Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing
Abstract
:1. Introduction
2. Proteins
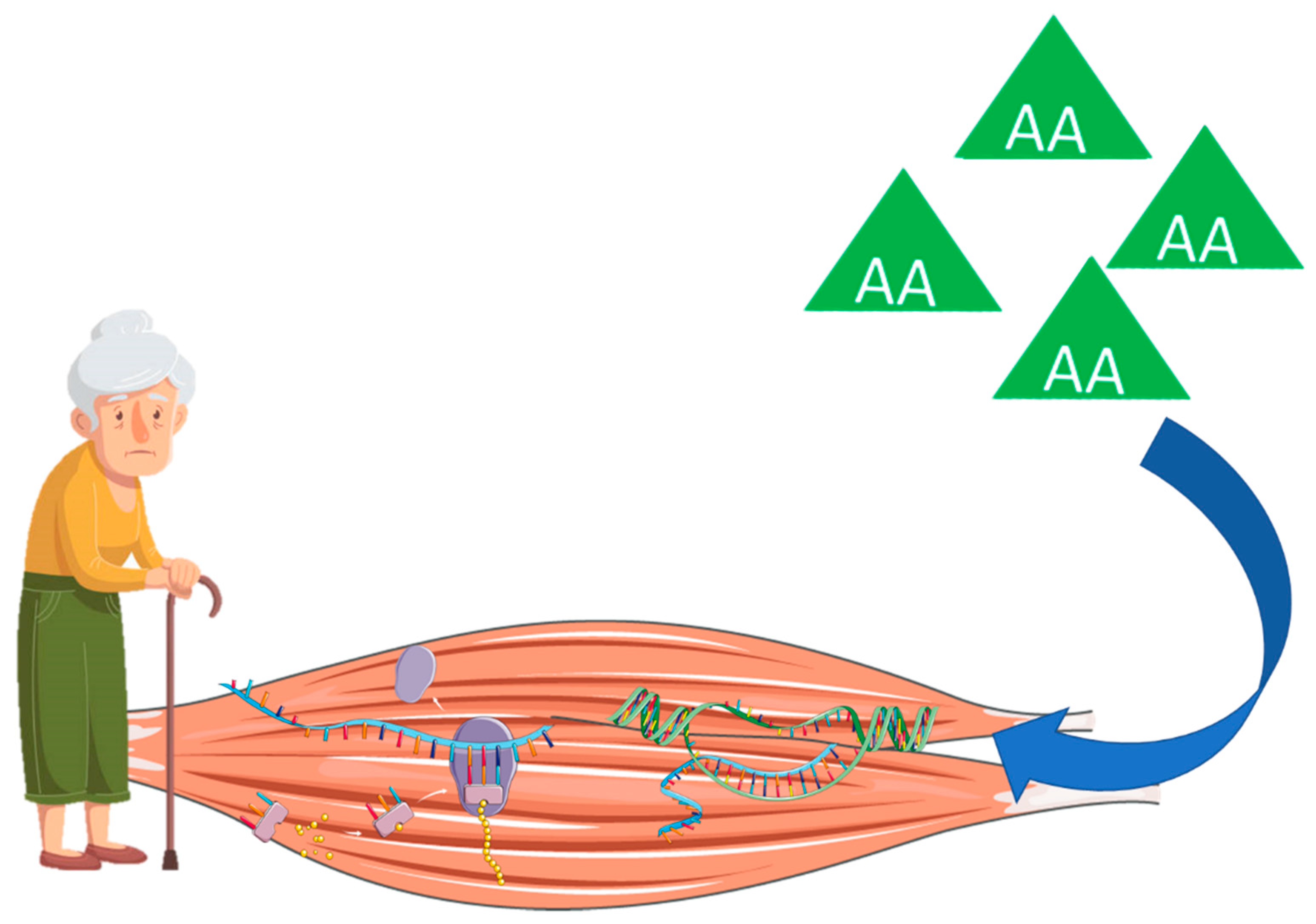
3. Age-Related Muscle Atrophy, Sarcopenia, and Frailty
4. How Much? Current Recommendations and Available Evidence
5. What Type? Animal-Based vs. Plant-Based Protein
6. When? Protein Distribution across Meals
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho-Junior, H.J.; Marzetti, E.; Picca, A.; Calvani, R.; Cesari, M.; Uchida, M. Prevalence of prefrailty and frailty in South America: A systematic review of observational studies. J. Frailty Aging 2020, 1–17. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a predictor of hospitalisation among community-dwelling older people: A systematic review and meta-analysis. J. Epidemiol. Commun. Health 2016, 70, 722–729. [Google Scholar] [CrossRef]
- Kojima, G.; Taniguchi, Y.; Iliffe, S.; Walters, K. Frailty as a Predictor of Alzheimer Disease, Vascular Dementia, and All Dementia Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 881–888. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a Predictor of Nursing Home Placement Among Community-Dwelling Older Adults. J. Geriatr. Phys. Ther. 2018, 41, 42–48. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a predictor of fractures among community-dwelling older people: A systematic review and meta-analysis. Bone 2016, 90, 116–122. [Google Scholar] [CrossRef]
- Kojima, G. Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 940–945. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a predictor of disabilities among community-dwelling older people: A systematic review and meta-analysis. Disabil. Rehabil. 2017, 39, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Frailty significantly increases the risk of fractures among middle-aged and older people. Evid. Based Nurs. 2017, 20, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein intake and muscle health in old age: From biological plausibility to clinical evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I.; Bautmans, I.; Beaudart, C.; Beckwée, D.; Beyer, I.; et al. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fougère, B.; Vellas, B.; van Kan, G.A.; Cesari, M. Identification of biological markers for better characterization of older subjects with physical frailty and sarcopenia. Transl. Neurosci. 2015, 6, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Picca, A.; Calvani, R.; Uchida, M.C.; Marzetti, E. If my muscle could talk: Myokines as a biomarker of frailty. Exp. Gerontol. 2019, 127, 110715. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef] [Green Version]
- Bohé, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose-response study. J. Physiol. 2003, 552, 315–324. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Greenhaff, P.L.; Karagounis, L.G.; Peirce, N.; Simpson, E.J.; Hazell, M.; Layfield, R.; Wackerhage, H.; Smith, K.; Atherton, P.; Selby, A.; et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E595–E604. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [Green Version]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.L.; Verdijk, L.B.; van Loon, L.J.C. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 1–22. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Lexell, J.; Taylor, C.C.; Sjöström, M. What is the cause of the ageing atrophy?. Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Klitgaard, H.; Mantoni, M.; Schiaffino, S.; Ausoni, S.; Gorza, L.; Laurent-Winter, C.; Schnohr, P.; Saltin, B. Function, morphology and protein expression of ageing skeletal muscle: A cross-sectional study of elderly men with different training backgrounds. Acta Physiol. Scand. 1990, 140, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.L.; van Kranenburg, J.; Verdijk, L.B.; Van Loon, L.J.C. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.; Stevens, J.; Binder-Macleod, S. Human Skeletal Muscle Fiber Type Classifications. Phys. Ther. 2001, 81, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.F.; Fielding, R.A. Skeletal muscle power: A critical determinant of physical functioning in older adults. Exerc. Sport Sci. Rev. 2012, 40, 4–12. [Google Scholar] [CrossRef]
