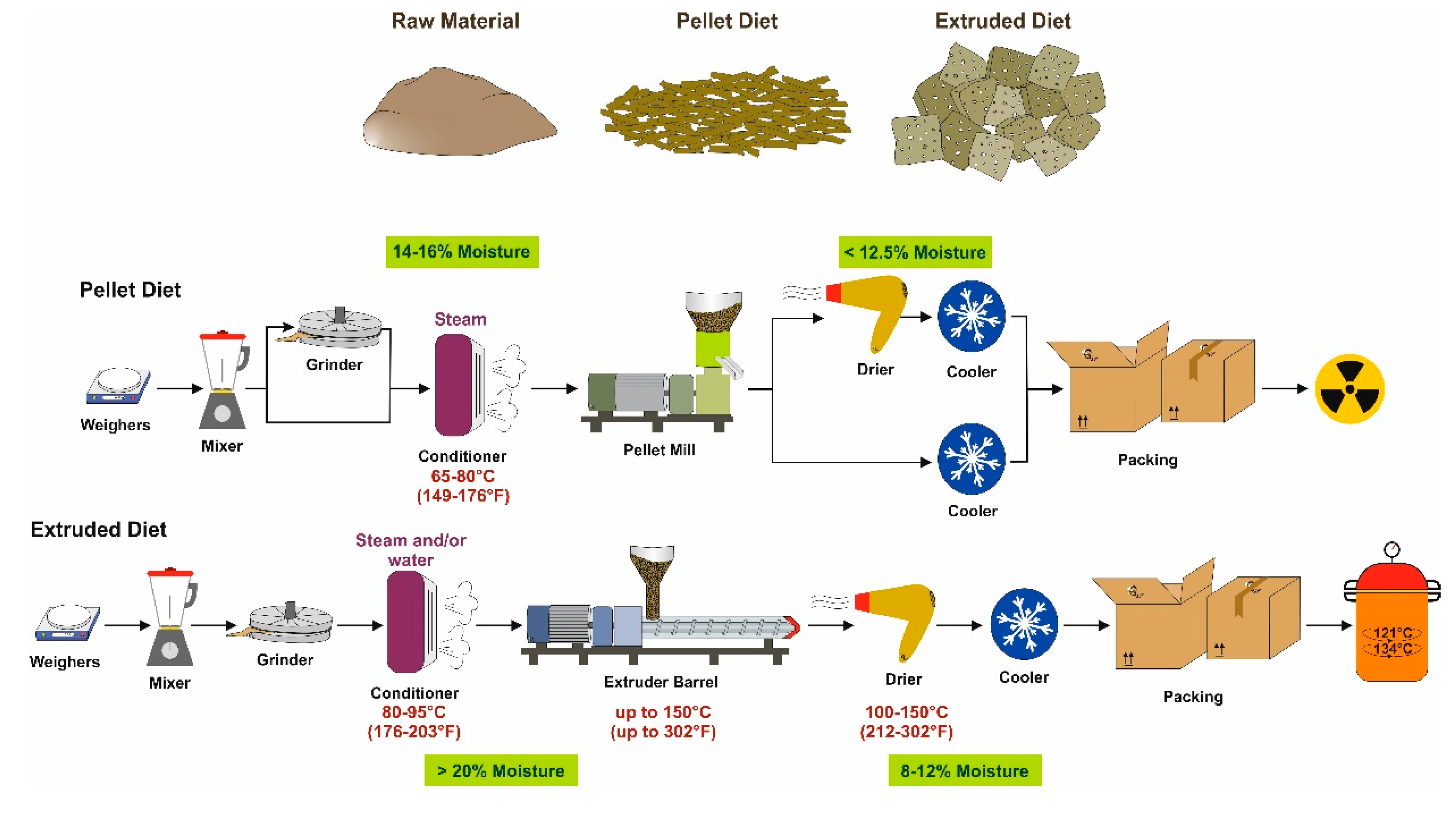
3. The Production Process
3.1. Grain-Based Diets
3.2. Purified Diets
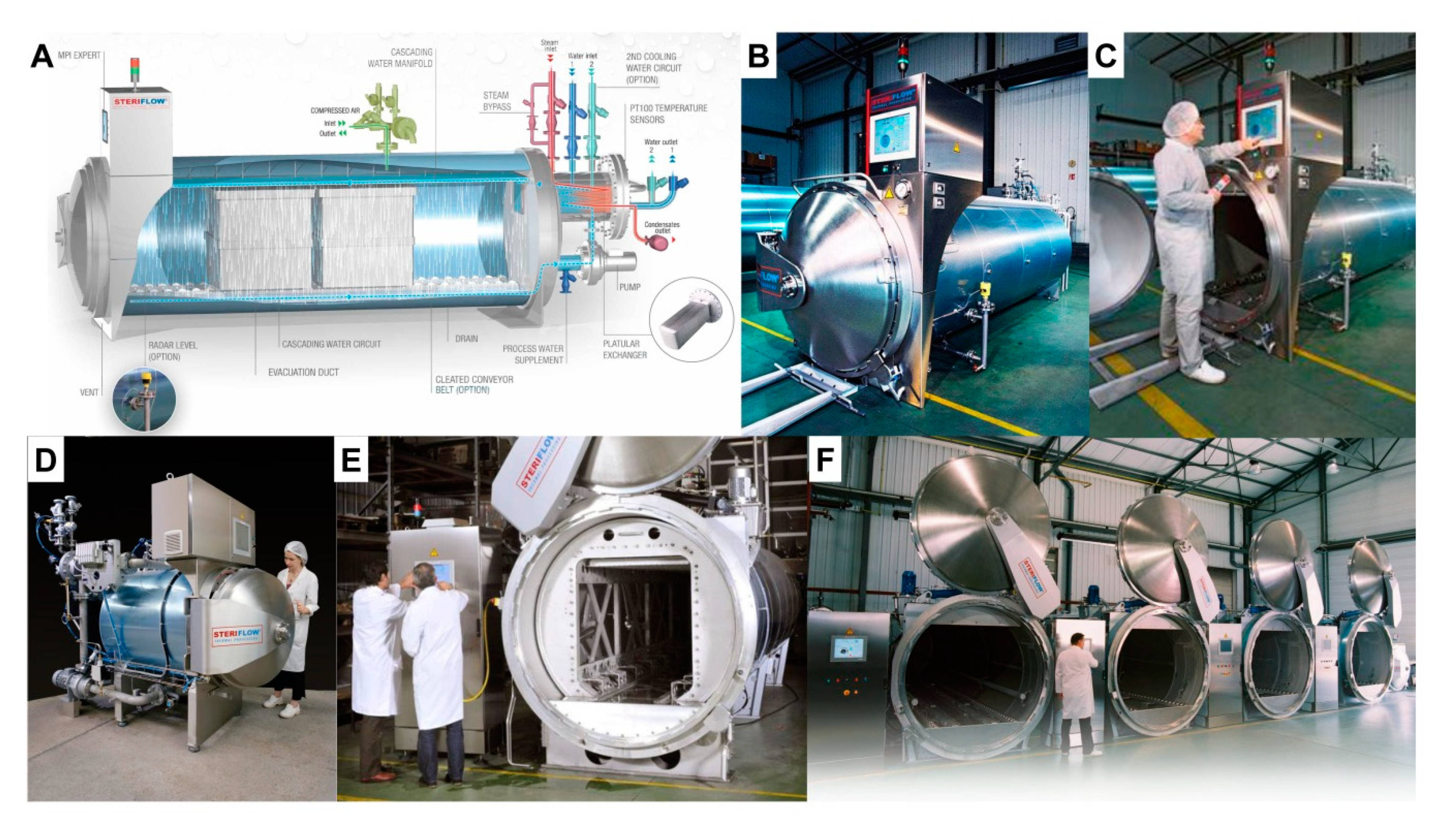
4. Pasteurization
5. Irradiation
6. General Remarks on the Origin of Nutrients in Purified Diets
6.1. Proteins
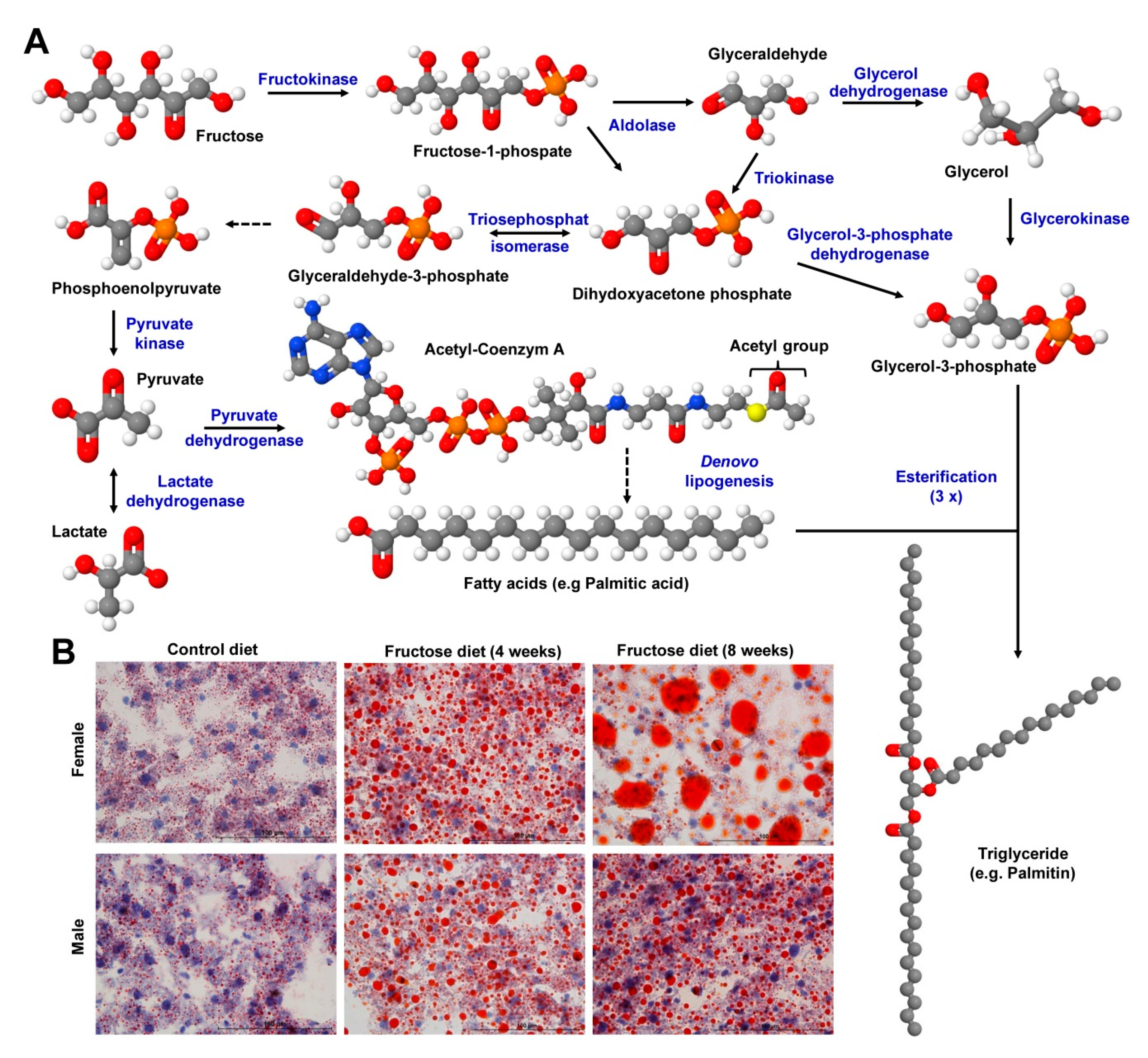
6.2. Carbohydrates
6.3. Fats
6.3.1. Lard
6.3.2. Corn Oil
6.3.3. Safflower Oil
6.3.4. Menhaden Oil
6.4. Vitamins
6.5. Minerals and Trace Elements
6.6. Fibers
7. Nutrient Requirements of the Mouse
8. Representative Examples of Diet-Induced Obesity and Fatty Liver Disease
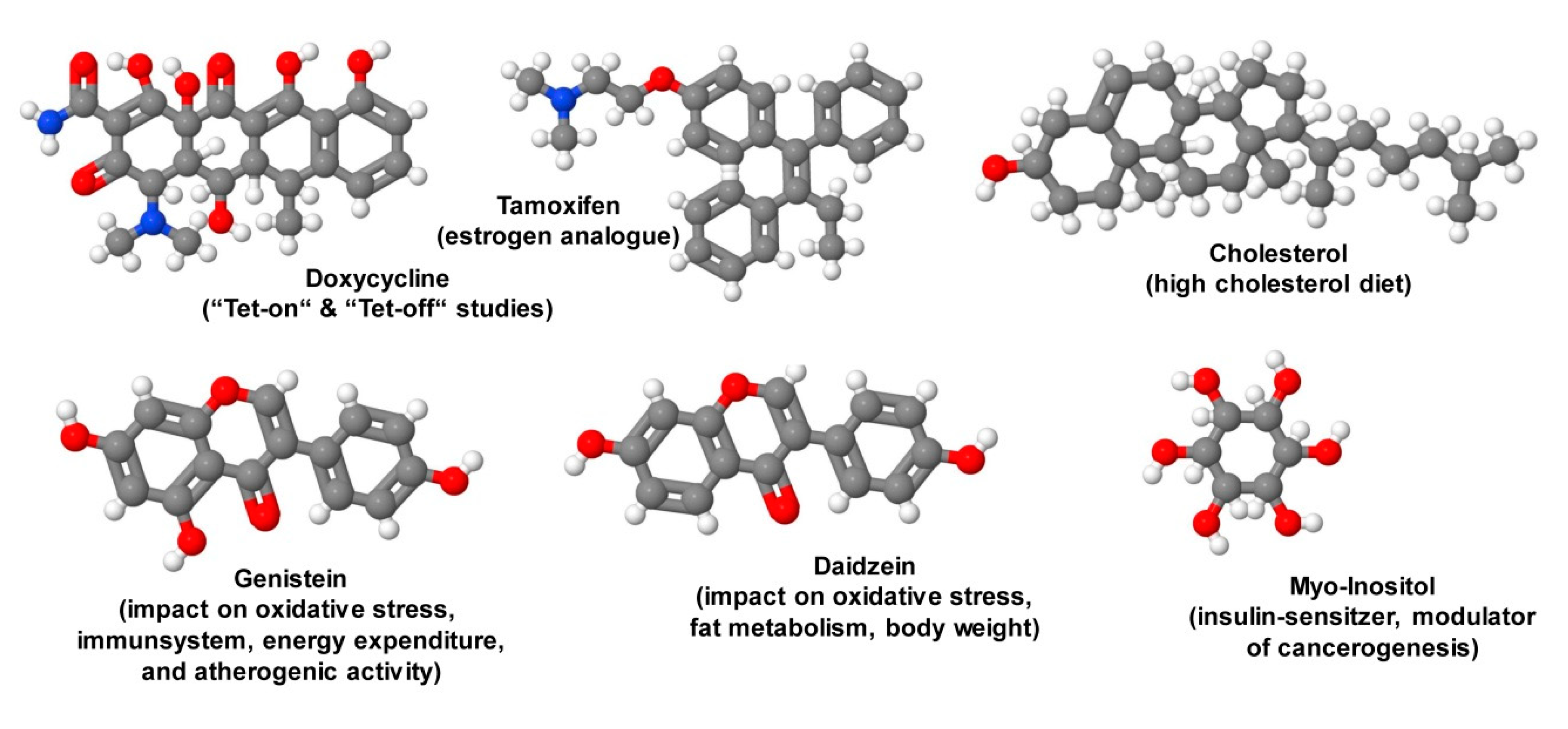
9. Special Ingredients
10. Diet Coloring
11. Diversity of Diet Ingredients may Confound Data Interpretation
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAALAC | Association for Assessment and Accreditation of Laboratory Animal Care |
| ADI | Acceptable daily intake |
| AIN | American Institute of Nutrition |
| ANSES | Agency for Food, Environmental and Occupational Health & Safety (L’Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail) |
| BARQA | British Association for Research Quality Assurance |
| CEN | European Committee for Standardization |
| CID | Compound identification |
| DHAc | Docosyhexaenoic acid |
| DIN | German Institute for Standardization (Deutsches Institut für Normierung) |
| DIO | Diet-induced obesity |
| EPA | Environmental Protection Agency |
| EPAc | Eicosapentaenoic acid |
| EU | European Union |
| FAO | Food and Agriculture Organization of the United Nations |
| FD&C | Federal Food, Drug and Cosmetic act |
| FDA | Food and Drug Administration |
| FFC | fat, fructose and cholesterol-rich diet |
| FSA | Food Standards Agency |
| GLP | Good laboratory practice |
| GMP | Good manufacturing practices |
| HDL | High density lipoprotein |
