Pterostilbene Prevents Early Diabetic Retinopathy Alterations in a Rabbit Experimental Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Administration and Measurement of Pterostilbene
2.3. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labelling (TUNEL) Assay
2.4. Biochemistry and Antioxidant Enzymes Activities
2.5. Oxidative Damage
2.6. Human Retina Endothelial Cells Culture
2.7. Hydrogen Peroxide Determination
2.8. Gene Expression Analysis. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. NRF2 Immunocytochemistry
2.10. Protein Extraction and Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Phosphorylated Pterostilbene Disodium Salt as a Source of Pterostilbene
3.2. Pterostilbene Reduces the Harmful Retinal Effects of Hyperglycaemia
3.3. Haematological Parameters and Pterostilbene Toxicity
3.4. Pterostilbene Inhibits Considerable Glucose-Oxidative Damage and Reduces Hydrogen Peroxide Production In Vivo
3.5. Pterostilbene Prevents Retinal Damage by Modulating Antioxidant Defences
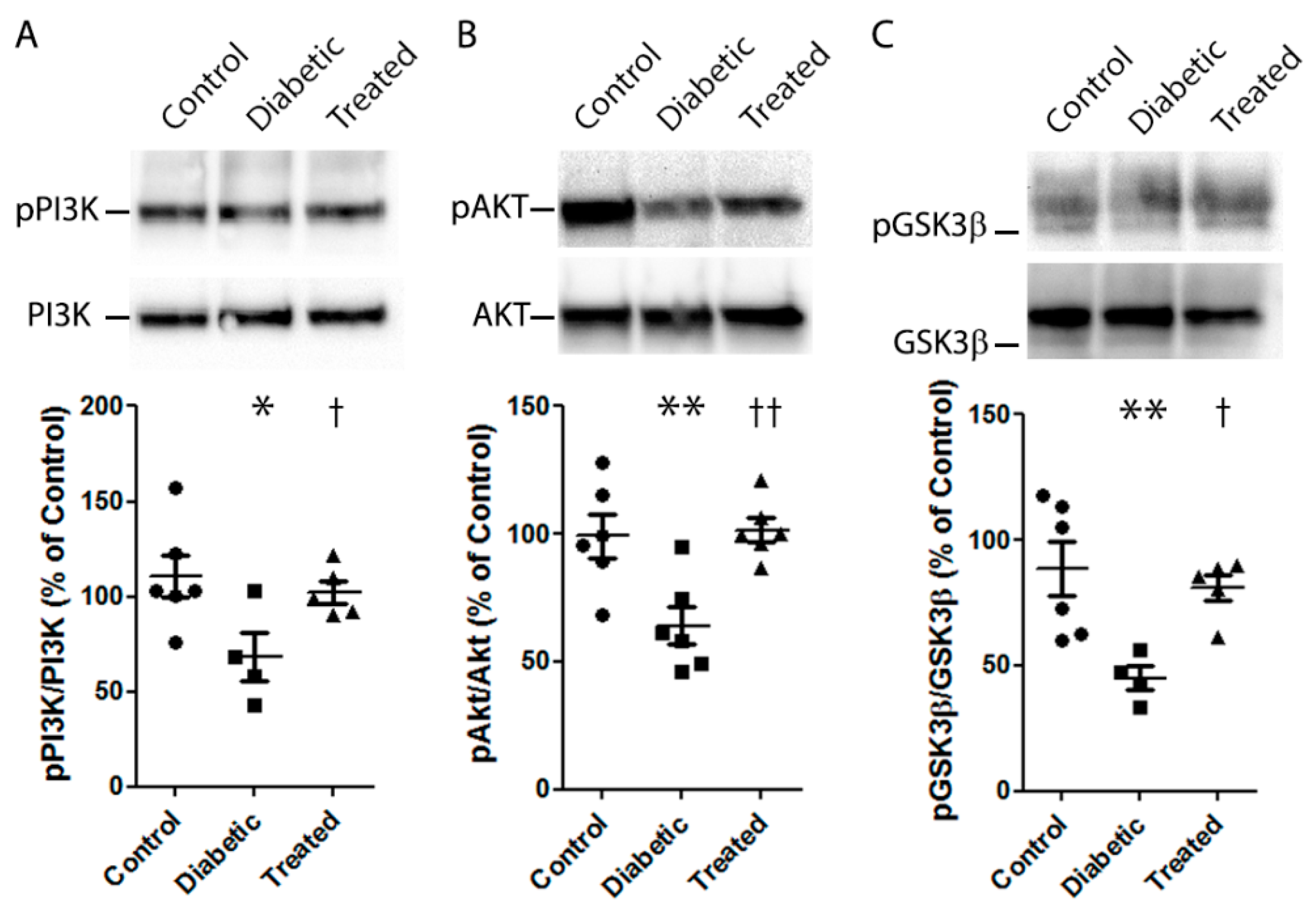
3.6. Molecular Mechanisms Involved in the Antioxidant Role of Pterostilbene
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Maric-Bilkan, C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin. Sci. (Lond.) 2017, 131, 833–846. [Google Scholar] [CrossRef]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal oxygen: Fundamental and clinical aspects. Arch. Ophthalmol. 2003, 121, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kisic, B.; Miric, D.; Zoric, L. Free Radical Biology of Eye Diseases. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3599–3623. [Google Scholar]
- Wu, L.; Fernandez-Loaiza, P.; Sauma, J.; Hernandez-Bogantes, E.; Masis, M. Classification of diabetic retinopathy and diabetic macular edema. World J. Diabetes 2013, 4, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Priscakova, P.; Minarik, G.; Repiska, V. Candidate gene studies of diabetic retinopathy in human. Mol. Biol. Rep. 2016, 43, 1327–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shabrawey, M.; Smith, S. Prediction of diabetic retinopathy: Role of oxidative stress and relevance of apoptotic biomarkers. EPMA J. 2010, 1, 56–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Tang, L.; Chen, B. Oxidative stress: Implications for the development of diabetic retinopathy and antioxidant therapeutic perspectives. Oxid. Med. Cell Longev. 2014, 2014, 752387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniadakis, N.; Konstantakopoulou, E. Cost Effectiveness of Treatments for Diabetic Retinopathy: A Systematic Literature Review. Pharmacoeconomics 2019, 37, 995–1010. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodriguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super Sanita 2007, 43, 348–361. [Google Scholar] [PubMed]
- Alabadi, J.A.; Miranda, F.J.; Llorens, S.; Centeno, J.M.; Marrachelli, V.G.; Alborch, E. Mechanisms underlying diabetes enhancement of endothelin-1-induced contraction in rabbit basilar artery. Eur. J. Pharmacol. 2004, 486, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, P.; Asensi, M.; Segarra, R.; Ortega, A.; Benlloch, M.; Obrador, E.; Varea, M.T.; Asensio, G.; Jorda, L.; Estrela, J.M. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia 2005, 7, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Cuevas, I.; Kuligowski, J.; Carcel, M.; Chafer-Pericas, C.; Asensi, M.; Solberg, R.; Cubells, E.; Nunez, A.; Saugstad, O.D.; Vento, M.; et al. Protein-bound tyrosine oxidation, nitration and chlorination by-products assessed by ultraperformance liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2016, 913, 104–110. [Google Scholar] [CrossRef]