- Bean, J.F.; Leveille, S.G.; Kiely, D.K.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. A Comparison of Leg Power and Leg Strength Within the InCHIANTI Study: Which Influences Mobility More? J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M728–M733. [Google Scholar] [CrossRef]
- Suetta, C.; Maier, A.B. Is muscle failure a better term than sarcopenia? J. Cachexia Sarcopenia Muscle 2019, 10, 1146–1147. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [Green Version]
- Hirani, V.; Blyth, F.; Naganathan, V.; Le Couteur, D.G.; Seibel, M.J.; Waite, L.M.; Handelsman, D.J.; Cumming, R.G. Sarcopenia Is Associated With Incident Disability, Institutionalization, and Mortality in Community-Dwelling Older Men: The Concord Health and Ageing in Men Project. J. Am. Med. Dir. Assoc. 2015, 16, 607–613. [Google Scholar] [CrossRef]
- Locquet, M.; Beaudart, C.; Petermans, J.; Reginster, J.-Y.; Bruyère, O. EWGSOP2 Versus EWGSOP1: Impact on the Prevalence of Sarcopenia and Its Major Health Consequences. J. Am. Med. Dir. Assoc. 2019, 20, 384–385. [Google Scholar] [CrossRef]
- Ethgen, O.; Beaudart, C.; Buckinx, F.; Bruyère, O.; Reginster, J.Y. The Future Prevalence of Sarcopenia in Europe: A Claim for Public Health Action. Calcif. Tissue Int. 2017, 100, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Chen, J.; Chen, X.; Hou, L.; Lin, X.; Yang, M. Prevalence and Associated Factors of Sarcopenia in Nursing Home Residents: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hirayama, K.; Han, T.-F.; Izutsu, M.; Yuki, M. Sarcopenia Prevalence and Risk Factors among Japanese Community Dwelling Older Adults Living in a Snow-Covered City According to EWGSOP2. J. Clin. Med. 2019, 8, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho-Junior, H.J.; Villani, E.R.; Calvani, R.; Carfì, A.; Picca, A.; Landi, F.; Bernabei, R.; Onder, G.; Marzetti, E. Sarcopenia-related parameters in adults with Down syndrome: A cross-sectional exploratory study. Exp. Gerontol. 2019, 119, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Rizzoli, R.; Bruyère, O.; Reginster, J.-Y.; Biver, E. Sarcopenia: Burden and challenges for public health. Arch. Public Health 2014, 72, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Bernabei, R.; Onder, G.; Marzetti, E. Sarcopenia as the Biological Substrate of Physical Frailty. Clin. Geriatr. Med. 2015, 31, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.-L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef]
- Faller, J.W.; Pereira, D.D.N.; de Souza, S.; Nampo, F.K.; Orlandi, F.D.S.; Matumoto, S. Instruments for the detection of frailty syndrome in older adults: A systematic review. PLoS ONE 2019, 14, e0216166. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Nadruz, W.; Kitzman, D.; Windham, B.G.; Kucharska-Newton, A.; Butler, K.; Palta, P.; Griswold, M.E.; Wagenknecht, L.E.; Heiss, G.; Solomon, S.D.; et al. Cardiovascular Dysfunction and Frailty among Older Adults in the Community: The ARIC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 958–964. [Google Scholar] [CrossRef] [Green Version]
- Zazzetta, M.S.; Gomes, G.A.O.; Orlandi, F.S.; Gratão, A.C.M.; Vasilceac, F.A.; Gramani-Say, K.; Ponti, M.A.; Castro, P.C.; Pavarini, S.C.I.; Menezes, A.L.C.; et al. Identifying Frailty Levels and Associated Factors in a Population Living in the Context of Poverty and Social Vulnerability. J. Frailty Aging 2017, 6, 29–32. [Google Scholar] [CrossRef]
