The Anti-Obesogenic Effect of Lean Fish Species Is Influenced by the Fatty Acid Composition in Fish Fillets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Diets
2.3. Mouse Study
2.4. Apparent Digestibility of Nitrogen and Fat
2.5. Insulin Tolerance Test (ITT)
2.6. Glucose Tolerance Test (GTT)
2.7. Histology and Immunohistochemistry
2.8. Real Time qPCR
2.9. Lipid Analyses
2.10. Plasma and Liver Analyses
2.11. Statistics
3. Results
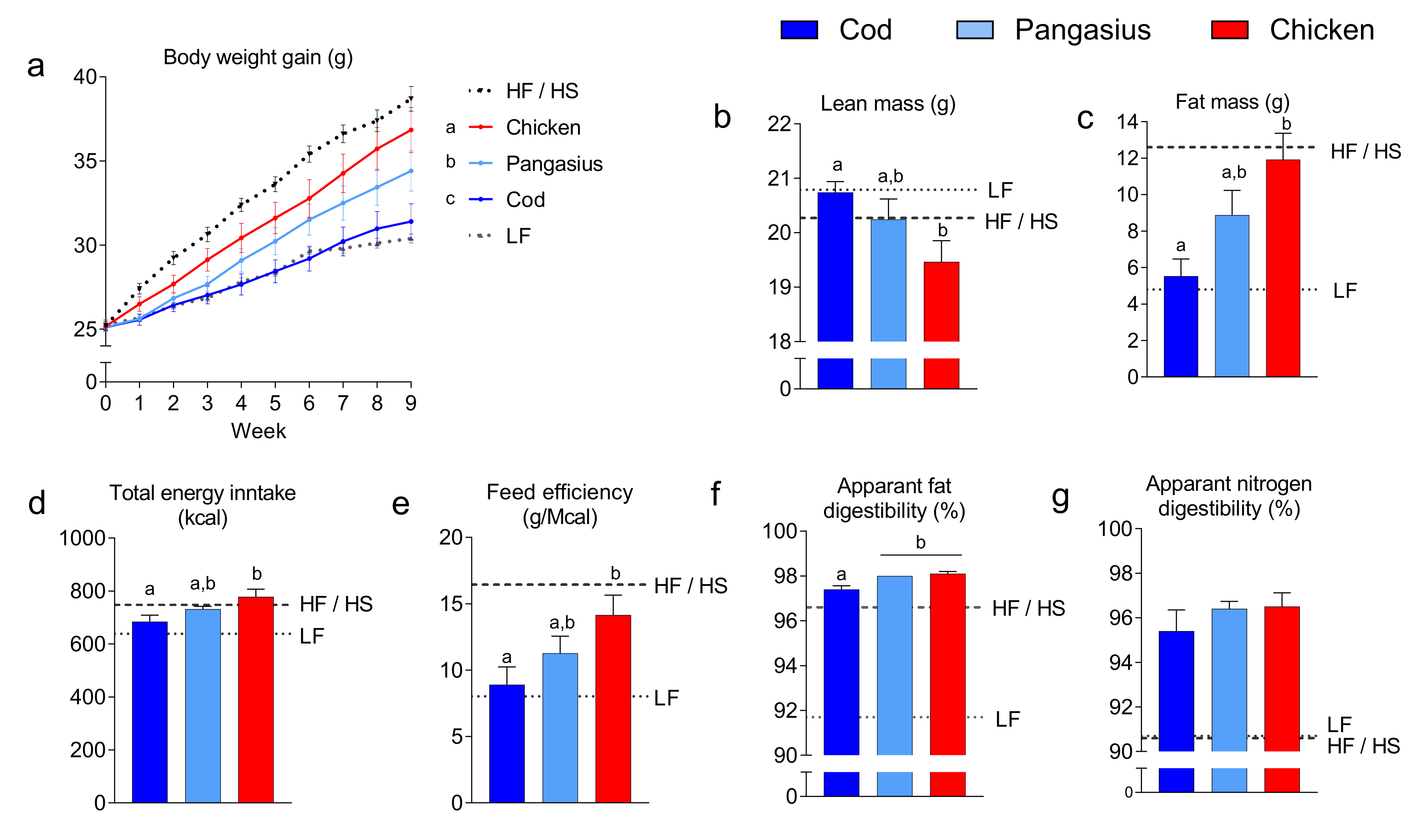
3.1. A High-Fat/High-Protein Diet Containing Cod is More Efficient than An Isoenergic Diet Containing Pangasius in Attenuating Development of Obesity and Glucose Intolerance
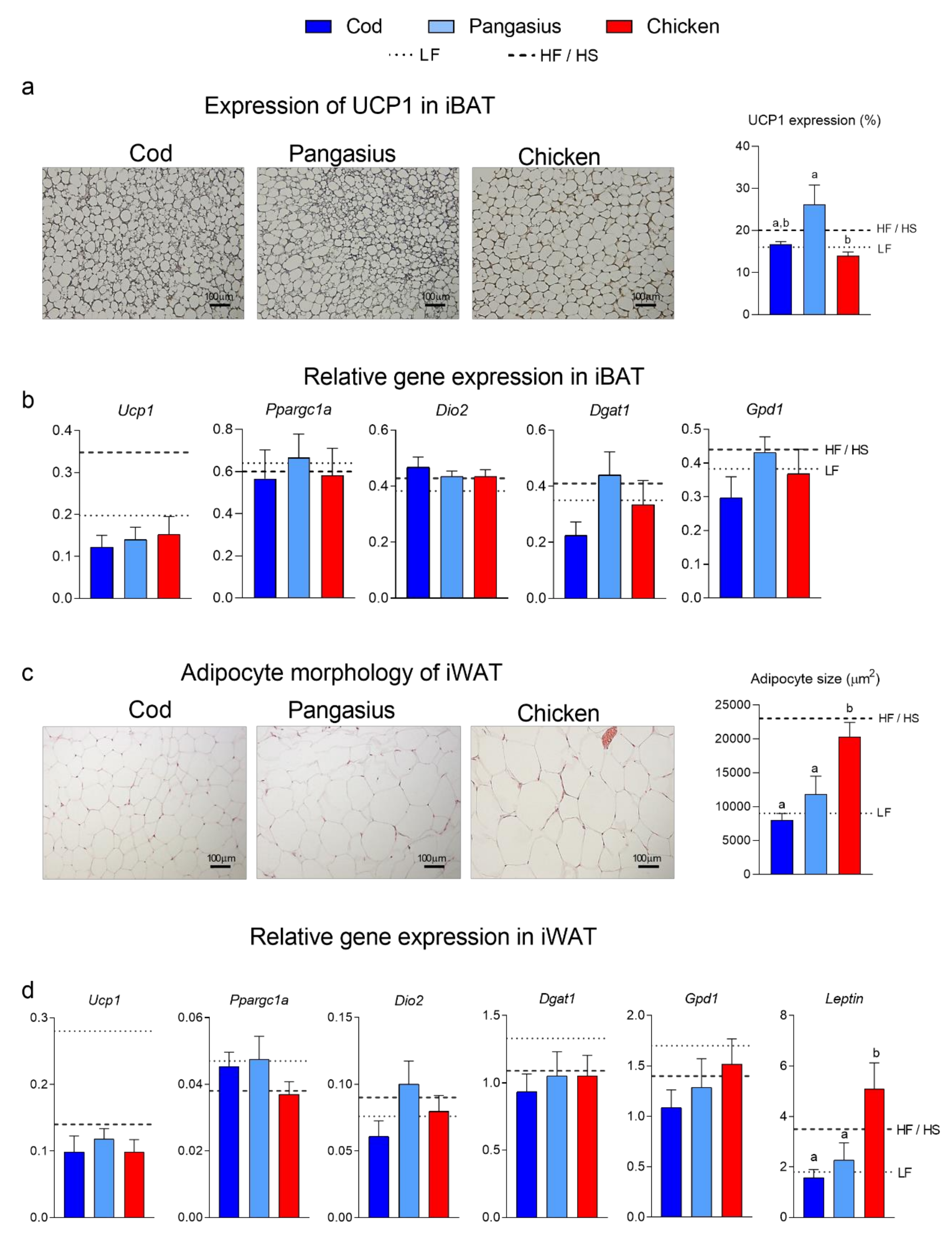
3.2. Mice fed a HF/HP Diet with Cod had Smaller Adipocytes than Mice fed a HF/HP Diet with Pangasius or Chicken
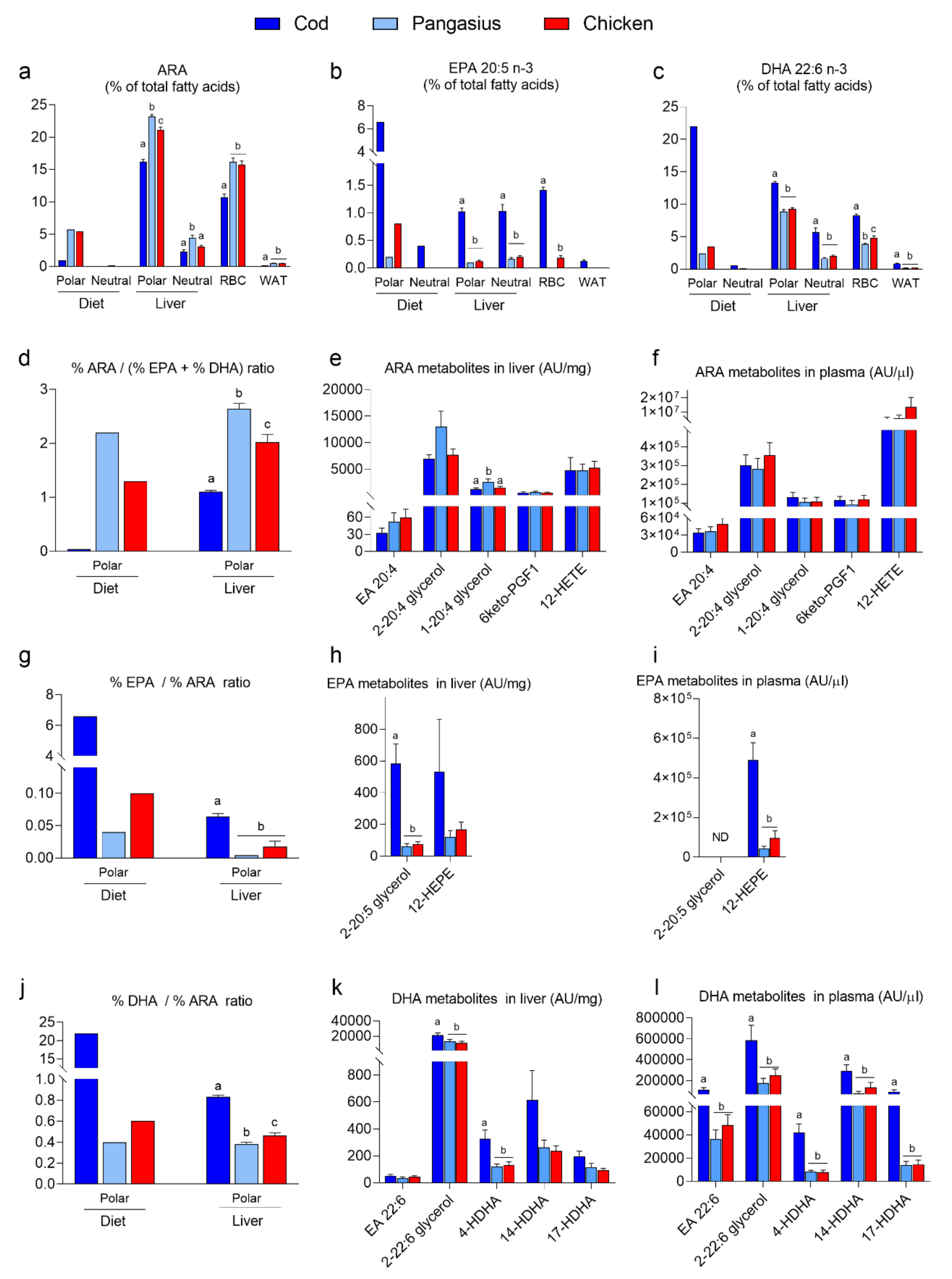
3.3. Feeding Mice a HF/HP Diet with Cod Increases Incorporation of Marine n-3 Fatty Acids in Mice Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fogelholm, M.; Anderssen, S.; Gunnarsdottir, I.; Lahti-Koski, M. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: A systematic literature review. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.D.; Hou, T.; Ludwig, D.S.; Rimm, E.B.; Willett, W.; Hu, F.B.; Mozaffarian, D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: Results from 3 prospective cohorts. Am. J. Clin. Nutr. 2015, 101, 1216–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liaset, B.; Øyen, J.; Jacques, H.; Kristiansen, K.; Madsen, L. Seafood intake and the development of obesity, insulin resistance and type 2 diabetes. Nutr. Res. Rev. 2019, 32, 146–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, L.; Petersen, R.K.; Kristiansen, K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim. Biophys. Acta 2005, 30, 266–286. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.W.; Chien, Y.S.; Chen, Y.J.; Ajuwon, K.M.; Mersmann, H.M.; Ding, S.T. Role of n-3 Polyunsaturated Fatty Acids in Ameliorating the Obesity-Induced Metabolic Syndrome in Animal Models and Humans. Int. J. Mol. Sci. 2016, 17, 1689. [Google Scholar] [CrossRef]
- Kuda, O.; Rossmeisl, M.; Kopecky, J. Omega-3 fatty acids and adipose tissue biology. Mol. Asp. Med. 2018, 64, 147–160. [Google Scholar] [CrossRef]
- Spitze, A.R.; Wong, D.L.; Rogers, Q.R.; Fascetti, A.J. Taurine concentrations in animal feed ingredients; cooking influences taurine content. J. Anim. Physiol. Anim. Nutr. 2003, 87, 251–262. [Google Scholar] [CrossRef]
