Daily Oral Administration of Protease-Treated Royal Jelly Protects Against Denervation-Induced Skeletal Muscle Atrophy
Abstract
:1. Introduction
2. Material and Methods
2.1. Denervation Model
2.2. Experimental Diet and pRJ Treatment
2.3. Histochemical Analysis
2.4. Cell Culture, Reagents, and Skeletal Muscle Differentiation
2.5. RNA Isolation and Quantitative Real-Time PCR (qPCR)
2.6. Immunocytochemistry Analysis
2.7. Western Blot Analysis
2.8. Cell Proliferation Assay
2.9. Statistical Analysis
3. Results
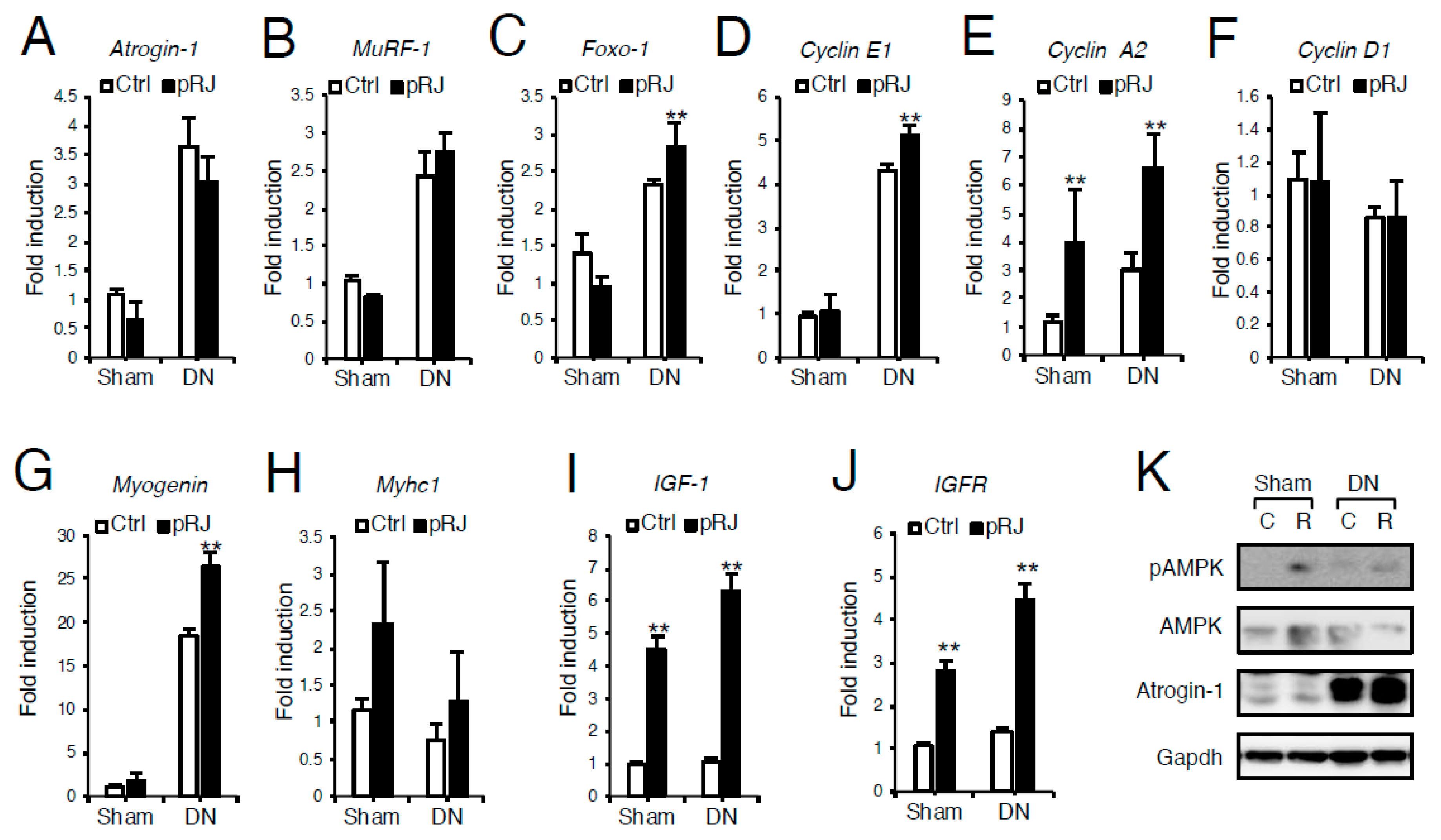
3.1. pRJ Attenuates Denervation-Induced Skeletal Muscle Atrophy
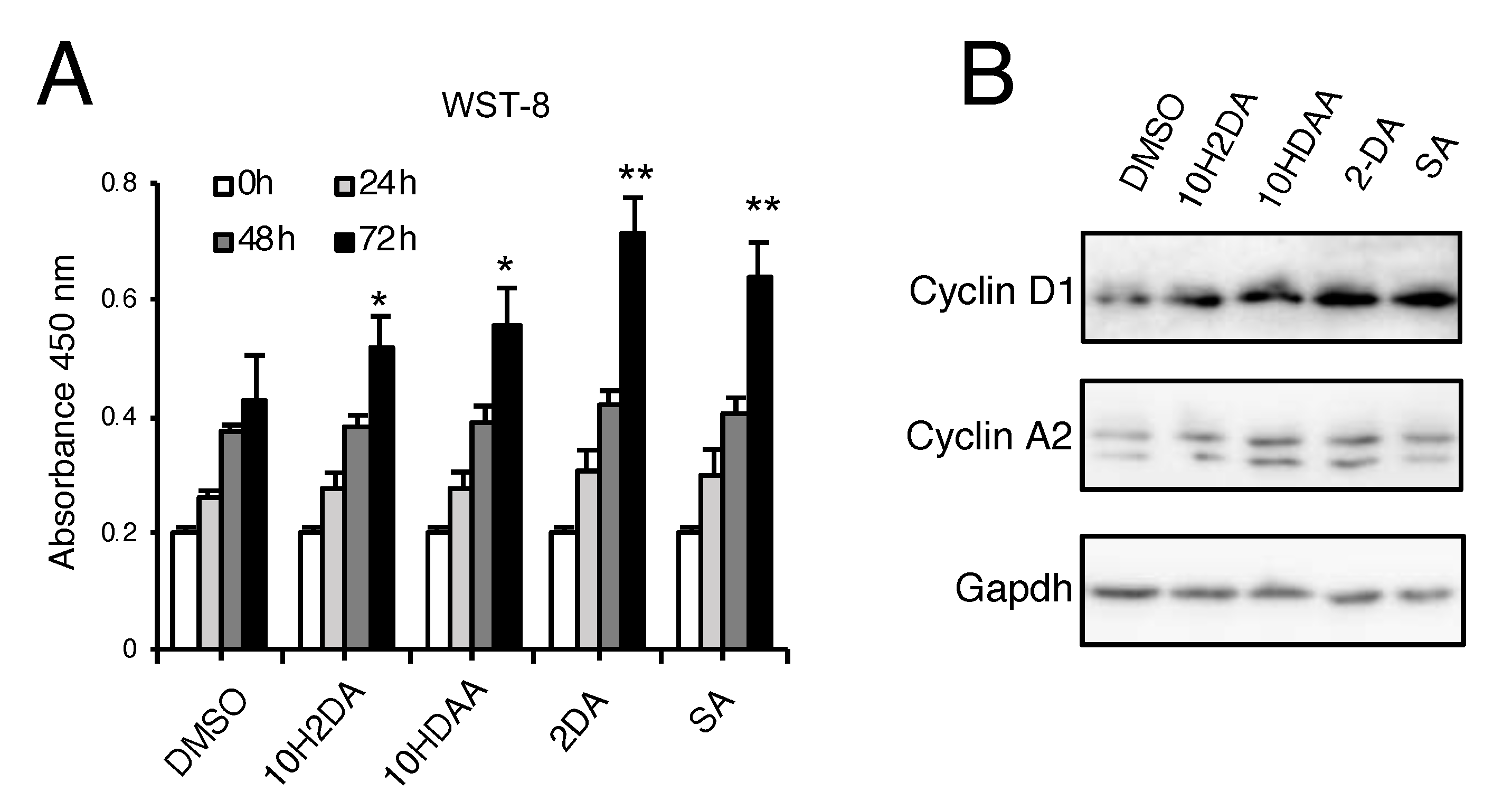
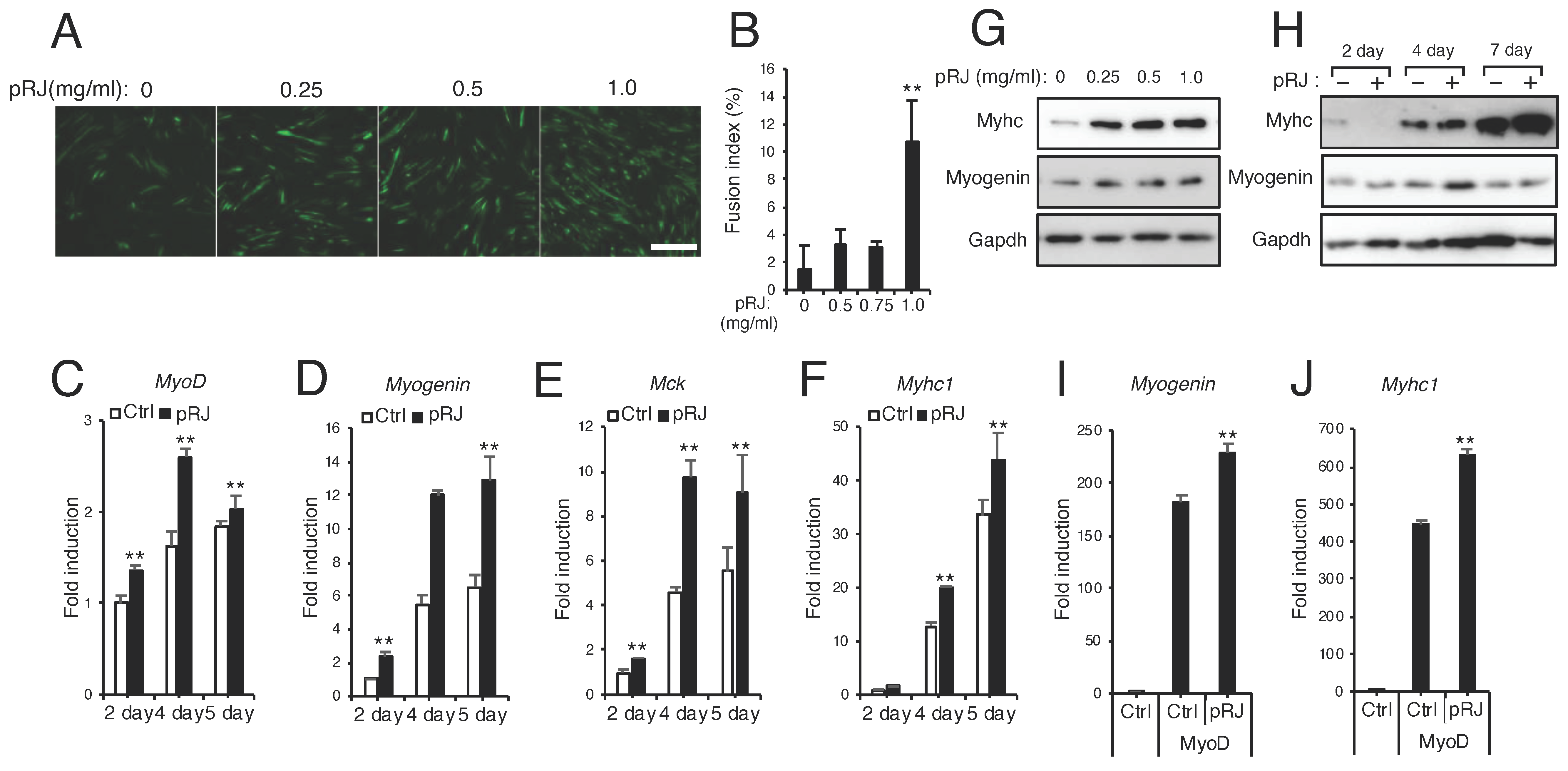
3.2. pRJ Stimulates Myoblast Proliferation
3.3. pRJ Stimulates Myoblast Differentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Royal jelly | RJ |
| Protease-treated RJ | pRJ |
| Trans-10-hydroxy-2-decenoic acid | 10H2DA |
| 10-hydroxydecanoic acid | 10HDAA |