- Furtado, G.E.; Caldo, A.; Rieping, T.; Filaire, E.; Hogervorst, E.; Teixeira, A.M.B.; Ferreira, J.P. Physical frailty and cognitive status over-60 age populations: A systematic review with meta-analysis. Arch. Gerontol. Geriatr. 2018, 78, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Cawthon, P.M.; Stone, K.L.; Hillier, T.A.; Cauley, J.A.; Hochberg, M.C.; Rodondi, N.; et al. Comparison of 2 Frailty Indexes for Prediction of Falls, Disability, Fractures, and Death in Older Women. Arch. Intern. Med. 2008, 168, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical Pathways of Sarcopenia and Their Modulation by Physical Exercise: A Narrative Review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Frisoli, A.; Chaves, P.H.; Ingham, S.J.M.; Fried, L.P. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: Results from the Women’s Health and Aging Study (WHAS) II. Bone 2011, 48, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Mijnarends, D.M.; Schols, J.M.G.A.; Meijers, J.M.M.; Tan, F.E.S.; Verlaan, S.; Luiking, Y.C.; Morley, J.E.; Halfens, R.J.G. Instruments to Assess Sarcopenia and Physical Frailty in Older People Living in a Community (Care) Setting: Similarities and Discrepancies. J. Am. Med. Dir. Assoc. 2015, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Table, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); National Academies Press: Washington, DC, USA, 2005; ISBN 030908525X. [Google Scholar]
- Dreyer, H.C.; Volpi, E. Role of Protein and Amino Acids in the Pathophysiology and Treatment of Sarcopenia. J. Am. Coll. Nutr. 2005, 24, 140S–145S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the prot-age study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.M.; Diekmann, R. Protein and Older Persons. Clin. Geriatr. Med. 2015, 31, 327–338. [Google Scholar] [CrossRef]
- Deer, R.R.; Volpi, E. Protein intake and muscle function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Campbell, W.W.; Crim, M.C.; Dallai, G.E.; Young, V.R.; Evans, W.J. Increased protein requirements in elderly people: New data and retrospective reassessments. Am. J. Clin. Nutr. 1994, 60, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Gersovitz, M.; Motil, K.; Munro, H.N.; Scrimshaw, N.S.; Young, V.R. Human protein requirements: Assessment of the adequacy of the current recommended dietary allowance for dietary protein in elderly men and women. Am. J. Clin. Nutr. 1982, 35, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Leung, J.; Woo, J.; Kwok, T. Associations of dietary protein intake on subsequent decline in muscle mass and physical functions over four years in ambulant older Chinese people. J. Nutr. Health Aging 2014, 18, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Jung, S.L.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gingrich, A.; Spiegel, A.; Gradl, J.E.; Skurk, T.; Hauner, H.; Sieber, C.C.; Volkert, D.; Kiesswetter, E. Daily and per-meal animal and plant protein intake in relation to muscle mass in healthy older adults without functional limitations: An enable study. Aging Clin. Exp. Res. 2019, 31, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorio, L.; Brindisi, J.; Kleppinger, A.; Sullivan, R.; Mangano, K.M.; Bihuniak, J.D.; Kenny, A.M.; Kerstetter, J.E.; Insogna, K.L. Adequate dietary protein is associated with better physical performance among post-menopausal women 60-90 years. J. Nutr. Health Aging 2014, 18, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Larocque, S.C.; Kerstetter, J.E.; Cauley, J.A.; Insogna, K.L.; Ensrud, K.; Lui, L.-Y.; Allore, H.G. Dietary Protein and Vitamin D Intake and Risk of Falls: A Secondary Analysis of Postmenopausal Women from the Study of Osteoporotic Fractures. J. Nutr. Gerontol. Geriatr. 2015, 34, 305–318. [Google Scholar] [CrossRef]