- Borck, P.C.; Vettorazzi, J.F.; Branco, R.C.S.; Batista, T.M.; Santos-Silva, J.C.; Nakanishi, V.Y.; Boschero, A.C.; Ribeiro, R.A.; Carneiro, E.M. Taurine supplementation induces long-term beneficial effects on glucose homeostasis in ob/ob mice. Amino Acids 2018, 50, 765–774. [Google Scholar] [CrossRef]
- Kim, K.S.; Doss, H.M.; Kim, H.J.; Yang, H.I. Taurine Stimulates Thermoregulatory Genes in Brown Fat Tissue and Muscle without an Influence on Inguinal White Fat Tissue in a High-Fat Diet-Induced Obese Mouse Model. Foods 2020, 9, 688. [Google Scholar] [CrossRef]
- Kim, K.S.; Jang, M.J.; Fang, S.; Yoon, S.G.; Kim, I.Y.; Seong, J.K.; Yang, H.I.; Hahm, D.H. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids 2019, 51, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Liaset, B.; Madsen, L.; Hao, Q.; Criales, G.; Mellgren, G.; Marschall, H.U.; Hallenborg, P.; Espe, M.; Frøyland, L.; Kristiansen, K. Fish protein hydrolysate elevates plasma bile acids and reduces visceral adipose tissue mass in rats. Biochim. Biophys. Acta 2009, 4, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Liaset, B.; Hao, Q.; Jørgensen, H.; Hallenborg, P.; Du, Z.Y.; Ma, T.; Marschall, H.U.; Kruhøffer, M.; Li, R.; Li, Q.; et al. Nutritional regulation of bile acid metabolism is associated with improved pathological characteristics of the metabolic syndrome. J. Biol. Chem. 2011, 286, 28382–28395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, Y.R.; Pérez-Torres, I.; Zúñiga-Muñoz, A.; Lans, V.G.; Díaz-Díaz, E.; Castro, E.S.; Espejel, R.V. Effect of Glycine on Adipocyte Hypertrophy in a Metabolic Syndrome Rat Model. Curr. Drug. Deliv. 2016, 13, 158–169. [Google Scholar] [CrossRef]
- Tastesen, H.S.; Keenan, A.H.; Madsen, L.; Kristiansen, K.; Liaset, B. Scallop protein with endogenous high taurine and glycine content prevents high-fat, high-sucrose-induced obesity and improves plasma lipid profile in male C57BL/6J mice. Amino Acids 2014, 46, 1659–1671. [Google Scholar] [CrossRef] [Green Version]