| HFD | High-fat diet |
| ISO | International Organization for Standardization |
| JECFA | Joint (FAO/WHO) Expert Committee for Food Additives |
| LDL | Low-density lipoprotein |
| LDLR | Low-density lipoprotein receptor |
| MCD | Methionine-choline-deficient diet |
| MND | Menadione nicotinamide bisulfite |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NRC | National Research Council |
| SAM | S-Adenosylmethionine |
| SPF | Specified pathogen free |
| SOP | Standard operating protocol(s) |
| WD | Western diet |
| WHO | World Health Organization |
References
- Guénet, J.-L.; Orth, A.; Bonhomme, F. Origins and phylogenetic relationships of the laboratory mouse. In The Laboratory Mouse; Hedrich, H.J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2012; pp. 3–20. ISBN 978-0-12-382008-2. [Google Scholar]
- Brinster, R.L.; Chen, H.Y.; Trumbauer, M.; Senear, A.W.; Warren, R.; Palmiter, R.D. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell 1981, 27, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Liedtke, C.; Luedde, T.; Sauerbruch, T.; Scholten, D.; Streetz, K.; Tacke, F.; Tolba, R.; Trautwein, C.; Trebicka, J.; Weiskirchen, R. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenes. Tissue Repair 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolba, R.H.; Riederer, B.M.; Weiskirchen, R. Standard operating procedures in experimental liver research: Time to achieve uniformity. Lab. Anim. 2015, 49, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Report from the Commission to the Council and the European Parliament. Seventh Report on the Statistics on the Number of Animals Used for Experimental and Other Scientific Purposes in the Member States of the European Union. 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52013DC0859&from=EN (accessed on 6 January 2020).