- Isanejad, M.; Mursu, J.; Sirola, J.; Kröger, H.; Rikkonen, T.; Tuppurainen, M.; Erkkilä, A.T. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br. J. Nutr. 2016, 115, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Rahi, B.; Morais, J.A.; Gaudreau, P.; Payette, H.; Shatenstein, B. Energy and protein intakes and their association with a decline in functional capacity among diabetic older adults from the NuAge cohort. Eur. J. Nutr. 2016, 55, 1729–1739. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Milano-Teixeira, L.; Rodrigues, B.; Bacurau, R.; Marzetti, E.; Uchida, M. Relative Protein Intake and Physical Function in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Haaf, D.S.M.; van Dongen, E.J.I.; Nuijten, M.A.H.; Eijsvogels, T.M.H.; de Groot, L.C.P.G.M.; Hopman, M.T.E. Protein Intake and Distribution in Relation to Physical Functioning and Quality of Life in Community-Dwelling Elderly People: Acknowledging the Role of Physical Activity. Nutrients 2018, 10, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanri, H.; Yamada, Y.; Yoshida, T.; Okabe, Y.; Nozawa, Y.; Itoi, A.; Yoshimura, E.; Watanabe, Y.; Yamaguchi, M.; Yokoyama, K.; et al. Sex Difference in the Association Between Protein Intake and Frailty: Assessed Using the Kihon Checklist Indexes Among Older Adults. J. Am. Med. Dir. Assoc. 2018, 19, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Rahi, B.; Colombet, Z.; Gonzalez-Colaco Harmand, M.; Dartigues, J.-F.; Boirie, Y.; Letenneur, L.; Feart, C. Higher Protein but Not Energy Intake Is Associated With a Lower Prevalence of Frailty Among Community-Dwelling Older Adults in the French Three-City Cohort. J. Am. Med. Dir. Assoc. 2016, 17, 672.e7–672.e11. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Insausti, H.; Perez-Tasigchana, R.F.; Lopez-Garcia, E.; Garcia-Esquinas, E.; Rodriguez-Artalejo, F.; Guallar-Castillon, P. Macronutrients Intake and Incident Frailty in Older Adults: A Prospective Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1329–1334. [Google Scholar] [CrossRef]
- Beasley, J.M.; Lacroix, A.Z.; Neuhouser, M.L.; Huang, Y.; Tinker, L.; Woods, N.; Michael, Y.; Curb, J.D.; Prentice, R.L. Protein intake and incident frailty in the women’s health initiative observational study. J. Am. Geriatr. Soc. 2010, 58, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Júnior, H.; Rodrigues, B.; Uchida, M.; Marzetti, E.; Coelho-Júnior, H.J.; Rodrigues, B.; Uchida, M.; Marzetti, E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1334. [Google Scholar] [CrossRef] [Green Version]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Distribution but not amount of protein intake is associated with frailty: A cross-sectional investigation in the region of Nürnberg. Nutr. J. 2013, 12, 109. [Google Scholar] [CrossRef] [Green Version]
- Shikany, J.M.; Barrett-Connor, E.; Ensrud, K.E.; Cawthon, P.M.; Lewis, C.E.; Dam, T.-T.L.; Shannon, J.; Redden, D.T. Macronutrients, diet quality, and frailty in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 695–701. [Google Scholar] [CrossRef]
- Millward, D.J.; Layman, D.K.; Tomé, D.; Schaafsma, G. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008, 87, 1576S–1581S. [Google Scholar] [CrossRef]