- Aakre, I.; Næss, S.; Kjellevold, M.; Markhus, M.W.; Alvheim, A.R.; Dalane, J.; Kielland, E.; Dahl, L. New data on nutrient composition in large selection of commercially available seafood products and its impact on micronutrient intake. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Lie, O.; Lambertsen, G. Fatty acid composition of glycerophospholipids in seven tissues of cod (Gadus morhua), determined by combined high-performance liquid chromatography and gas chromatography. J. Chromatogr. 1991, 565, 119–129. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xu, J.; Wang, Y.M.; Xue, C.H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid. Res. 2019, 75, 20. [Google Scholar] [CrossRef]
- Fauske, K.R.; Bernhard, A.; Fjaere, E.; Myrmel, L.S.; Froyland, L.; Kristiansen, K.; Liaset, B.; Madsen, L. Effects of Frozen Storage on Phospholipid Content in Atlantic Cod Fillets and the Influence on Diet-Induced Obesity in Mice. Nutrients 2018, 10, 695. [Google Scholar] [CrossRef] [Green Version]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Ghasemifard, S.; Turchini, G.M.; Sinclair, A.J. Omega-3 long chain fatty acid “bioavailability”: A review of evidence and methodological considerations. Prog. Lipid. Res. 2014, 56, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Ulven, S.M.; Holven, K.B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc. Health Risk. Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liisberg, U.; Fauske, K.R.; Kuda, O.; Fjaere, E.; Myrmel, L.S.; Norberg, N.; Froyland, L.; Graff, I.E.; Liaset, B.; Kristiansen, K.; et al. Intake of a Western diet containing cod instead of pork alters fatty acid composition in tissue phospholipids and attenuates obesity and hepatic lipid accumulation in mice. J. Nutr. Biochem. 2016, 33, 119–127. [Google Scholar] [CrossRef]
- Rossmeisl, M.; Jilkova, Z.M.; Kuda, O.; Jelenik, T.; Medrikova, D.; Stankova, B.; Kristinsson, B.; Haraldsson, G.G.; Svensen, H.; Stoknes, I.; et al. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: Possible role of endocannabinoids. PLoS ONE 2012, 7, e38834. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.Y.; Ding, L.; Shi, H.H.; Xu, J.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Eicosapentaenoic acid in the form of phospholipids exerts superior anti-atherosclerosis effects to its triglyceride form in ApoE(-/-) mice. Food Funct. 2019, 10, 4177–4188. [Google Scholar] [CrossRef]
- Batetta, B.; Griinari, M.; Carta, G.; Murru, E.; Ligresti, A.; Cordeddu, L.; Giordano, E.; Sanna, F.; Bisogno, T.; Uda, S.; et al. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J. Nutr. 2009, 139, 1495–1501. [Google Scholar] [CrossRef] [Green Version]