- Escobar, J.; Sanchez-Illana, A.; Kuligowski, J.; Torres-Cuevas, I.; Solberg, R.; Garberg, H.T.; Huun, M.U.; Saugstad, O.D.; Vento, M.; Chafer-Pericas, C. Development of a reliable method based on ultra-performance liquid chromatography coupled to tandem mass spectrometry to measure thiol-associated oxidative stress in whole blood samples. J. Pharm. Biomed. Anal. 2016, 123, 104–112. [Google Scholar] [CrossRef]
- Yu, G.; Guang, Y.; Li, J.; Xing, J.; Bai, Z. The protective effect of low-energy shock wave on testicular ischemia-reperfusion injury is mediated by the PI3K/AKT/NRF2 pathway. Life Sci. 2018, 213, 142–148. [Google Scholar] [CrossRef]
- Li, H.; Tang, Z.; Chu, P.; Song, Y.; Yang, Y.; Sun, B.; Niu, M.; Qaed, E.; Shopit, A.; Han, G.; et al. Neuroprotective effect of phosphocreatine on oxidative stress and mitochondrial dysfunction induced apoptosis in vitro and in vivo: Involvement of dual PI3K/Akt and Nrf2/HO-1 pathways. Free Radic. Biol. Med. 2018, 120, 228–238. [Google Scholar] [CrossRef]
- Padiya, R.; Chowdhury, D.; Borkar, R.; Srinivas, R.; Pal Bhadra, M.; Banerjee, S.K. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS ONE 2014, 9, e94228. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, W.; Wang, Z.; Jin, X.; Xu, J.; Bai, L.; Li, Y.; Cui, J.; Cai, L. Resveratrol attenuates testicular apoptosis in type 1 diabetic mice: Role of Akt-mediated Nrf2 activation and p62-dependent Keap1 degradation. Redox Biol. 2017, 14, 609–617. [Google Scholar] [CrossRef]
- Soufi, F.G.; Mohammad-Nejad, D.; Ahmadieh, H. Resveratrol improves diabetic retinopathy possibly through oxidative stress - nuclear factor kappaB-apoptosis pathway. Pharmacol. Rep. 2013, 64, 1505–1514. [Google Scholar] [CrossRef]
- Li, W.; Jiang, D. Effect of resveratrol on Bcl-2 and VEGF expression in oxygen-induced retinopathy of prematurity. J. Pediatr. Ophthalmol. Strabismus 2011, 49, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Raju, R.; Nagineni, K.K.; Kommineni, V.K.; Cherukuri, A.; Kutty, R.K.; Hooks, J.J.; Detrick, B. Resveratrol Suppresses Expression of VEGF by Human Retinal Pigment Epithelial Cells: Potential Nutraceutical for Age-related Macular Degeneration. Aging Dis. 2014, 5, 88–100. [Google Scholar] [PubMed]
- Silva, K.C.; Rosales, M.A.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.; Faria, J.B.; Faria, J.M. Green tea is neuroprotective in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Jun, J.H.; Jung, E.H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.C.; Chang, W.C.; Hung, K.H.; Yang, D.M.; Cheng, Y.H.; Liao, Y.W.; Woung, L.C.; Tsai, C.Y.; Hsu, C.C.; Lin, T.C.; et al. The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: Identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress. Front. Aging Neurosci. 2014, 6, 191. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. (Lond.) 2007, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, P.; Shen, Y.; Lin, B.Q.; Zhang, W.Y.; Chiou, G.C. Effect of quercetin on formation of choroidal neovascularization (CNV) in age-related macular degeneration (AMD). Eye Sci. 2011, 26, 23–29. [Google Scholar]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Ying, J.; Shi, T.; Wang, P. Resveratrol Prevents ROS-Induced Apoptosis in High Glucose-Treated Retinal Capillary Endothelial Cells via the Activation of AMPK/Sirt1/PGC-1. Oxid. Med. Cell Longev. 2017, 2017, 7584691. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Z.K.; Liang, S. Epigallocatechin-3-gallate protects retinal vascular endothelial cells from high glucose stress in vitro via the MAPK/ERK-VEGF pathway. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Lee, M.; Yun, S.; Lee, H.; Yang, J. Quercetin Mitigates Inflammatory Responses Induced by Vascular Endothelial Growth Factor in Mouse Retinal Photoreceptor Cells through Suppression of Nuclear Factor Kappa B. Int. J. Mol. Sci. 2017, 18, 2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.M.; Chang, H.H.; Wang, V.C.; Huang, C.L.; Hung, C.F. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRbeta, PI3K/Akt and MAPK pathways. PLoS ONE 2013, 8, e56819. [Google Scholar]
- Bucolo, C.; Drago, F.; Maisto, R.; Romano, G.L.; D’Agata, V.; Maugeri, G.; Giunta, S. Curcumin prevents high glucose damage in retinal pigment epithelial cells through ERK1/2-mediated activation of the Nrf2/HO-1 pathway. J. Cell Physiol. 2019, 234, 17295–17304. [Google Scholar] [CrossRef] [PubMed]
- Xue, E.X.; Lin, J.P.; Zhang, Y.; Sheng, S.R.; Liu, H.X.; Zhou, Y.L.; Xu, H. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget 2017, 8, 41988–42000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupi, M.; Perez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Rong, H. Pterostilbene impact on retinal endothelial cells under high glucose environment. Int. J. Clin. Exp. Pathol. 2016, 8, 12589–12594. [Google Scholar]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Pari, L.; Satheesh, M.A. Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats. Life Sci. 2006, 79, 641–645. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Long, S.R.; Teng, W.; Ge, H.; Wang, Y.; Yu, S.; Xue, Y.; Zhang, Y.; Li, X.; et al. Antidiabetic effects of pterostilbene through PI3K/Akt signal pathway in high fat diet and STZ-induced diabetic rats. Eur. J. Pharmacol. 2019, 859, 172526. [Google Scholar] [CrossRef]
- Tastekin, B.; Pelit, A.; Polat, S.; Tuli, A.; Sencar, L.; Alparslan, M.M.; Daglioglu, Y.K. Therapeutic Potential of Pterostilbene and Resveratrol on Biomechanic, Biochemical, and Histological Parameters in Streptozotocin-Induced Diabetic Rats. Evid. Based Complement. Alternat. Med. 2018, 2018, 9012352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarnath Satheesh, M.; Pari, L. The antioxidant role of pterostilbene in streptozotocin-nicotinamide-induced type 2 diabetes mellitus in Wistar rats. J. Pharm. Pharmacol. 2006, 58, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, M.J.; Fernandez, M.; Pico, Y.; Manes, J.; Asensi, M.; Carda, C.; Asensio, G.; Estrela, J.M. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.J.; Chiu, H.W.; Lee, Y.L.; Li, C.Y.; Wang, Y.J.; Lee, Y.H. Pterostilbene Attenuates Hexavalent Chromium-Induced Allergic Contact Dermatitis by Preventing Cell Apoptosis and Inhibiting IL-1beta-Related NLRP3 Inflammasome Activation. J. Clin. Med. 2018, 7, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, P.A.; Khan, J.A.; Ullah, I.; Ullah, S. Synergistic combinatorial antihyperlipidemic study of selected natural antioxidants; modulatory effects on lipid profile and endogenous antioxidants. Lipids Health Dis. 2016, 15, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riche, D.M.; McEwen, C.L.; Riche, K.D.; Sherman, J.J.; Wofford, M.R.; Deschamp, D.; Griswold, M. Analysis of safety from a human clinical trial with pterostilbene. J. Toxicol. 2013, 2013, 463595. [Google Scholar] [CrossRef]
- Hougee, S.; Faber, J.; Sanders, A.; de Jong, R.B.; van den Berg, W.B.; Garssen, J.; Hoijer, M.A.; Smit, H.F. Selective COX-2 inhibition by a Pterocarpus marsupium extract characterized by pterostilbene, and its activity in healthy human volunteers. Planta Med. 2005, 71, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wan, R.; Mo, Y.; Zhang, Q.; Sherwood, L.C.; Chien, S. Creating a long-term diabetic rabbit model. Exp. Diabetes Res. 2010, 2010, 289614. [Google Scholar] [CrossRef]
- El-Fayoumi, D.; Badr Eldine, N.M.; Esmael, A.F.; Ghalwash, D.; Soliman, H.M. Retinal Nerve Fiber Layer and Ganglion Cell Complex Thicknesses Are Reduced in Children with Type 1 Diabetes with No Evidence of Vascular Retinopathy. Invest. Ophthalmol. Vis. Sci. 2016, 57, 5355–5360. [Google Scholar] [CrossRef]
- van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Garvin, M.K.; Sonka, M.; Lee, K.; Devries, J.H.; Michels, R.P.; van Velthoven, M.E.; Schlingemann, R.O.; et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest. Ophthalmol. Vis. Sci. 2010, 51, 3660–3665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, Y.; Tan, H.Y.; Li, S.; Wang, N.; Zhang, Y.; Feng, Y. Neuroprotective effect of He-Ying-Qing-Re formula on retinal ganglion cell in diabetic retinopathy. J. Ethnopharmacol. 2017, 214, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, L.; Huang, J.; Lv, H.; Deng, X.; Ci, X. Pterostilbene Reduces Acetaminophen-Induced Liver Injury by Activating the Nrf2 Antioxidative Defense System via the AMPK/Akt/GSK3beta Pathway. Cell Physiol. Biochem. 2018, 49, 1943–1958. [Google Scholar] [CrossRef] [PubMed]
- Kosuru, R.; Kandula, V.; Rai, U.; Prakash, S.; Xia, Z.; Singh, S. Pterostilbene Decreases Cardiac Oxidative Stress and Inflammation via Activation of AMPK/Nrf2/HO-1 Pathway in Fructose-Fed Diabetic Rats. Cardiovasc. Drugs Ther. 2018, 32, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, C.; Wang, B.; Ma, Z.; Wang, D.; Gong, B.; Di, S.; Jiang, S.; Li, Y.; Li, T.; et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 827–837. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Shalini, D.; Sekar, T.V.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. Therapeutic potential of pterostilbene against pancreatic beta-cell apoptosis mediated through Nrf2. Br. J. Pharmacol. 2014, 171, 1747–1757. [Google Scholar] [CrossRef]
- Nakagami, Y. Nrf2 Is an Attractive Therapeutic Target for Retinal Diseases. Oxid. Med. Cell Longev. 2016, 2016, 7469326. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell Physiol. 2019. [Google Scholar] [CrossRef]