- Checa-López, M.; Oviedo-Briones, M.; Pardo-Gómez, A.; Gonzales-Turín, J.; Guevara-Guevara, T.; Carnicero, J.A.; Alamo-Ascencio, S.; Landi, F.; Cesari, M.; Grodzicki, T.; et al. FRAILTOOLS study protocol: A comprehensive validation of frailty assessment tools to screen and diagnose frailty in different clinical and social settings and to provide instruments for integrated care in older adults. BMC Geriatr. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Witard, O.C. Characterising the Muscle Anabolic Potential of Dairy, Meat and Plant-Based Protein Sources in Older Adults. In Proceedings of the Nutrition Society; Cambridge University Press: Cambridge, UK, 2018; Volume 77, pp. 20–31. [Google Scholar]
- Anthony, T.G.; McDaniel, B.J.; Knoll, P.; Bunpo, P.; Paul, G.L.; McNurlan, M.A. Feeding Meals Containing Soy or Whey Protein after Exercise Stimulates Protein Synthesis and Translation Initiation in the Skeletal Muscle of Male Rats. J. Nutr. 2007, 137, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.J.; Della Gatta, P.A.; Petersen, A.C.; Cameron-Smith, D.; Markworth, J.F. Soy protein ingestion results in less prolonged p70S6 kinase phosphorylation compared to whey protein after resistance exercise in older men. J. Int. Soc. Sports Nutr. 2015, 12, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bos, C.; Metges, C.C.; Gaudichon, C.; Petzke, K.J.; Pueyo, M.E.; Morens, C.; Everwand, J.; Benamouzig, R.; Tomé, D. Postprandial Kinetics of Dietary Amino Acids Are the Main Determinant of Their Metabolism after Soy or Milk Protein Ingestion in Humans. J. Nutr. 2003, 133, 1308–1315. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Moughan, P.J. Available versus digestible dietary amino acids. Br. J. Nutr. 2012, 108, S298–S305. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Fang, A.P.; Ma, W.J.; Wu, S.L.; Li, C.L.; Chen, Y.M.; Zhu, H.L. Amount Rather than Animal vs Plant Protein Intake Is Associated with Skeletal Muscle Mass in Community-Dwelling Middle-Aged and Older Chinese Adults: Results from the Guangzhou Nutrition and Health Study. J. Acad. Nutr. Diet. 2019, 119, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Junior, H.J.; Calvani, R.; Gonçalves, I.O.; Rodrigues, B.; Picca, A.; Landi, F.; Bernabei, R.; Uchida, M.C.; Marzetti, E. High relative consumption of vegetable protein is associated with faster walking speed in well-functioning older adults. Aging Clin. Exp. Res. 2019, 31, 837–844. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Tipton, K.D.; Miller, S.L.; Wolf, S.E.; Wolfe, R.R. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J. Appl. Physiol. 2000, 88, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.; Reynolds, N.; Downie, S.; Patel, A.; Rennie, M.J. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am. J. Physiol. Endocrinol. Metab. 1998, 275, E73–E78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borack, M.S.; Volpi, E. Efficacy and Safety of Leucine Supplementation in the Elderly. J. Nutr. 2016, 146, 2625S–2629S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolster, D.R.; Vary, T.C.; Kimball, S.R.; Jefferson, L.S. Leucine Regulates Translation Initiation in Rat Skeletal Muscle Via Enhanced eIF4G Phosphorylation. J. Nutr. 2004, 134, 1704–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dardevet, D.; Sornet, C.; Balage, M.; Grizard, J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J. Nutr. 2000, 130, 2630–2635. [Google Scholar] [CrossRef]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575, 305–315. [Google Scholar] [CrossRef]
- Atherton, P.J.; Kumar, V.; Selby, A.L.; Rankin, D.; Hildebrandt, W.; Phillips, B.E.; Williams, J.P.; Hiscock, N.; Smith, K. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clin. Nutr. 2017, 36, 888–895. [Google Scholar] [CrossRef]
- Kimball, S.R.; Shantz, L.M.; Horetsky, R.L.; Jefferson, L.S. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J. Biol. Chem. 1999, 274, 11647–11652. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.B.; Tarnopolsky, M.A.; MacDonald, M.J.; MacDonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef]