- Piscitelli, F.; Carta, G.; Bisogno, T.; Murru, E.; Cordeddu, L.; Berge, K.; Tandy, S.; Cohn, J.S.; Griinari, M.; Banni, S.; et al. Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice. Nutr. Metab. 2011, 8, 1743–7075. [Google Scholar] [CrossRef] [Green Version]
- Alvheim, A.R.; Malde, M.K.; Osei-Hyiaman, D.; Lin, Y.H.; Pawlosky, R.J.; Madsen, L.; Kristiansen, K.; Froyland, L.; Hibbeln, J.R. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring) 2012, 20, 1984–1994. [Google Scholar] [CrossRef] [Green Version]
- Rossmeisl, M.; Medrikova, D.; van Schothorst, E.M.; Pavlisova, J.; Kuda, O.; Hensler, M.; Bardova, K.; Flachs, P.; Stankova, B.; Vecka, M.; et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim. Biophys. Acta 2014, 2, 267–278. [Google Scholar] [CrossRef]
- Tillander, V.; Bjørndal, B.; Burri, L.; Bohov, P.; Skorve, J.; Berge, R.K.; Alexson, S.E. Fish oil and krill oil supplementations differentially regulate lipid catabolic and synthetic pathways in mice. Nutr. Metab. 2014, 11, 1743–7075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef] [Green Version]
- Liisberg, U.; Myrmel, L.S.; Fjaere, E.; Ronnevik, A.K.; Bjelland, S.; Fauske, K.R.; Holm, J.B.; Basse, A.L.; Hansen, J.B.; Liaset, B.; et al. The protein source determines the potential of high protein diets to attenuate obesity development in C57BL/6J mice. Adipocyte 2016, 5, 196–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein--beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Fjaere, E.; Aune, U.L.; Roen, K.; Keenan, A.H.; Ma, T.; Borkowski, K.; Kristensen, D.M.; Novotny, G.W.; Mandrup-Poulsen, T.; Hudson, B.D.; et al. Indomethacin Treatment Prevents High Fat Diet-induced Obesity and Insulin Resistance but Not Glucose Intolerance in C57BL/6J Mice. J. Biol. Chem. 2014, 289, 16032–16045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillefosse, H.H.; Tastesen, H.S.; Du, Z.Y.; Ditlev, D.B.; Thorsen, F.A.; Madsen, L.; Kristiansen, K.; Liaset, B. Hydrolyzed casein reduces diet-induced obesity in male C57BL/6J mice. J. Nutr. 2013, 143, 1367–1375. [Google Scholar] [CrossRef] [Green Version]
- Dumlao, D.S.; Buczynski, M.W.; Norris, P.C.; Harkewicz, R.; Dennis, E.A. High-throughput lipidomic analysis of fatty acid derived eicosanoids and N-acylethanolamines. Biochim. Biophys. Acta 2011, 1811, 724–736. [Google Scholar] [CrossRef] [Green Version]