- Tayler, K.; Gordon, N.; Langley, G.; Higgins, W. Estimates for worldwide laboratory animal use in 2005. Altern. Lab. Anim. 2008, 36, 327–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Reprinted by UFAW, Potters Bar, Herts, 1992; Methuen & Co Ltd.: London, UK, 1959. [Google Scholar]
- Herrmann, K.; Pistollato, F.; Stephens, M.L. Beyond the 3Rs: Expanding the use of human-relevant replacement methods in biomedical research. ALTEX 2019, 36, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormandy, E.H.; Schuppli, C.A. Public Attitudes toward Animal Research: A Review. Animals 2014, 4, 391–408. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Subcommittee on Laboratory Animal Nutrition; National Academy Press: Washington, DC, USA, 1995; ISBN 0-309-05126-6. [Google Scholar]
- Pellizzon, M.A.; Ricci, M.R. The common use of improper control diets in diet-induced metabolic disease research confounds data interpretation: The fiber factor. Nutr. Metab. 2018, 15, 3. [Google Scholar] [CrossRef]
- Pellizzon, M. Choice of laboratory animal diet influences intestinal health. Lab. Anim. 2016, 45, 238–239. [Google Scholar] [CrossRef] [Green Version]
- Ramadori, P.; Weiskirchen, R.; Trebicka, J.; Streetz, K. Mouse models of metabolic liver injury. Lab. Anim. 2015, 49, 47–58. [Google Scholar] [CrossRef]
- Drew, J.E.; Reichardt, N.; Williams, L.M.; Mayer, C.D.; Walker, A.W.; Farquharson, A.J.; Kastora, S.; Farquharson, F.; Milligan, G.; Morrison, D.J.; et al. Dietary fibers inhibit obesity in mice, but host responses in the cecum and liver appear unrelated to fiber-specific changes in cecal bacterial taxonomic composition. Sci. Rep. 2018, 8, 15566. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, J.D.; Yanckello, L.M.; Chlipala, G.; Hammond, T.C.; McCulloch, S.D.; Parikh, I.; Sun, S.; Morganti, J.M.; Green, S.J.; Lin, A.L. Dietary inulin alters the gut microbiome, enhances systemic metabolism and reduces neuroinflammation in an APOE4 mouse model. PLoS ONE 2019, 14, e0221828. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Sandhu, Y.K.; Korzun, W.J.; Ghosh, S. Dietary supplementation with galactooligosaccharides attenuates high-fat, high-cholesterol diet-induced glucose intolerance and disruption of colonic mucin layer in C57BL/6 mice and reduces atherosclerosis in Ldlr-/- mice. J. Nutr. 2019, nxz233. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Meyer, J.G.; Wang, G.X.; Gupta, M.K.; Batista, T.M.; Lauritzen, H.P.M.M.; Fujisaka, S.; Serra, D.; Herrero, L.; Willoughby, J.; et al. Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab. 2019, 30, 735. [Google Scholar] [CrossRef] [PubMed]
- Muehlmann, A.M.; Bliznyuk, N.; Duerr, I.; Yang, T.P.; Lewis, M.H. Early exposure to a methyl donor supplemented diet and the development of repetitive motor behavior in a mouse model. Dev. Psychobiol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Report of the American Institute of Nutrition ad hoc Committee on Standards for Nutritional Studies. J. Nutr. 1977, 107, 1340–1348. [CrossRef]
- Kumar, P.; Sandeep, K.P. Thermal principles and kinetics. In Processing: Principles and Applications, 2nd ed.; Clark, S., Jung, S., Lamsal, B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 17–31. ISBN 978-0-470-67114-6. [Google Scholar]
- Kurtz, D.M.; Glascoe, R.; Caviness, G.; Locklear, J.; Whiteside, T.; Ward, T.; Adsit, F.; Lih, F.; Deterding, L.J.; Churchwell, M.I.; et al. Acrylamide production in autoclaved rodent feed. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 703–711. [Google Scholar] [CrossRef]
- Ford, D.J. Effect of autoclaving and physical structure of diets on their utilization by mice. Lab. Anim. 1977, 11, 235–239. [Google Scholar] [CrossRef]
- Tusnio, A.; Taciak, M.; Barszcz, M.; Paradziej-Lukowicz, J.; Oledzka, I.; Wiczkowski, W.; Szumska, M.; Patusuzewska, B.; Skomial, J. Thermal sterilization affects the content of selected compounds in diets for laboratory animals. J. Anim. Feed Sci. 2014, 23, 351–360. [Google Scholar] [CrossRef]
- Twaddle, N.C.; Churchwell, M.I.; McDaniel, L.P.; Doerge, D.R. Autoclave sterilization produces acrylamide in rodent diets: Implications for toxicity testing. J. Agric. Food Chem. 2004, 52, 4344–4349. [Google Scholar] [CrossRef]
- Caulfield, C.D.; Cassidy, J.P.; Kelly, J.P. Effects of gamma irradiation and pasteurization on the nutritive composition of commercially available animal diets. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 61–66. [Google Scholar] [PubMed]
- Kilcast, D. Effect of irradiation on vitamins. Food Chem. 1994, 49, 157–164. [Google Scholar] [CrossRef]
- Hammer, C.T.; Wills, E.D. The effect of ionizing radiation on the fatty acid composition of natural fats and on lipid peroxide formation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1979, 35, 323–332. [Google Scholar] [CrossRef]
- Key, D. Environmental enrichment options for laboratory rats and mice. Lab. Anim. 2004, 33, 39–44. [Google Scholar] [CrossRef]
- Fjære, E.; Myrmel, L.S.; Lützhøft, D.O.; Andersen, H.; Holm, J.B.; Kiilerich, P.; Hannisdal, R.; Liaset, B.; Kristiansen, K.; Madsen, L. Effects of exercise and dietary protein sources on adiposity and insulin sensitivity in obese mice. J. Nutr. Biochem. 2019, 66, 98–109. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein proteins: structural and functional aspects. Chapter 1. In Milk Proteins—From Structure to Biological Properties and Health Aspects; Gigli, I., Ed.; IntechOpen: London, UK, 2015; ISBN 978-953-51-2537-2. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, T.; Fukui, K.; Takamatsu, K.; Hashimoto, Y.T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition 2000, 16, 349–354. [Google Scholar] [CrossRef]
- Kuang, H.; Yang, F.; Zhang, Y.; Wang, T.; Chen, G. The impact of egg nutrient composition and its consumption on cholesterol homeostasis. Cholesterol 2018, 2018, 6303810. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Azuma, Y. Egg white hydrolysate improves glucose tolerance in type-2 diabetic NSY mice. J. Nutr. Sci. Vitaminol. 2017, 63, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Park, J.K.; Kim, H.W.; Lee, W.Y. Effects of egg white consumption on allergy, immune modulation, and blood cholesterol levels in BALB/c mice. Korean J. Food Sci. Anim. Resour. 2014, 34, 630–637. [Google Scholar] [CrossRef]
- Maeta, A.; Matsushima, M.; Katahira, R.; Sakamoto, N.; Takahashi, K. Diets supplemented with 1% egg white induce oral desensitization and immune tolerance in an egg white-specific allergic mouse model. Int. Arch. Allergy Immunol. 2018, 176, 205–214. [Google Scholar] [CrossRef]
- Harzer, G.; Kauer, H. Binding of zinc to casein. Am. J. Clin. Nutr. 1982, 35, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomastowski, P.; Sprynskyy, M.; Buszewski, B. The study of zinc ions binding to casein. Colloids Surf. B Biointerfaces 2014, 120, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Diet & Fitness Today. Online Health and Fitness. Available online: http://www.dietandfitnesstoday.com/zinc-in-egg-whites.php (accessed on 6 January 2020).
- Rasmussen, M.V.; Barker, T.T.; Silbart, L.K. High affinity binding site-mediated prevention of chemical absorption across the gastrointestinal tract. Toxicol. Lett. 2001, 125, 51–59. [Google Scholar] [CrossRef]
- Hirakawa, D.A.; Olson, L.M.; Baker, D.H. Comparative utilization of a crystalline amino acid diet and a methionine-fortified casein diet by young rats and mice. Nutr. Res. 1984, 4, 891–895. [Google Scholar] [CrossRef]
- Maddy, K.H.; Elvehjem, C.A. Studies on growth of mice fed rations containing free amino acids. J. Biol. Chem. 1949, 177, 577–590. [Google Scholar]
- Glendinning, J.I.; Breinager, L.; Kyrillou, E.; Lacuna, K.; Rocha, R.; Sclafani, A. Differential effects of sucrose and fructose on dietary obesity in four mouse strains. Physiol. Behav. 2010, 101, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Gibson, S.; Gunn, P.; Wittekind, A.; Cottrell, R. The effects of sucrose on metabolic health: A systematic review of human intervention studies in healthy adults. Crit. Rev. Food Sci. Nutr. 2013, 53, 591–614. [Google Scholar] [CrossRef] [Green Version]
- Tappy, L. Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J. Exp. Biol. 2018, 221, jeb164202. [Google Scholar] [CrossRef] [Green Version]
- Lambertz, J.; Berger, T.; Mak, T.W.; van Helden, J.; Weiskirchen, R. Lipocalin-2 in fructose-induced fatty liver disease. Front. Physiol. 2017, 8, 964. [Google Scholar] [CrossRef] [Green Version]