- Aubertin-Leheudre, M.; Adlercreutz, H. Relationship between animal protein intake and muscle mass index in healthy women. Br. J. Nutr. 2009, 102, 1803–1810. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, N.V.; Eelderink, C.; Singh-Povel, C.M.; Navis, G.J.; Bakker, S.J.L.; Corpeleijn, E. Dietary protein sources and muscle mass over the life course: The lifelines cohort study. Nutrients 2018, 10, 1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, R.R.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; Sahni, S. Dietary Protein Intake Is Protective Against Loss of Grip Strength Among Older Adults in the Framingham Offspring Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 356–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hengeveld, L.M.; Wijnhoven, H.A.H.; Olthof, M.R.; Brouwer, I.A.; Simonsick, E.M.; Kritchevsky, S.B.; Houston, D.K.; Newman, A.B.; Visser, M. Prospective Associations of Diet Quality With Incident Frailty in Older Adults: The Health, Aging, and Body Composition Study. J. Am. Geriatr. Soc. 2019, 67, 1835–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, E.; Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; Bracone, F.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M.; et al. Socioeconomic and psychosocial determinants of adherence to the Mediterranean diet in a general adult Italian population. Eur. J. Public Health 2019, 29, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Mukherjea, A.; Underwood, K.C.; Stewart, A.L.; Ivey, S.L.; Kanaya, A.M. Asian Indian views on diet and health in the United States: Importance of understanding cultural and social factors to address disparities. Fam. Commun. Health 2013, 36, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.G.M.; Houser, R.F.; Mattei, J.; Lichtenstein, A.H.; Folta, S.C. Qualitative exploration of cultural factors influencing diet among African-, Caribbean- and US-born Blacks living in the northeast USA. J. Nutr. Sci. 2019, 8, e23. [Google Scholar] [CrossRef] [Green Version]
- Juárez-Ramírez, C.; Théodore, F.L.; Villalobos, A.; Allen-Leigh, B.; Jiménez-Corona, A.; Nigenda, G.; Lewis, S. The importance of the cultural dimension of food in understanding the lack of adherence to diet regimens among Mayan people with diabetes. Public Health Nutr. 2019, 22, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Zou, P. Diet and Blood Pressure Control in Chinese Canadians: Cultural Considerations. J. Immigr. Minor. Health 2017, 19, 477–483. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Calvani, R.; Picca, A.; Gonçalves, I.O.; Landi, F.; Bernabei, R.; Cesari, M.; Uchida, M.C.; Marzetti, E. Association between dietary habits and physical function in brazilian and italian older women. Nutrients 2020, 12, 1635. [Google Scholar] [CrossRef]
- Cardoso, L.D.O.; Carvalho, M.S.; Cruz, O.G.; Melere, C.; Luft, V.C.; Molina, M.D.C.B.; Faria, C.P.D.; Benseñor, I.M.; Matos, S.M.A.; Fonseca, M.D.J.M.D.; et al. Eating patterns in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): An exploratory analysis. Cad. Saude Publica 2016, 32, e00066215. [Google Scholar] [CrossRef] [Green Version]