- Holm, J.B.; Ronnevik, A.; Tastesen, H.S.; Fjaere, E.; Fauske, K.R.; Liisberg, U.; Madsen, L.; Kristiansen, K.; Liaset, B. Diet-induced obesity, energy metabolism and gut microbiota in C57BL/6J mice fed Western diets based on lean seafood or lean meat mixtures. J. Nutr. Biochem. 2016, 31, 127–136. [Google Scholar] [CrossRef]
- Myrmel, L.S.; Fauske, K.R.; Fjære, E.; Bernhard, A.; Liisberg, U.; Hasselberg, A.E.; Øyen, J.; Kristiansen, K.; Madsen, L. The Impact of Different Animal-Derived Protein Sources on Adiposity and Glucose Homeostasis during Ad Libitum Feeding and Energy Restriction in Already Obese Mice. Nutrients 2019, 11, 1153. [Google Scholar] [CrossRef] [Green Version]
- Fjaere, E.; Myrmel, L.S.; Lutzhoft, D.O.; Andersen, H.; Holm, J.B.; Kiilerich, P.; Hannisdal, R.; Liaset, B.; Kristiansen, K.; Madsen, L. Effects of exercise and dietary protein sources on adiposity and insulin sensitivity in obese mice. J. Nutr. Biochem. 2019, 66, 98–109. [Google Scholar] [CrossRef]
- Flachs, P.; Horakova, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.; Ruzickova, J.; Sponarova, J.; Drahota, Z.; Vlcek, C.; et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.F.; Xin, X.; McLennan, P.; Storlien, L. Role of fat amount and type in ameliorating diet-induced obesity: Insights at the level of hypothalamic arcuate nucleus leptin receptor, neuropeptide Y and pro-opiomelanocortin mRNA expression. Diabetes Obes. Metab. 2004, 6, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kondo, H.; Hase, T.; Tokimitsu, I.; Murase, T. Dietary fish oil upregulates intestinal lipid metabolism and reduces body weight gain in C57BL/6J mice. J. Nutr. 2007, 137, 2629–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossmeisl, M.; Jelenik, T.; Jilkova, Z.; Slamova, K.; Kus, V.; Hensler, M.; Medrikova, D.; Povysil, C.; Flachs, P.; Mohamed-Ali, V.; et al. Prevention and reversal of obesity and glucose intolerance in mice by DHA derivatives. Obesity (Silver Spring) 2009, 17, 1023–1031. [Google Scholar] [CrossRef]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Veck, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004, 39, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Ukropec, J.; Reseland, J.E.; Gasperikova, D.; Demcakova, E.; Madsen, L.; Berge, R.K.; Rustan, A.C.; Klimes, I.; Drevon, C.A.; Sebokova, E. The hypotriglyceridemic effect of dietary n-3 FA is associated with increased beta-oxidation and reduced leptin expression. Lipids 2003, 38, 1023–1029. [Google Scholar] [CrossRef]