- Lambertz, J.; Weiskirchen, S.; Landert, S.; Weiskirchen, R. Fructose: A dietary sugar in crosstalk with microbiota contributing to the development and progression of non-alcoholic liver disease. Front. Immunol. 2017, 8, 1159. [Google Scholar] [CrossRef] [Green Version]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit. Rev. Biotechnol. 2016, 36, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieffer, D.A.; Piccolo, B.D.; Marco, M.L.; Kim, E.B.; Goodson, M.L.; Keenan, M.J.; Dunn, T.N.; Knudsen, K.E.; Martin, R.J.; Adams, S.H. Mice fed a high-fat diet supplemented with resistant starch display marked shifts in the liver metabolome concurrent with altered gut bacteria. J. Nutr. 2016, 146, 2476–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubant, R.; Poon, A.N.; Sánchez-Hernández, D.; Domenichiello, A.F.; Huot, P.S.; Pannia, E.; Cho, C.E.; Hunschede, S.; Bazinet, R.P.; Anderson, G.H. A comparison of effects of lard and hydrogenated vegetable shortening on the development of high-fat diet-induced obesity in rats. Nutr. Diabetes 2015, 5, e188. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, X.; Gao, Q.; Fan, L.; Jin, H.; Guo, Y. Differential effects of olive oil, soybean oil, corn oil and lard oil on carbon tetrachloride induced liver fibrosis in mice. Biosci. Rep. 2019, BSR20191913. [Google Scholar] [CrossRef] [Green Version]
- Ghazani, S.M.; Marangoni, A.G. Healthy Fats and Oils. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 257–267. ISBN 978-0-12-394437-5. [Google Scholar]
- Dupont, J.; White, P.J.; Carpenter, M.P.; Schaefer, E.J.; Meydani, S.N.; Elson, C.E.; Woods, M.; Gorbach, S.L. Food uses and health effects of corn oil. J. Am. Coll. Nutr. 1990, 9, 438–470. [Google Scholar] [CrossRef]
- Si, H.; Zhang, L.; Liu, S.; LeRoith, T.; Virgous, C. High corn oil dietary intake improves health and longevity of aging mice. Exp. Gerontol. 2014, 58, 244–249. [Google Scholar] [CrossRef]
- Drescher, H.K.; Weiskirchen, R.; Fülöp, A.; Hopf, C.; de San Román, E.G.; Huesgen, P.F.; de Bruin, A.; Bongiovanni, L.; Christ, A.; Tolba, R.; et al. The influence of different fat sources on steatohepatitis and fibrosis development in the Western diet mouse model of non-alcoholic steatohepatitis (NASH). Front. Physiol. 2019, 10, 770. [Google Scholar] [CrossRef]
- Wong, C.K.; Botta, A.; Pither, J.; Dai, C.; Gibson, W.T.; Ghosh, S. A high-fat diet rich in corn oil reduces spontaneous locomotor activity and induces insulin resistance in mice. J. Nutr. Biochem. 2015, 26, 319–326. [Google Scholar] [CrossRef]
- Kim, S.K.; Cha, J.Y.; Jeong, S.J.; Chung, C.H.; Choi, Y.R.; Cho, Y.S. Properties of the chemical composition of safflower (Carthamus tinctorius L.) sprout. Korean J. Life Sci. 2000, 10, 68–73. [Google Scholar]
- Ide, T.; Origuchi, I. Physiological effects of an oil rich in γ-linolenic acid on hepatic fatty acid oxidation and serum lipid levels in genetically hyperlipidemic mice. J. Clin. Biochem. Nutr. 2019, 64, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucchi, D.; Camacho-Muñoz, D.; Certo, M.; Niven, J.; Smith, J.; Nicolaou, A.; Mauro, C. Omega-3 polyunsaturated fatty acids impinge on CD4+ T cell motility and adipose tissue distribution via direct and lipid mediator-dependent effects. Cardiovasc. Res. 2019, cvz208. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Dasari, S.; Lanza, I.R. EPA and DHA elicit distinct transcriptional responses to high-fat feeding in skeletal muscle and liver. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E460–E472. [Google Scholar] [CrossRef]
- Depner, C.M.; Torres-Gonzalez, M.; Tripathy, S.; Milne, G.; Jump, D.B. Menhaden oil decreases high-fat diet-induced markers of hepatic damage, steatosis, inflammation, and fibrosis in obese Ldlr-/- mice. J. Nutr. 2012, 142, 1495–1503. [Google Scholar] [CrossRef] [Green Version]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Lonsdale, D. A review of the biochemistry, metabolism and clinical benefits of thiamin(e) and its derivatives. Evid. Based Complement Alternat. Med. 2006, 3, 49–59. [Google Scholar] [CrossRef]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, M.K.; Jacobson, E.L. Vitamin B3 in health and disease: Toward the second century of discovery. Methods Mol. Biol. 2018, 1813, 3–8. [Google Scholar] [CrossRef]
- Stover, P.J.; Field, M.S. Vitamin B-6. Adv. Nutr. 2015, 6, 132–133. [Google Scholar] [CrossRef]
- Wilson, M.P.; Plecko, B.; Mills, P.B.; Clayton, P.T. Disorders affecting vitamin B6 metabolism. J. Inherit. Metab. Dis. 2019, 42, 629–646. [Google Scholar] [CrossRef] [Green Version]
- Saleem, F.; Soos, M.P. Biotin Deficiency. StatPearls Publishing. Available online: http://www.ncbi.nlm.nih.gov/books/NBK547751/ (accessed on 6 January 2020).
- Stover, P.J. Physiology of folate and vitamin B12 in health and disease. Nutr. Rev. 2004, 62, S3–S12. [Google Scholar] [CrossRef]
- Moreno-Garcia, M.A.; Rosenblatt, D.S.; Jerome-Majewska, L.A. Vitamin B12 metabolism during pregnancy and in embryonic mouse models. Nutrients 2013, 5, 3531–3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinder, R.; Cooley, R.; Vlad, L.G.; Molnar, J.A. Vitamin A and Wound Healing. Nutr. Clin. Pract. 2019, 34, 839–849. [Google Scholar] [CrossRef]
- Polcz, M.E.; Barbul, A. The role of vitamin A in wound healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, L.; Li, C.; Gai, Z.; Li, Y. Vitamin D and vitamin D receptor: New insights in the treatment of hypertension. Curr. Protein Pept. Sci. 2019, 20, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D: A micronutrient regulating genes. Curr. Pharm. Des. 2019, 25, 1740–1746. [Google Scholar] [CrossRef]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef]
- Hirota, Y.; Suhara, Y. New aspects of vitamin K research with synthetic ligands: transcriptional activity via SXR and neural differentiation activity. Int. J. Mol. Sci. 2019, 20, 3006. [Google Scholar] [CrossRef] [Green Version]
- Ottaway, P.B. Stability of vitamins in food. In The Technology of Vitamins in Food; Ottaway, P.B., Ed.; Springer: Boston, MA, USA, 1993; pp. 90–113. ISBN 978-1-4613-5889-3. [Google Scholar]
- Riaz, M.N.; Asif, M.; Ali, R. Stability of vitamins during extrusion. Crit. Rev. Food Sci. Nutr. 2009, 49, 361–368. [Google Scholar] [CrossRef]
- Hadinata Lie, A.; VChandra-Hioe, M.; Arcot, J. Sorbitol enhances the physicochemical stability of B12 vitamers. Int. J. Vitam. Nutr. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.M.; Horner, K.; De Vito, G.; Conway, G.E. The role of mineral and trace element supplementation in exercise and athletic performance: A systematic review. Nutrients 2019, 11, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hervik, A.K.; Svihus, B. The role of fiber in energy balance. J. Nutr. Metab. 2019, 2019, 4983657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Matallana-González, M.C.; Morales, P. Dietary fiber sources and human benefits: The case study of cereal and pseudocereals. Adv. Food Nutr. Res. 2019, 90, 83–134. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Wine, R.H. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar]
- Konishi, F.; Oku, T.; Hosoya, N. Hypertrophic effect of unavailable carbohydrate on cecum and colon in rats. J. Nutr. Sci. Vitaminol. 1984, 30, 373–379. [Google Scholar] [CrossRef]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef] [Green Version]
- Ellacott, K.L.; Morton, G.J.; Woods, S.C.; Tso, P.; Schwartz, M.W. Assessment of feeding behavior in laboratory mice. Cell Metab. 2010, 12, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Stebegg, M.; Silva-Cayetano, A.; Innocentin, S.; Jenkins, T.P.; Cantacessi, C.; Gilbert, C.; Linterman, M.A. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat. Commun. 2019, 10, 2443. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Sun, A.Y. Synaptosomal plasma membranes: Acyl group composition of phosphoglycerides and (Na+ plus K+)-ATPase activity during fatty acid deficiency. J. Neurochem. 1974, 22, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.G.; Potter, A.J.; Rabinovitch, P.S. Splenocyte glutathione and CD3-mediated cell proliferation are reduced in mice fed a protein-deficient diet. J. Nutr. 1997, 127, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Millis, R.M.; Offiah, G.U. Dietary protein deficiency in pregnant mice and offspring. Life Sci. 2007, 80, 1184–1188. [Google Scholar] [CrossRef]
- Guo, F.; Cavener, D.R. The GCN2 eIF2α kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007, 5, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Meng, Q.; Wang, C.; Li, H.; Huang, Z.; Chen, S.; Xiao, F.; Guo, F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010, 59, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Huang, Z.; Li, H.; Yu, J.; Wang, C.; Chen, S.; Meng, Q.; Cheng, Y.; Gao, X.; Li, J.; et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes 2011, 60, 746–756. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Du, Y.; Lv, Z.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F. Effects of essential amino acids on lipid metabolism in mice and humans. J. Mol. Endocrinol. 2016, 57, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.S.; Byun, H.S.; Yoon, K.H.; Lee, J.S.; Choi, K.C.; Jeung, E.B. Dietary calcium and vitamin D2 supplementation with enhanced Lentinula edodes improves osteoporosis-like symptoms and induces duodenal and renal active calcium transport gene expression in mice. Eur. J. Nutr. 2009, 48, 75–83. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A.; Mills, B.G. Magnesium deficiency: effect on bone and mineral metabolism in the mouse. Calcif. Tissue Int. 2003, 72, 32–41. [Google Scholar] [CrossRef]