- Neutzling, M.B.; Rombaldi, A.J.; Azevedo, M.R.; Hallal, P.C. Fatores associados ao consumo de frutas, legumes e verduras em adultos de uma cidade no Sul do Brasil. Cad. Saude Publica 2009, 25, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D. Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.D.S.G. O consumo da carne no Brasil: Entre valores sócios culturais e nutricionais. Demetra Aliment. Nutr. Saúde 2013, 8, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Symons, T.B.; Sheffield-Moore, M.; Wolfe, R.R.; Paddon-Jones, D. A Moderate Serving of High-Quality Protein Maximally Stimulates Skeletal Muscle Protein Synthesis in Young and Elderly Subjects. J. Am. Diet. Assoc. 2009, 109, 1582–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loenneke, J.P.; Loprinzi, P.D.; Murphy, C.H.; Phillips, S.M. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin. Nutr. 2016, 35, 1506–1511. [Google Scholar] [CrossRef]
- Gaytán-González, A.; Ocampo-Alfaro, M.D.J.; Torres-Naranjo, F.; Arroniz-Rivera, M.; González-Mendoza, R.G.; Gil-Barreiro, M.; López-Taylor, J.R. The Consumption of Two or Three Meals per Day with Adequate Protein Content Is Associated with Lower Risk of Physical Disability in Mexican Adults Aged 60 Years and Older. Geriatrics 2020, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Farsijani, S.; Payette, H.; Morais, J.A.; Shatenstein, B.; Gaudreau, P.; Chevalier, S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: The Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study). Am. J. Clin. Nutr. 2017, 106, 113–124. [Google Scholar] [CrossRef]
- Farsijani, S.; Morais, J.A.; Payette, H.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K.; Chevalier, S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am. J. Clin. Nutr. 2016, 104, 694–703. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Picca, A.; Gonçalves, I.O.; Landi, F.; Bernabei, R.; Cesari, M.; Uchida, M.C.; Marzetti, E. Protein-related dietary parameters and frailty status in older community-dwellers across different frailty instruments. Nutrients 2020, 12, 508. [Google Scholar] [CrossRef] [Green Version]
- Rhee, C.M.; Ahmadi, S.F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.; Hodson, E.M.; Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 2018, 10, CD001892. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, Y.S.; Kim, Y.H.; Chung, W.; Park, S.K.; Choi, K.H.; Ahn, C.; Oh, K.H. Dietary protein intake, protein energy wasting, and the progression of chronic kidney disease: Analysis from the know-ckd study. Nutrients 2019, 11, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.P.; Xue, Q.-L.; Cappola, A.R.; Ferrucci, L.; Chaves, P.; Varadhan, R.; Guralnik, J.M.; Leng, S.X.; Semba, R.D.; Walston, J.D.; et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1049–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoufour, J.D.; Franco, O.H.; Kiefte-De Jong, J.C.; Trajanoska, K.; Stricker, B.; Brusselle, G.; Rivadeneira, F.; Lahousse, L.; Voortman, T. The association between dietary protein intake, energy intake and physical frailty: Results from the Rotterdam Study. Br. J. Nutr. 2019, 121, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, M.L.; Coppinger, T.; McCarthy, A.L. The role of nutrition and physical activity in frailty: A review. Clin. Nutr. ESPEN 2020, 35, 1–11. [Google Scholar] [CrossRef]
- Otsuka, R.; Tange, C.; Tomida, M.; Nishita, Y.; Kato, Y.; Yuki, A.; Ando, F.; Shimokata, H.; Arai, H. Dietary Factors Associated with the Development of Physical Frailty in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 89–95. [Google Scholar] [CrossRef]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low nutrient intake is an essential component of frailty in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef]
- Lahmann, N.A.; Tannen, A.; Suhr, R. Underweight and malnutrition in home care: A multicenter study. Clin. Nutr. 2016, 35, 1140–1146. [Google Scholar] [CrossRef]
- Sewo Sampaio, P.Y.; Sampaio, R.A.C.; Yamada, M.; Arai, H. Systematic review of the Kihon Checklist: Is it a reliable assessment of frailty? Geriatr. Gerontol. Int. 2016, 16, 893–902. [Google Scholar] [CrossRef]