- Wang, H.; Storlien, L.H.; Huang, X.F. Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am. J. Physiol. Endocrinol Metab. 2002, 282, E1352–E1359. [Google Scholar] [CrossRef] [Green Version]
- Salem, N.; Kuratko, C.N. A reexamination of krill oil bioavailability studies. Lipids Health Dis. 2014, 13. [Google Scholar] [CrossRef] [Green Version]
- Alvheim, A.R.; Torstensen, B.E.; Lin, Y.H.; Lillefosse, H.H.; Lock, E.-J.; Madsen, L.; Hibbeln, J.R.; Malde, M.K. Dietary linoleic acid elevates endogenous 2-arachidonoylglycerol and anandamide in Atlantic salmon (Salmo salar L.) and mice, and induces weight gain and inflammation in mice. Br. J. Nutr. 2013, 109, 1508–1517. [Google Scholar] [CrossRef] [Green Version]
- Midtbø, L.K.; Borkowska, A.G.; Bernhard, A.; Rønnevik, A.K.; Lock, E.-J.; Fitzgerald, M.L.; Torstensen, B.E.; Liaset, B.; Brattelid, T.; Pedersen, T.L.; et al. Intake of farmed Atlantic salmon fed soybean oil increases hepatic levels of arachidonic acid-derived oxylipins and ceramides in mice. J. Nutr. Biochem. 2015, 26, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Midtbø, L.K.; Ibrahim, M.M.; Myrmel, L.S.; Aune, U.L.; Alvheim, A.R.; Liland, N.S.; Torstensen, B.E.; Rosenlund, G.; Liaset, B.; Brattelid, T. Intake of farmed Atlantic salmon fed soybean oil increases insulin resistance and hepatic lipid accumulation in mice. PLoS ONE 2013, 8, e53094. [Google Scholar] [CrossRef] [PubMed]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Fatty Acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int. J. Endocrinol 2013, 2013, 361895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravinet Trillou, C.; Delgorge, C.; Menet, C.; Arnone, M.; Soubrie, P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravinet Trillou, C.; Arnone, M.; Delgorge, C.; Gonalons, N.; Keane, P.; Maffrand, J.P.; Soubrie, P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R345–R353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cota, D.; Marsicano, G.; Tschop, M.; Grubler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thone-Reineke, C.; Ortmann, S.; et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Invest. 2003, 112, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Goparaju, S.K.; Wang, L.; Liu, J.; Batkai, S.; Jarai, Z.; Fezza, F.; Miura, G.I.; Palmiter, R.D.; Sugiura, T.; et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001, 410, 822–825. [Google Scholar] [CrossRef]