- Günther, T.; Ising, H.; Mohr-Nawroth, F.; Chahoud, I.; Merker, H.J. Embryotoxic effects of magnesium deficiency and stress on rats and mice. Teratology 1981, 24, 225–233. [Google Scholar] [CrossRef]
- Stubbs, J.R.; Liu, S.; Tang, W.; Zhou, J.; Wang, Y.; Yao, X.; Quarles, L.D. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J. Am. Soc. Nephrol. 2007, 18, 2116–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, A.; Miyado, K.; Yamatoya, K.; Kawano, N.; Umezawa, A. Breast milk stimulates growth hormone secretion in infant mice, and phosphorus insufficiency disables this ability and causes dwarfism-like symptoms. Regen. Ther. 2015, 2, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Capelo, A.; Castells, M.T.; Cremades, A.; Peñafiel, R. Hypokalemia decreases testosterone production in male mice by altering luteinizing hormone secretion. Endocrinology 1996, 137, 3738–3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matak, P.; Zumerle, S.; Mastrogiannaki, M.; El Balkhi, S.; Delga, S.; Mathieu, J.R.; Canonne-Hergaux, F.; Poupon, J.; Sharp, P.A.; Vaulont, S.; et al. Copper deficiency leads to anemia, duodenal hypoxia, upregulation of HIF-2α and altered expression of iron absorption genes in mice. PLoS ONE 2013, 8, e59538. [Google Scholar] [CrossRef] [Green Version]
- Gibson, J.N.; Jellen, L.C.; Unger, E.L.; Morahan, G.; Mehta, M.; Earley, C.J.; Allen, R.P.; Lu, L.; Jones, B.C. Genetic analysis of iron-deficiency effects on the mouse spleen. Mamm. Genome 2011, 22, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahdila, D.; Markowitz, K.; Pawar, S.; Chavan, K.; Fine, D.H.; Velliyagounder, K. The effect of iron deficiency anemia on experimental dental caries in mice. Arch. Oral Biol. 2019, 105, 13–19. [Google Scholar] [CrossRef]
- Kemmerer, A.R.; Elvehjem, C.A.; Hart, E.B. Studies on the relation of manganese to the nutrition of the mouse. J. Biol. Chem. 1931, 92, 623–630. [Google Scholar]
- Shils, M.E.; McCollum, E.V. Further studies on the symptoms of manganese deficiency in the rat and mouse: Five figures. J. Nutr. 1943, 26, 1–19. [Google Scholar] [CrossRef]
- Bolick, D.T.; Kolling, G.L.; Moore, J.H.; de Oliveira, L.A.; Tung, K.; Philipson, C.; Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J.; Guerrant, R.L. Zinc deficiency alters host response and pathogen virulence in a mouse model of enteroaggregative Escherichia coli-induced diarrhea. Gut Microbes 2014, 5, 618–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, R.T., Jr.; Stevenson, J.K. Development of carcinoma of the thyroid in iodine-deficient mice. Cancer 1966, 19, 1063–1080. [Google Scholar] [CrossRef]
- Maier, J.; van Steeg, H.; van Oostrom, C.; Paschke, R.; Weiss, R.E.; Krohn, K. Iodine deficiency activates antioxidant genes and causes DNA damage in the thyroid gland of rats and mice. Biochim. Biophys. Acta 2007, 1773, 990–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, S.M.; Aliesky, H.; Banuelos, B.; Hee, S.S.Q.; Rapoport, B. Variable effects of dietary selenium in mice that spontaneously develop a spectrum of thyroid autoantibodies. Endocrinology 2017, 158, 3754–3764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, S.H.; Clish, C.B.; Gladyshev, V.N. Selenium deficiency is associated with pro-longevity mechanisms. Cell Rep. 2019, 27, 2785–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustafa, M.E.; Carlson, B.A.; Anver, M.R.; Bobe, G.; Zhong, N.; Ward, J.M.; Perella, C.M.; Hoffmann, V.J.; Rogers, K.; Combs, G.F., Jr.; et al. Selenium and selenoprotein deficiencies induce widespread pyogranuloma formation in mice, while high levels of dietary selenium decrease liver tumor size driven by TGFα. PLoS ONE 2013, 8, e57389. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Vazquez, A.J.; Garcia-Sanchez, J.A.; Moreno-Arriola, E.; Salvador-Adriano, A.; Ortega-Cuellar, D.; Velazquez-Arellano, A. Thiamine deprivation produces a liver ATP deficit and metabolic and genomic effects in mice: findings are parallel to those of biotin deficiency and have implications for energy disorders. J. Nutrigenet. Nutrigenom. 2016, 9, 287–299. [Google Scholar] [CrossRef]
- Ochoa-Ruiz, E.; Díaz-Ruiz, R.; Hernández-Vázquez Ade, J.; Ibarra-González, I.; Ortiz-Plata, A.; Rembao, D.; Ortega-Cuéllar, D.; Viollet, B.; Uribe-Carvajal, S.; Corella, J.A.; et al. Biotin deprivation impairs mitochondrial structure and function and has implications for inherited metabolic disorders. Mol. Genet. Metab. 2015, 116, 204–214. [Google Scholar] [CrossRef]
- Elhusseini, H.; Elkafas, H.; Abdelaziz, M.; Halder, S.; Atabiekov, I.; Eziba, N.; Ismail, N.; El Andaloussi, A.; Al-Hendy, A. Diet-induced vitamin D deficiency triggers inflammation and DNA damage profile in murine myometrium. Int. J. Womens Health 2018, 10, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mihalcioiu, M.; Li, L.; Zakikhani, M.; Camirand, A.; Kremer, R. Vitamin D prevents lipid accumulation in murine muscle through regulation of PPARγ and perilipin-2 expression. J. Steroid Biochem. Mol. Biol. 2018, 177, 116–124. [Google Scholar] [CrossRef]
- Nakamoto, A.; Shuto, E.; Tsutsumi, R.; Nakamoto, M.; Nii, Y.; Sakai, T. Vitamin A Deficiency Impairs Induction of Oral Tolerance in Mice. J. Nutr. Sci. Vitaminol. 2015, 61, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citelli, M.; Bittencourt, L.L.; da Silva, S.V.; Pierucci, A.P.; Pedrosa, C. Vitamin A modulates the expression of genes involved in iron bioavailability. Biol. Trace Elem. Res. 2012, 149, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; O’Connell, J.F.; Carlson, O.D.; González-Mariscal, I.; Kim, Y.; Moaddel, R.; Ghosh, P.; Egan, J.M. Linoleic acid in diets of mice increases total endocannabinoid levels in bowel and liver: Modification by dietary glucose. Obes. Sci. Pract. 2019, 5, 383–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raubenheimer, P.J.; Nyirenda, M.J.; Walker, B.R. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes 2006, 55, 2015–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuura, K.; Suetsugu, A.; Satake, T.; Nakamura, M.; Kunisada, T.; Shimizu, M.; Hoffman, R.M. Choline-deficient-diet decreases fibroblasts in the circulating tumor cell (CTC) microenvironment. Anticancer Res. 2019, 39, 4061–4064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhla, A.; Hahn, S.; Butschkau, A.; Lange, S.; Wree, A.; Vollmar, B. Lifelong caloric restriction reprograms hepatic fat metabolism in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.; Ji, S.; Maudsley, S.; Mattson, M.P. Control laboratory rodents are metabolically morbid: Why it matters. Proc. Natl. Acad. Sci. USA 2010, 107, 6127–6133. [Google Scholar] [CrossRef] [Green Version]
- Ritskes-Hotinga, M.; Tobin, G.; Jensen, T.L.; Mikkelsen, L.F. Nutrition of the Laboratory Mouse; The laboratory mouse; Hedrich, H.J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2012; pp. 567–599. ISBN 978-0-12-382008-2. [Google Scholar]
- Machado, M.V.; Michelotti, G.A.; Xie, G.; Almeida Pereira, T.; Boursier, J.; Bohnic, B.; Guy, C.D.; Diehl, A.M. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS ONE 2015, 10, e0127991. [Google Scholar] [CrossRef] [Green Version]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Nizar, J.M.; Dong, W.; McClellan, R.B.; Labarca, M.; Zhou, Y.; Wong, J.; Goens, D.G.; Zhao, M.; Velarde, N.; Bernstein, D.; et al. Na+-sensitive elevation in blood pressure is ENaC independent in diet-induced obesity and insulin resistance. Am. J. Physiol. Renal. Physiol. 2016, 310, F812–F820. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castañeda, T.R.; Tong, J.; Datta, R.; Culler, M.; Tschöp, M.H. Ghrelin in the regulation of body weight and metabolism. Front. Neuroendocrinol. 2010, 31, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Temel, R.E.; Rudel, L.L. Diet effects on atherosclerosis in mice. Curr. Drug Targets 2007, 8, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Diet and murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumiyoshi, M.; Sakanaka, M.; Kimura, Y. Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice. J. Nutr. 2006, 136, 582–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Byun, H.R.; Ying, Z.; Blencowe, M.; Zhao, Y.; Hong, J.; Shu, L.; Chella Krishnan, K.; Gomez-Pinilla, F.; Yang, X. Differential metabolic and multi-tissue transcriptomic responses to fructose consumption among genetically diverse mice. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165569. [Google Scholar] [CrossRef]