- Robledinos-Anton, N.; Fernandez-Gines, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Chen, Z.W.; Miu, H.F.; Wang, H.P.; Wu, Z.N.; Wang, W.J.; Ling, Y.J.; Xu, X.H.; Sun, H.J.; Jiang, X. Pterostilbene protects against uraemia serum-induced endothelial cell damage via activation of Keap1/Nrf2/HO-1 signaling. Int Urol. Nephrol. 2017, 50, 559–570. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Dineshkumar, K.; Karthik, S.; Sireesh, D.; Hopper, W.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene-mediated Nrf2 activation: Mechanistic insights on Keap1:Nrf2 interface. Bioorg. Med. Chem. 2016, 24, 3378–3386. [Google Scholar] [CrossRef] [PubMed]
- Ullah, O.; Li, Z.; Ali, I.; Xu, L.; Liu, H.; Shah, S.Z.A.; Fang, N. Pterostilbene alleviates hydrogen peroxide-induced oxidative stress via nuclear factor erythroid 2 like 2 pathway in mouse preimplantation embryos. J. Reprod. Dev. 2018, 65, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Control | Diabetic | Treated | |
|---|---|---|---|
| ALT (U/L) | 49.0 ± 3.6 | 70.8 ± 6.3 * | 50.3 ± 2.6 † |
| GOT | 16.5 ± 0.9 | 20.7 ± 2.7 | 22.7 ± 1.2 |
| Alkaline phosphatase (U/L) | 102.0 ± 8.9 | 137.7 ± 6.1 * | 53.0 ± 4.9 ** ††† |
| Total bilirubin (mg/dL) | 0.1 ± 0.01 | 0.1 ± 0.02 | 0.2 ± 0.02 |
| Albumin (g/L) | 47.0 ± 0.7 | 44.2 ± 1.0 | 35.3 ± 0.9 *** ††† |
| Total protein (g/L) | 60.8 ± 2.2 | 55.2 ± 1.1 | 67.3 ± 1.9 |
| Chloride (mmol/L) | 102.3 ± 2.3 | 96.6 ± 1.2 | 100.7 ± 2.7 |
| Blood Urea Nitrogen (mg/dL) | 22.7 ± 2.5 | 20.9 ± 1.7 | 20.1 ± 2.8 |
| Creatinine (mg/dL) | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| Calcium (mg/dL) | 11.9 ± 0.1 | 11.9 ± 0.3 | 12.1 ± 0.3 |
| Phosphorus (mg/dL) | 5.5 ± 0.2 | 6.1 ± 0.2 | 5.9 ± 0.4 |
| Sodium (mmol/L) | 140.8 ± 0.5 | 138.4 ± 0.4 | 138.3 ± 0.3 |
| Potassium (mmol/L) | 3.7 ± 0.1 | 4.1 ± 0.1 | 3.9 ± 0.1 |
| HDL cholesterol (mg/dL) | 19.2 ± 2.8 | 19.0 ± 2.3 | 25.4 ± 3.8 |
| Total cholesterol (mg/dL) | 41.5 ± 4.4 | 42.4 ± 3.9 | 63.7 ± 14.2 |
| Urea (mg/dL) | 48.5 ± 5.4 | 44.8 ± 3.6 | 43.0 ± 6.1 |
| Uric acid (mg/dL) | 3.6 ± 0.4 | 4.2 ± 0.3 | 4.4 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán, I.; Desco, M.d.C.; Torres-Cuevas, I.; Pérez, S.; Pulido, I.; Mena-Mollá, S.; Mataix, J.; Asensi, M.; Ortega, Á.L. Pterostilbene Prevents Early Diabetic Retinopathy Alterations in a Rabbit Experimental Model. Nutrients 2020, 12, 82. https://doi.org/10.3390/nu12010082
Millán I, Desco MdC, Torres-Cuevas I, Pérez S, Pulido I, Mena-Mollá S, Mataix J, Asensi M, Ortega ÁL. Pterostilbene Prevents Early Diabetic Retinopathy Alterations in a Rabbit Experimental Model. Nutrients. 2020; 12(1):82. https://doi.org/10.3390/nu12010082
Chicago/Turabian StyleMillán, Iván, María del Carmen Desco, Isabel Torres-Cuevas, Salvador Pérez, Inés Pulido, Salvador Mena-Mollá, Jorge Mataix, Miguel Asensi, and Ángel Luis Ortega. 2020. "Pterostilbene Prevents Early Diabetic Retinopathy Alterations in a Rabbit Experimental Model" Nutrients 12, no. 1: 82. https://doi.org/10.3390/nu12010082
APA StyleMillán, I., Desco, M. d. C., Torres-Cuevas, I., Pérez, S., Pulido, I., Mena-Mollá, S., Mataix, J., Asensi, M., & Ortega, Á. L. (2020). Pterostilbene Prevents Early Diabetic Retinopathy Alterations in a Rabbit Experimental Model. Nutrients, 12(1), 82. https://doi.org/10.3390/nu12010082