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Batkai, S.; Harvey-White, J.; Mackie, K.; Offertaler, L.; Wang, L.; et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Invest. 2005, 115, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Perwitz, N.; Wenzel, J.; Wagner, I.; Büning, J.; Drenckhan, M.; Zarse, K.; Ristow, M.; Lilienthal, W.; Lehnert, H.; Klein, J. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes. Metab. 2010, 12, 158–166. [Google Scholar] [CrossRef]
- Tedesco, L.; Valerio, A.; Cervino, C.; Cardile, A.; Pagano, C.; Vettor, R.; Pasquali, R.; Carruba, M.O.; Marsicano, G.; Lutz, B.; et al. Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes 2008, 57, 2028–2036. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.Y.; Li, B.Y.; Peng, W.Q.; Guo, L.; Tang, Q.Q. Taurine-mediated browning of white adipose tissue is involved in its anti-obesity effect in mice. J. Biol. Chem. 2019, 294, 15014–15024. [Google Scholar] [CrossRef]


| FA (mg/g) | Cod | Pangasius | Chicken | Cod | Pangasius | Chicken |
|---|---|---|---|---|---|---|
| Neutral Lipid Fraction 5 | Polar Lipid Fraction 6 | |||||
| Sum total FAs 1 | 10 | 50 | 60 | 8 | 12 | 18 |
| Sum SFAs 2 | 2.3 | 20.3 | 18.1 | 2.0 | 3.1 | 5.4 |
| Sum MUFAs 3 | 1.9 | 19.9 | 27.2 | 1.3 | 2.9 | 4.3 |
| 18:1 n-9 | 0.7 | 18.2 | 22.6 | 0.6 | 2.5 | 3.4 |
| Sum n-6 PUFAs 4 | 0.4 | 7.6 | 12.5 | 0.3 | 3.9 | 5.1 |
| 18:2 n-6 | 0.1 | 5.3 | 11.8 | 0.1 | 1.1 | 2.8 |
| 20:4 n-6 | 0.2 | 0.7 | 0.3 | 0.1 | 1.1 | 1.5 |
| Sum n-3 PUFAs4 | 5.46 | 0.83 | 1.48 | 4.31 | 0.68 | 1.56 |
| 18:3 n-3 | 0.26 | 0.28 | 1.04 | 0.01 | 0.03 | 0.06 |
| 20:5 n-3 | 1.76 | 0.06 | 0.05 | 0.87 | 0.04 | 0.17 |
| 22:6 n-3 | 3.40 | 0.21 | 0.09 | 3.24 | 0.44 | 0.82 |
| Amino acids (mg/g) | Cod | Pangasius | Chicken |
|---|---|---|---|
| Indispensable | |||
| Leucine | 72.8 | 76.1 | 70.4 |
| Isoleucine | 40.3 | 43.6 | 40.7 |
| Valine | 46.3 | 46.8 | 43.9 |
| Lysine | 93.1 | 94.6 | 85.3 |
| Methionine | 28.3 | 27.7 | 24.7 |
| Phenylalanine | 34.6 | 38.1 | 34.9 |
| Threonine | 39.6 | 43.1 | 39.6 |
| Tryptophan | 9.5 | 9.9 | 10.3 |
| Histidine | 16.9 | 19.5 | 23.7 |
| Dispensable | |||
| Alanine | 54.0 | 52.7 | 50.9 |
| Arginine | 53.7 | 56.9 | 53.8 |
| Aspartate | 101.8 | 101.0 | 89.0 |
| Cysteine | 1.0 | 1.0 | 1.0 |
| Glutamate | 146.9 | 148.3 | 135.2 |
| Glycine | 38.4 | 42.3 | 36.4 |
| Proline | 30.0 | 32.8 | 30.8 |