- Brandt, A.; Jin, C.J.; Nolte, K.; Sellmann, C.; Engstler, A.J.; Bergheim, I. Short-term intake of a fructose-, fat- and cholesterol-rich diet causes hepatic steatosis in mice: Effect of antibiotic treatment. Nutrients 2017, 9, 1013. [Google Scholar] [CrossRef] [Green Version]
- Rivière, S.; Soubeyre, V.; Jarriault, D.; Molinas, A.; Léger-Charnay, E.; Desmoulins, L.; Grebert, D.; Meunier, N.; Grosmaitre, X. High fructose diet inducing diabetes rapidly impacts olfactory epithelium and behavior in mice. Sci. Rep. 2016, 6, 34011. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, N.; Cheishvili, D.; Arakelian, A.; Tanvir, I.; Khan, H.A.; Pépin, A.S.; Szyf, M.; Rabbani, S.A. Methyl donor S-adenosylmethionine (SAM) supplementation attenuates breast cancer growth, invasion, and metastasis in vivo; therapeutic and chemopreventive applications. Oncotarget 2017, 9, 5169–5183. [Google Scholar] [CrossRef] [Green Version]
- De Conti, A.; Pogribny, I.P. Epigenetics of dietary methyl-group donor deficiency and liver cancer. In Handbook of Nutrition, Diet, and Epigenetics; Patel, V., Preedy, V., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Mbikay, M.; Mayne, J.; Sirois, F.; Fedoryak, O.; Raymond, A.; Noad, J.; Chrétien, M. Mice Fed a High-cholesterol diet supplemented with quercetin-3-glucoside show attenuated hyperlipidemia and hyperinsulinemia associated with differential regulation of PCSK9 and LDLR in their liver and pancreas. Mol. Nutr. Food Res. 2018, 62, e1700729. [Google Scholar] [CrossRef]
- Matsuzawa, N.; Takamura, T.; Kurita, S.; Misu, H.; Ota, T.; Ando, H.; Yokoyama, M.; Honda, M.; Zen, Y.; Nakanuma, Y.; et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 2007, 46, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Shockley, K.R.; Witmer, D.; Burgess-Herbert, S.L.; Paigen, B.; Churchill, G.A. Effects of atherogenic diet on hepatic gene expression across mouse strains. Physiol. Genom. 2009, 39, 172–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warden, C.H.; Fisler, J.S. Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 2008, 7, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redelsperger, I.M.; Taldone, T.; Riedel, E.R.; Lepherd, M.L.; Lipman, N.S.; Wolf, F.R. Stability of doxycycline in feed and water and minimal effective doses in tetracycline-inducible systems. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 467–474. [Google Scholar] [PubMed]
- Feil, S.; Valtcheva, N.; Feil, R. Inducible Cre mice. Methods Mol. Biol. 2009, 530, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, Z.J.; Chen, M.Q.; Chen, J.J.; Liang, Z.M.; Ding, X.T.; Zhou, M.; Li, S.J.; Li, X.W.; Yang, J.M.; et al. The effects of tamoxifen on mouse behavior. Genes Brain Behav. 2019, e12620. [Google Scholar] [CrossRef]
- Ganai, A.A.; Farooqi, H. Bioactivity of genistein: A review of in vitro and in vivo studies. Biomed. Pharmacother. 2015, 76, 30–38. [Google Scholar] [CrossRef]
- Polkowski, K.; Mazurek, A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol. Pharm. 2000, 57, 135–155. [Google Scholar]
- Marini, H.; Polito, F.; Adamo, E.B.; Bitto, A.; Squadrito, F.; Benvenga, S. Update on genistein and thyroid: An overall message of safety. Front. Endocrinol. 2012, 3, 94. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, S.; Broderick, T.L.; Al-Nakkash, L. Feeding obese diabetic mice a genistein diet induces thermogenic and metabolic change. J. Med. Food 2018, 21, 332–339. [Google Scholar] [CrossRef]
- Luo, T.; Miranda-Garcia, O.; Sasaki, G.; Wang, J.; Shay, N.F. Genistein and daidzein decrease food intake and body weight gain in mice, and alter LXR signaling in vivo and in vitro. Food Funct. 2018, 9, 6257–6267. [Google Scholar] [CrossRef] [PubMed]
- Day, J.K.; Bauer, A.M.; DesBordes, C.; Zhuang, Y.; Kim, B.E.; Newton, L.G.; Nehra, V.; Forsee, K.M.; MacDonald, R.S.; Besch-Williford, C.; et al. Genistein alters methylation patterns in mice. J. Nutr. 2002, 132, 2419S–2423S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, W.; Blackburn, G.L.; Zhou, J.R. Effects of a dadzein-rich isoflavone aglycone extract on diet-induced obesity in an ovarierectomized mouse model. Clin. Exp. Pharm. Physiol. 2007, 34, S55–S57. [Google Scholar] [CrossRef]
- Croze, M.L.; Vella, R.E.; Pillon, N.J.; Soula, H.A.; Hadji, L.; Guichardant, M.; Soulage, C.O. Chronic treatment with myo-inositol reduces white adipose tissue accretion and improves insulin sensitivity in female mice. J. Nutr. Biochem. 2013, 24, 457–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unver, N.; Delgado, O.; Zeleke, K.; Cumpian, A.; Tang, X.; Caetano, M.S.; Wang, H.; Katayama, H.; Yu, H.; Szabo, E.; et al. Reduced IL-6 levels and tumor-associated phospho-STAT3 are associated with reduced tumor development in a mouse model of lung cancer chemoprevention with myo-inositol. Int. J. Cancer 2018, 142, 1405–1417. [Google Scholar] [CrossRef] [Green Version]
- EFSA panel on additives and products or substances used in animal feed (FEEDAP). Safety and efficacy of iron oxide black, red and yellow for animal species. EFSA J. 2016, 14, 4482. [Google Scholar]
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Jacobs, G.H.; Williams, G.A.; Cahill, H.; Nathans, J. Emergence of novel color vision in mice engineered to express a human cone photopigment. Science 2007, 315, 1723–1725. [Google Scholar] [CrossRef]
- Riera, C.E.; Tsaousidou, E.; Halloran, J.; Follett, P.; Hahn, O.; Pereira, M.M.A.; Ruud, L.E.; Alber, J.; Tharp, K.; Anderson, C.M.; et al. The sense of smell impacts metabolic health and obesity. Cell Metab. 2017, 26, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.G.; Faria, R.X.; Ferreira, N.C.; Soares-Bezerra, R.J. Brilliant Blue dyes in daily food: How could purinergic system be affected? Int. J. Food Sci. 2016, 2016, 7548498. [Google Scholar] [CrossRef] [Green Version]
- Hess, S.M.; Fitzhugh, O.G. Absorption and excretion of certain triphenylmethane colors in rats and dogs. J. Pharmacol. Exp. Ther. 1955, 114, 38–42. [Google Scholar] [PubMed]
- Kobylewski, S.; Jacobson, M.F.; Center for Science in the Public Interest. Food Dyes. A Rainbow of Risks. Available online: https://cspinet.org/sites/default/files/attachment/food-dyes-rainbow-of-risks.pdf (accessed on 6 January 2020).
- Angarita, S.A.K.; Duarte, S.; Russell, T.A.; Ruchala, P.; Elliott, I.A.; Whitelegge, J.P.; Zarrinpar, A. Quantitative measure of intestinal permeability using blue food coloring. J. Surg. Res. 2019, 233, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasiak, N.; Kukula-Koch, W.; Glowniak, K. Modern industrial and pharmacological application of indigo dye and its derivatives—A review. Acta Poloniae Pharm. Drug Res. 2014, 71, 215–221. [Google Scholar]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk. Assess. 2017, 34, 335–355. [Google Scholar] [CrossRef] [Green Version]
- FAO. Toxicological evaluation of some food colours, emulsifiers, stabilizers, anti-caking agents and certain other substances. FAO Nutr. Meet. Rep. Ser. 1970, 46, 1–161. [Google Scholar]
- Hansen, W.H.; Long, E.L.; Davis, K.J.; Nelson, A.A.; Fitzhugh, O.G. Chronic toxicity of three food colourings: Guinea Green B, Light Green SF Yellowish and Fast Green FCF in rats, dogs and mice. Food Cosmet. Toxicol. 1966, 4, 389–410. [Google Scholar] [CrossRef]
- IPCS INCHEM Home. Fast Green FCF. Available online: http://www.inchem.org/documents/jecfa/jecmono/v16je12.htm (accessed on 6 January 2020).
- Chequer, F.M.; Venâncio, V.P.; Bianchi, M.L.; Antunes, L.M. Genotoxic and mutagenic effects of erythrosine B, a xanthene food dye, on HepG2 cells. Food Chem. Toxicol. 2012, 50, 3447–3451. [Google Scholar] [CrossRef]
- Certified Color Manufacturers Association. A Consideration of the Data Relating to the Thyroid Effects of FD&C Red No. 3.; File 76N-O366; United States Food and Drug Administration: Washington, DC, USA, 1983. [Google Scholar]
- Lin, G.H.; Brusick, D.J. Mutagenicity studies on FD&C red No.3. Mutagenesis 1986, 1, 253–259. [Google Scholar] [CrossRef] [Green Version]
- IPCS INCHEM Home. Erythrosine. Available online: http://www.inchem.org/documents/jecfa/jecmono/v28je12.htm (accessed on 6 January 2020).
- Doell, D.L.; Folmer, D.E.; Lee, H.S.; Butts, K.M.; Carberry, S.E. Exposure estimate for FD&C colour additives for the US population. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk. Assess. 2016, 33, 782–797. [Google Scholar] [CrossRef]