- Ferreira, R.S.; Da Silva Coqueiro, R.; Barbosa, A.R.; Pinheiro, P.A.; Fernandes, M.H. Relationship between BMI and physical performance among older adults. Geriatr. Nurs. 2013, 34, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Schrager, M.A.; Schrack, J.A.; Simonsick, E.M.; Ferrucci, L. Association between energy availability and physical activity in older adults. Am. J. Phys. Med. Rehabil. 2014, 93, 876–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paslakis, G.; Richardson, C.; Nöhre, M.; Brähler, E.; Holzapfel, C.; Hilbert, A.; de Zwaan, M. Prevalence and psychopathology of vegetarians and vegans—Results from a representative survey in Germany. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisay, T.; Tolessa, T.; Mekonen, W. Changes in biochemical parameters by gender and time: Effect of short-term vegan diet adherence. PLoS ONE 2020, 15, e0237065. [Google Scholar] [CrossRef]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-inflammatory effects of a vegan diet versus the american heart association–recommended diet in coronary artery disease trial. J. Am. Heart Assoc. 2018, 7, e001367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawthon, P.M.; Blackwell, T.; Cummings, S.R.; Orwoll, E.S.; Duchowny, K.A.; Kado, D.M.; Stone, K.L.; Ensrud, K.E.; Cauley, J.A.; Evans, W.J. Muscle mass assessed by D3-Creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community dwelling older men. J. Gerontol. A Biol. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Hellerstein, M.; Evans, W. Recent advances for measurement of protein synthesis rates, use of the “Virtual Biopsy” approach, and measurement of muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 191–200. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Park, S.; Jang, J.; Wolfe, R.R. Understanding Muscle Protein Dynamics: Technical Considerations for Advancing Sarcopenia Research. Ann. Geriatr. Med. Res. 2020. [Google Scholar] [CrossRef]
- Rodgers, G.P.; Collins, F.S. Precision Nutrition—The Answer to “What to Eat to Stay Healthy. ” JAMA 2020, 324, 735–736. [Google Scholar] [CrossRef]
- Dragsted, L.O. The metabolic nature of individuality. Nat. Food 2020, 1, 327–328. [Google Scholar] [CrossRef]
- Cuparencu, C.; Praticó, G.; Hemeryck, L.Y.; Sri Harsha, P.S.C.; Noerman, S.; Rombouts, C.; Xi, M.; Vanhaecke, L.; Hanhineva, K.; Brennan, L.; et al. Biomarkers of meat and seafood intake: An extensive literature review. Genes Nutr. 2019, 14, 1–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Perez, I.; Posma, J.M.; Chambers, E.S.; Mathers, J.C.; Draper, J.; Beckmann, M.; Nicholson, J.K.; Holmes, E.; Frost, G. Dietary metabotype modelling predicts individual responses to dietary interventions. Nat. Food 2020, 1, 355–364. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho-Junior, H.J.; Marzetti, E.; Picca, A.; Cesari, M.; Uchida, M.C.; Calvani, R. Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing. Nutrients 2020, 12, 2915. https://doi.org/10.3390/nu12102915
Coelho-Junior HJ, Marzetti E, Picca A, Cesari M, Uchida MC, Calvani R. Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing. Nutrients. 2020; 12(10):2915. https://doi.org/10.3390/nu12102915
Chicago/Turabian StyleCoelho-Junior, Hélio J., Emanuele Marzetti, Anna Picca, Matteo Cesari, Marco C. Uchida, and Riccardo Calvani. 2020. "Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing" Nutrients 12, no. 10: 2915. https://doi.org/10.3390/nu12102915
APA StyleCoelho-Junior, H. J., Marzetti, E., Picca, A., Cesari, M., Uchida, M. C., & Calvani, R. (2020). Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing. Nutrients, 12(10), 2915. https://doi.org/10.3390/nu12102915