| Serine | 39.4 | 38.5 | 35.1 |
| Tyrosine | 30.0 | 31.5 | 28.9 |
| Sum BCAAs 1 | 159.3 | 166.5 | 155.0 |
| Hydroxyproline | 1.5 | 3.6 | 1.7 |
| Taurine | 4.8 | 2.6 | 0.6 |
| Component (g/kg) | LF | HF/HS | HF/HP Cod | HF/HP Pangasius | HF/HP Chicken |
|---|---|---|---|---|---|
| Casein # | 207 | 207 | |||
| Cod # | 482 | ||||
| Pangasius # | 502 | ||||
| Chicken # | 501 | ||||
| Protein from protein source * | 200 | 200 | 400 | 400 | 400 |
| Fat from protein source ** | 1 | 1 | 8.4 | 31.5 | 40.0 |
| Corn oil | 69 | 249 | 242 | 219 | 210 |
| Sucrose | 92 | 439 | 140 | 140 | 140 |
| Dextrin | 531 | 5 | 36.0 | 38.7 | 48.2 |
| L-cystine | 3 | 3 | 3 | 3 | 3 |
| Cellulose | 50 | 50 | 50 | 50 | 50 |
| t-butylhydroquinone | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Mineral mix † | 35 | 35 | 35 | 35 | 35 |
| Vitamin mix ‡ | 10 | 10 | 10 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Analyzed $ | |||||
| Protein (g/100g) | 19.8 ± 0.3 | 20.0 ± 0.2 | 40.6 ± 0.5 | 39.5 ± 0.4 | 39.6 ± 0.4 |
| Energy (kcal/g) | 4.3 ± 0.2 | 5.8 ± 0.2 | 5.7 ± 0.3 | 5.7 ± 0.3 | 5.8 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fjære, E.; Myrmel, L.S.; Dybing, K.; Kuda, O.; Holbech Jensen, B.A.; Rossmeisl, M.; Frøyland, L.; Kristiansen, K.; Madsen, L. The Anti-Obesogenic Effect of Lean Fish Species Is Influenced by the Fatty Acid Composition in Fish Fillets. Nutrients 2020, 12, 3038. https://doi.org/10.3390/nu12103038
Fjære E, Myrmel LS, Dybing K, Kuda O, Holbech Jensen BA, Rossmeisl M, Frøyland L, Kristiansen K, Madsen L. The Anti-Obesogenic Effect of Lean Fish Species Is Influenced by the Fatty Acid Composition in Fish Fillets. Nutrients. 2020; 12(10):3038. https://doi.org/10.3390/nu12103038
Chicago/Turabian StyleFjære, Even, Lene Secher Myrmel, Karianne Dybing, Ondrej Kuda, Benjamin Anderschou Holbech Jensen, Martin Rossmeisl, Livar Frøyland, Karsten Kristiansen, and Lise Madsen. 2020. "The Anti-Obesogenic Effect of Lean Fish Species Is Influenced by the Fatty Acid Composition in Fish Fillets" Nutrients 12, no. 10: 3038. https://doi.org/10.3390/nu12103038
APA StyleFjære, E., Myrmel, L. S., Dybing, K., Kuda, O., Holbech Jensen, B. A., Rossmeisl, M., Frøyland, L., Kristiansen, K., & Madsen, L. (2020). The Anti-Obesogenic Effect of Lean Fish Species Is Influenced by the Fatty Acid Composition in Fish Fillets. Nutrients, 12(10), 3038. https://doi.org/10.3390/nu12103038