- WHO. Evaluation of Certain Food Additives. Eighty-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO technical report series 1000; WHO: Geneva, Switzerland, 2016; ISBN 9-78924-121-0003. [Google Scholar]
- Bastaki, M.; Farrell, T.; Bhusari, S.; Pant, K.; Kulkarni, R. Lack of genotoxicity in vivo for food color additive Allura Red AC. Food Chem. Toxicol. 2017, 105, 308–314. [Google Scholar] [CrossRef]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Tanaka, T. Reproductive and neurobehavioural toxicity study of tartrazine administered to mice in the diet. Food Chem. Toxicol. 2006, 44, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Poul, M.; Jarry, G.; Elhkim, M.O.; Poul, J.M. Lack of genotoxic effect of food dyes amaranth, sunset yellow and tartrazine and their metabolites in the gut micronucleus assay in mice. Food Chem. Toxicol. 2009, 47, 443–448. [Google Scholar] [CrossRef] [PubMed]
- EFSA ANS Panel (Panel on Food Additives and Nutrient Sources added to Food). Scientific opinion on the reconsideration of the temporary ADI and refined exposure assessment for Sunset Yellow FCF (E 110). EFSA J. 2014, 12, 3765. [Google Scholar] [CrossRef]
- Sharratt, M.; Frazer, A.C.; Paranjoti, I.S. Biological effects of Citrus Red No. 2 in the mouse. Food Cosmet. Toxicol. 1966, 4, 493–502. [Google Scholar] [CrossRef]
- Kobylewski, S.; Jacobson, M.F. Toxicology of food dyes. Int. J. Occup. Environ. Health 2012, 18, 220–246. [Google Scholar] [CrossRef]
- Park, M.; Park, H.R.; Kim, S.J.; Kim, M.S.; Kong, K.H.; Kim, H.S.; Gong, E.J.; Kim, M.E.; Kim, H.S.; Lee, B.M.; et al. Risk assessment for the combinational effects of food color additives: Neural progenitor cells and hippocampal neurogenesis. J. Toxicol. Environ. Health 2009, 72, 1412–1423. [Google Scholar] [CrossRef]
- Borzelleca, J.F.; Depukat, K.; Hallagan, J.B. Lifetime toxicity/carcinogenicity studies of FD & C Blue, No. 1 (brilliant blue FCF) in rats and mice. Food Chem Toxicol. 1990, 28, 221–234. [Google Scholar] [CrossRef]
- Chassaing, B.; Miles-Brown, J.; Pellizzon, M.; Ulman, E.; Ricci, M.; Zhang, L.; Patterson, A.D.; Vijay-Kumar, M.; Gewirtz, A.T. Lack of soluble fiber drives diet-induced adiposity in mice. Am. J. Physiol. Gastrointest Liver Physiol. 2015, 309, G528–G541. [Google Scholar] [CrossRef]








| Company | Principal Office | Homepage |
|---|---|---|
| Research Diets, Inc. | New Brunswick, NJ, USA | https://www.researchdiets.com/ |
| Ssniff Spezialdiäten GmbH | Soest, Germany | http://www.ssniff.com/ |
| Altromin Spezialfutter GmbH & Co. KG | Lage, Germany | https://altromin.de/ |
| BioServ | Flemington, NJ, USA | https://www.bio-serv.com/ |
| Envigo Teklad (formerly Harlan Teklad) | Madison, WI, USA | https://www.envigo.com/ |
| CLEA Japan, Inc. | Tokyo, Japan | https://www.clea-japan.com/ |
| Specialty Feeds | Glen Forest, Western Australia, Australia | http://www.specialtyfeeds.com |
| Safe | Augy, France | www.safe-diets.com/ |
| LabDiet | St. Louis, MO, USA | https://www.labdiet.com/ |
| TestDiet | St. Louis, MO, USA | https://www.testdiet.com |
| Dyets, Inc. | Bethlehem, PA, USA | https://dyets.com/ |
| Special Diets Services (SDS) | London, UK | http://www.sdsdiets.com/ |
| Substance Class | Representative Compounds |
|---|---|
| Proteins | Casein, soy protein isolate, egg white protein; crystalline amino acids |
| Carbohydrates | Sucrose, fructose, corn starch, (nondigestable oligosaccharides) |
| Fats | Lard, corn oil, safflower oil, Menhaden oil; soybean oil |
| Vitamins | Vitamin A, vitamin D, vitamin E, vitamin K, vitamin B12, biotin, choline, folates, niacin, pantothenic acid, vitamin B6 (pyridoxine, pyridoxal, pyridoxamine), riboflavin, thiamine, vitamin C |
| Minerals | Dicalcium phosphate, sodium selenite |
| Fibers | Cellulose, guar gum, pectin, carboxymethylcellulose, carrageenan, xanthan gum, gum arabic, inulin, fructooligosaccharides |
| Additives | Genistein, daidzein, cholesterol, myo-inositol |
| Special additives | Doxycycline, tamoxifen |
| Vitamin Sub-Class | Vitamin | Compound (Alternate Names)/Members | Biochemical Function | Reference |
|---|---|---|---|---|
| Water-soluble | Vitamin C | Ascorbic acid | Maintenance of redox balance; co-substrate for several enzymes; intracellular antioxidant; electron donor | [64] |
| Vitamin B1 | Thiamine | Coenzyme in the catabolism of sugars and amino acids | [65] | |
| Vitamin B2 | Riboflavin | Coenzyme in flavoprotein enzyme reactions (e.g., FAD); antioxidant | [66] | |
| Vitamin B3 | Niacin (nicotinic acid) | Coenzyme involved in protein, fat and carbohydrate metabolism (e.g., Nicotinamide adenine dinucleotide (NAD); hydrogen carrier; antioxidant, reducing agent | [67] | |
| Vitamin B5 | Pantothenic acid | Coenzyme in synthesis and metabolism of proteins, carbohydrates and fats; required for synthesis of coenzyme A | [68] | |
| Vitamin B6 | pyridoxine, pyridoxal, pyridoxamine and their respective mono-phosphorylated derivatives | Coenzyme in many enzymatic reactions (decarboxylations, transaminations, eliminations, racemizations, transsulfurations, interconversions); antioxidant | [69] | |
| Vitamin B7 | Biotin (vitamin H) | Coenzyme for carboxylases: pyruvate carboxylase, 3-methylcrotonyl-CoA carboxylase, propionyl-CoA carboxylase, and coenzyme for acetyl-CoA carboxylase 1 and 2 | [70] | |
| Vitamin B9 | Folic acid (folacin), folate pteroyl-L-glutamic acid, pteroyl-L-glutamate, pteroylmonoglutamic acid | Coenyzme in single-carbon group (methyl-, methylene-, formyl group) transfer reactions | [71] | |
| Vitamin B12 | Cobalamin | Coenzyme for the methionine synthase and methylmalonyl-CoA mutase | [72] | |
| Fat-soluble | Vitamin A | Retinol, retinal, retinoic acid, provitamin A carotenoids | Modulator of immune system; low-light and color vision; wound healing; hormone (binding to retinoic acid receptors); metabolic effects; reproduction | [73,74] |
| Vitamin D | Group of secosteroids (e.g., ergocalciferol, cholecalciferol and others); | Binding to vitamin D receptor acting as a transcription factor; calcium and phosphate homeostasis; immune system; cell proliferation and differentiation; bone formation; innate and adaptive immunity | [75,76] | |
| Vitamin E | α/β/γ/δ-tocopherols, α/β/γ/δ-tocotrienols | Antioxidant and radical scavenger; modulator of gene expression; enzyme activity regulator (e.g., protein kinase C) | [77] | |
| Vitamin K | Phylloquinone (vitamin K1), menaquinones MK-4 through MK-10 (vitamin K2) | γ-glutamyl carboxylation; relevant in blood coagulation and bone metabolism; modulator of transcriptional activity; agonist of steroid and xenobiotic nuclear receptor; neural stem cell differentiation modulator | [78] |
| Vitamin | Factors Affecting Vitamin Stability during Processing and Storage |
|---|---|
| Vitamin C (ascorbic acid) | Moisture, heat, oxidation, light, iron |
| Vitamin B1 (thiamine) | Oxidation |
| Vitamin B2 (riboflavin) | Light |
| Vitamin B3 (niacin) | Rather stable |
| Vitamin B5 (pantothenate) | Oxidation, light |
| Vitamin B6 (pyridoxine) | Oxidation, reduction |
| Vitamin B7 (biotin) | Oxidation |
| Vitamin B9 (folic acid) | Oxidation, light, microbial |
| Vitamin B12 (cobalamin) | UV light, interaction with other water-soluble vitamins, heat, pH |
| Vitamin A (retinol, retinal, retinoic acid, provitamin A carotenoids) | Oxidation, light, (trace elements) |
| Vitamin D (cholecalciferol) | Stable |
| Vitamin E (α/β/γ/δ-tocopherols, α/β/γ/δ-tocotrienols) | Oxidation, light, oxidized fat |
| Vitamin K (menadione) | Oxidation |
| Nutrient | Amount (per kg Diet) |
|---|---|
| Lipid | 50 g |
| Linoleic acid | 6.8 g |
| Protein (N × 6.25) ** | 180–200 g |
| Amino acids | Arginine: 3 g; histidine: 2 g; isoleucine: 4 g; leucine: 7 g; valine: 5 g; threonine: 4 g; lysine: 4 g; methionine: 5 g; phenylalanine: 7.6 g; tryptophan: 1 g |
| Minerals | Calcium: 5 g; chloride: 0.5 g; magnesium: 0.5 g; phosphorus: 3 g; potassium: 2 g; sodium: 0.5 g; copper: 6 mg; iron: 35 mg; manganese: 10 mg; zinc: 10 mg; iodine: 150 µg; molybdenum: 150 µg; selenium: 150 µg |
| Vitamins | Retinol: 0.72 mg (= 2400 IU); cholecalciferol: 0.025 mg (1000 IU); RRR-α-tocopherol: 22 mg (= 32 IU); phylloquionone: 1 mg; biotin: 0.1 mg; choline: 2 g; folic acid: 0.5 mg; niacin: 15 mg; Ca-pantothenate: 16 mg; riboflavin: 7 mg; thiamine-HCl: 5 mg; pyridoxine-HCl: 8 mg; cobalamin: 10 µg |
| Component | Consequences of Insufficient Supply | References |
|---|---|---|
| Fat | Alterations in composition and functionality of synaptosomal plasma membranes | [93] |
| Protein | Decreased host immune defense; reduced numbers of splenocytes, lower quantities of glutathione in several organs; less food ingestion and weight gain during pregnancy resulting in fewer viable pups | [94,95] |
| Phe, Thr, Trp, Met, Lys, Leu, Ile, Val | Massive reduction of abdominal fat mass after 7 days, most likely via increased energy expenditure | [96,97,98,99] |
| Calcium | Development of osteoporosis-like symptoms with reduced femur length and reduced density of various bones | [100] |
| Magnesium | Hypomagnesemia reduced bone growth and chondrocyte functionality; During embryogenesis embryotoxic effects (retardation, disturbed bone development and skeletal malformations) are induced | [101,102] |
| Phosphorus | Severe growth retardation with a 50% reduction in body weight; reduced formation of milk droplets | [103,104] |
| Potassium | Hypokalemia results in decrease in luteinizing hormone and testosterone provoking testicular impairments that is also seen in a fall in the weight of seminal vesicles | [105] |
| Copper | Development of anemia, duodenal hypoxia and alterations in intestinal iron absorption | [106] |
| Iron | Development of pronounced splenomegaly; anemia with reduction in hemoglobin and hematocrit levels with higher risk of developing deep dental caries | [107,108] |
| Manganese | Growth retardation and malfunction of reproductive organs and ovulation; congenital debility of young animals with loss of both equilibrium and coordination | [109,110] |
| Zinc | Reduced immune responses after challenging with pathogen indicated by greater weight loss, stool shedding, mucus production and diarrhea | [111] |
| Iodine | Development of carcinoma of the thyroid; formation of oxidative stress and DNA modifications | [112,113] |
| Selenium | Potentiate the development of autoantibodies; reduction of amino acid levels and elevation of mononucleotides resulting in dysregulated metabolomes and age-associated decline of protein synthesis; development of widespread pyogranulomas | [114,115,116] |
| Thiamine | Reduction of energy state in the liver; reduction of blood glucose, insulin, triglycerides, cholesterol, liver glycogen; increase of serum lactate | [117] |
| Biotin | Impairment of mitochondrial structure and function; intoxication with propionyl-CoA; systemic inflammation | [118] |
| Vitamin D | Increased expression of sex steroid receptors in myometrium; increased expression of proliferation-related genes; promotion of fibrosis, inflammation, and immunosuppression; enhancement of DNA damage; increased lipid deposition in skeletal muscle and muscle fiber disorganization | [119,120] |
| Vitamin A | Breakdown of oral tolerance; reduction of iron absorption | [121,122] |
| Linoleic acid | Reduction results in lower concentrations of circulating, small bowel and hepatic endocannabinoids; lower feed efficiency and weight | [123] |
| Choline | Amplification of liver fat accumulation in phases of high fat consumption; lowering of fasting plasma insulin; improvement of glucose tolerance; reduction of fibroblast-like cells in circulating tumor cells and less metastasis | [124,125] |
| Component | Control Diet | DIO ** Diet | WD Diet | FFC Diet | MCD Diet | |
|---|---|---|---|---|---|---|
| Crude nutrients [%] | Crude protein (N × 6.25) | 17.6 | 24.4 | 17.3 | 19.7 | 15.0 |
| Crude fat | 7.1 | 34.6 | 21.1 | 20.0 | 10.0 | |
| Crude fibre | 5.0 | 6.0 | 5.0 | 5.1 | 3.0 | |
| Crude ash | 3.2 | 5.3 | 4.2 | 4.4 | 3.3 | |
| Starch | 38.2 | 0.1 | 14.4 | 0.1 | 19.2 | |
| Dextrin | 13.1 | 15.4 | 0 | 10.9 | 0 | |
| Sugar | 11.2 | 9.4 | 34.3 | 34.2 | 45.1 | |
| N free extracts | 63.1 | 26.3 | 49.8 | 46.2 | 67.3 | |
| Minerals [%] | Calcium | 0.55 | 0.92 | 0.76 | 0.78 | 0.59 |
| Phosphorus | 0.37 | 0.64 | 0.45 | 0.50 | 0.46 | |
| Sodium | 0.15 | 0.20 | 0.24 | 0.20 | 0.16 | |
| Magnesium | 0.10 | 0.23 | 0.10 | 0.13 | 0.06 | |
| Potassium | 0.55 | 0.97 | 0.54 | 0.77 | 0.36 | |
| Fatty acids [%] | C 4:0 | - | - | 0.80 | - | - |
| C 6:0 | - | - | 0.53 | - | - | |
| C 8:0 | - | - | 0.29 | - | - | |
| C10:0 | - | - | 0.63 | - | - | |
| C 12:0 | - | 0.07 | 0.72 | 0.02 | - | |
| C 14:0 | 0.02 | 0.44 | 2.22 | 0.07 | - | |
| C 16:0 | 0.84 | 7.93 | 5.60 | 2.04 | 1.09 | |
| C 17:0 | 0.01 | 0 | 0.14 | 0.01 | 0.01 | |
| C 18:0 | 0.26 | 4.37 | 2.05 | 2.38 | 0.19 | |
| C 20:0 | 0.03 | 0.11 | 0.04 | 0.14 | 0.03 | |
| C 16:1 | 0.01 | 0.94 | 0.38 | 0.08 | 0.01 | |
| C 18:1 | 1.78 | 13.97 | 4.65 | 11.65 | 2.58 | |
| C 18:2 | 3.69 | 4.64 | 0.38 | 2.14 | 5.53 | |
| C 18:3 | 0.41 | 0.49 | 0.11 | 0.19 | 0.09 | |
| Amino acids [%] | Lysine | 1.47 | 2.20 | 1.43 | 1.63 | 1.40 |
| Methionine | 0.55 | 0.86 | 0.93 | 0.61 | 0 | |
| Cystine | 0.37 | 0.45 | 0.07 | 0.43 | 0.35 | |
| Threonine | 0.78 | 1.07 | 0.76 | 0.86 | 0.80 | |
| Tryptophan | 0.23 | 0.33 | 0.22 | 0.26 | 0.18 | |
| Arginine | 0.69 | 0.95 | 0.67 | 0.77 | 0.98 | |
| Histidine | 0.53 | 0.74 | 0.52 | 0.59 | 0.45 | |
| Valine | 1.23 | 1.70 | 1.20 | 1.37 | 0.81 | |
| Isoleucine | 1.00 | 1.38 | 0.97 | 1.11 | 0.80 | |
| Leucine | 1.75 | 2.42 | 1.71 | 1.95 | 1.10 | |
| Phenylalanine | 0.92 | 1.27 | 0.89 | 1.02 | 0.74 | |
| Phenylalanine +Tyrosine | 1.85 | 2.56 | 1.80 | 2.06 | 1.24 | |
| Glycine | 0.35 | 0.52 | 0.34 | 0.39 | 2.31 | |
| Glutamic acid (+Glutamine) | 3.97 | 5.50 | 3.88 | 4.43 | 3.96 | |
| Aspartic acid (+Asparagine) | 1.31 | 1.82 | 1.28 | 1.45 | 0.95 | |
| Proline | 2.02 | 2.80 | 1.97 | 2.25 | 0.35 | |
| Serine | 1.06 | 1.46 | 1.03 | 1.18 | 0.35 | |
| Alanine | 0.53 | 0.81 | 0.52 | 0.59 | 0.35 | |
| Vitamins [mg per kg] | Vitamin A *** | 1.20 | 4.50 | 4.50 | 1.32 | 5.85 |
| Vitamin D3 **** | 0.025 | 0.0375 | 0.0375 | 0.0275 | 0.055 | |
| Vitamin E | 75 | 150 | 150 | 90 | 135 | |
| Vitamin K (as MNB) | 4 | 20 | 20 | 4 | 45 | |
| Thiamine (B1) | 12 | 25 | 26 | 13 | 22 | |
| Riboflavin (B2) | 16 | 16 | 16 | 18 | 22 | |
| Pyridoxine (B6) | 7 | 16 | 16 | 7 | 22 | |
| Cobalamin (B12) | 0.025 | 0.03 | 0.03 | 0.028 | 0.03 | |
| Nicotinic acid | 29 | 47 | 49 | 32 | 98 | |
| Pantothenic acid | 15 | 55 | 55 | 17 | 60 | |
| Folic acid | 2 | 16 | 16 | 2 | 2 | |
| Biotin | 0.2 | 0.3 | 0.3 | 0.2 | 0.4 | |
| Choline | 1130 | 1140 | 920 | 920 | 0 | |
| Trace elements [mg per kg] | Iron | 49 | 168 | 49 | 68 | 42 |
| Manganese | 22 | 95 | 22 | 30 | 53 | |
| Zinc | 41 | 65 | 41 | 58 | 29 | |
| Copper | 10 | 13 | 11 | 14 | 6 | |
| Iodine | 0.3 | 1.2 | 0.3 | 0.4 | 0.2 | |
| Selenium | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | |
| Other ingredients [mg/kg] | Cholesterol | 0 | ~230 | 2070 | 20,000 | 0 |
| Food and Drug Administration (FDA) Name | Organic Compound | Color | E Number | ADI * (mg/kg bw/d) | Chemical Abstracts Service (CAS) Number |
|---|---|---|---|---|---|
| Federal Food, Drug and Cosmetic Act (FD&C) Blue No. 1 | Brilliant Blue FCF (Acid Blue 9, D&C Blue No. 4, Atracid Blue FG) | blue | E133 | 0–6 | 3844-45-9 |
| FD&C Blue No. 2 | Indigotine (Indigo Carmine, Acid Blue 74, Murabba, Sachsischblau) | indigo | E132 | 0–5 | 860-22-0 |
| FD&C Green No. 3 | Fast Green FCF (Food Green FCF, Food Green 3, Green 1724) | green | E143 *** | 0–12.5 | 2353-45-9 |
| FD&C Red No. 3 | Erythrosine (Erythrosin B, Acid Red 51, Pyrosin B, Food Red No. 3) | pink | E127 | 0–0.1 | 16423-68-0 |
| FD&C Red No. 40 | Allura Red AC (Food Red 17, Curry Red) | red | E129 | 0–7 | 25956-17-6 |
| FD&C Yellow No. 5 | Tartrazine (Tatrazol Yellow, Acid Yellow 23, Food Yellow 4) | yellow | E102 | 0–10 | 1934-21-0 |
| FD&C Yellow No. 6 | Sunset Yellow FCF (Orange Yellow S, Food Yellow 3) | orange | E110 | 0–4 | 2783-94-0 |
| Citrus Red 2 ** | Citrus Red (2,5-Dimethoxy-1-Phenylazo-2-naphthol, CI 12156, CI Solvent Red 80) | red | E121 *** | Only approved for use to color orange peels (US) | 6358-53-8 |
| Orange B ** | Acid Orange 137 (LS-128771, Schembl132534) | orange | not allowed *** | Only approved for use in hot dog and sausage casings (US) | 53060-70-1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiskirchen, S.; Weiper, K.; Tolba, R.H.; Weiskirchen, R. All You Can Feed: Some Comments on Production of Mouse Diets Used in Biomedical Research with Special Emphasis on Non-Alcoholic Fatty Liver Disease Research. Nutrients 2020, 12, 163. https://doi.org/10.3390/nu12010163
Weiskirchen S, Weiper K, Tolba RH, Weiskirchen R. All You Can Feed: Some Comments on Production of Mouse Diets Used in Biomedical Research with Special Emphasis on Non-Alcoholic Fatty Liver Disease Research. Nutrients. 2020; 12(1):163. https://doi.org/10.3390/nu12010163
Chicago/Turabian StyleWeiskirchen, Sabine, Katharina Weiper, René H. Tolba, and Ralf Weiskirchen. 2020. "All You Can Feed: Some Comments on Production of Mouse Diets Used in Biomedical Research with Special Emphasis on Non-Alcoholic Fatty Liver Disease Research" Nutrients 12, no. 1: 163. https://doi.org/10.3390/nu12010163
APA StyleWeiskirchen, S., Weiper, K., Tolba, R. H., & Weiskirchen, R. (2020). All You Can Feed: Some Comments on Production of Mouse Diets Used in Biomedical Research with Special Emphasis on Non-Alcoholic Fatty Liver Disease Research. Nutrients, 12(1), 163. https://doi.org/10.3390/nu12010163