| 2-decenedioic acid | 2DA |
| Sebacic acid | SA |
| Decanoic acid | DA |
| Docosahexaenoic acid | DHA |
| Muscle creatine kinase | Mck |
| Myosin heavy chain | Myhc |
| Muscle ring finger protein 1 | Murf1 |
| Forkhead box O1 | Foxo1 |
| Quantitative real time PCR | qPCR |
| messenger RNA | mRNA |
| Insulin-like growth factor-1 | IGF-1 |
| Insulin-like growth factor receptor | IGFR |
References
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; A Tylavsky, F.; Newman, A.B. Alternative Definitions of Sarcopenia, Lower Extremity Performance, and Functional Impairment with Aging in Older Men and Women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Woo, J. Sarcopenia. Clin. Geriatr. Med. 2017, 33, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Soendenbroe, C.; Heisterberg, M.F.; Schjerling, P.; Karlsen, A.; Kjaer, M.; Andersen, J.L.; Mackey, A.L. Molecular indicators of denervation in aging human skeletal muscle. Muscle Nerve 2019, 60, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Model. Mech. 2013, 6, 1339–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, D.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; García-Prat, L.; Serrano, A.L.; Perdiguero, E.; Muñoz-Cánoves, P. Muscle stem cell aging: Regulation and rejuvenation. Trends Endocrinol. Metab. 2015, 26, 287–296. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Neves, J.; Sousa-Victor, P. Understanding muscle regenerative decline with aging: New approaches to bring back youthfulness to aged stem cells. FEBS J. 2020, 287, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.M.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle. The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef]
- Montarras, D.; L’Honoré, A.; Buckingham, M. Lying low but ready for action: The quiescent muscle satellite cell. FEBS J. 2013, 280, 4036–4050. [Google Scholar] [CrossRef]
- Zammit, P.S.; Golding, J.P.; Nagata, Y.; Hudon, V.; Partridge, T.A.; Beauchamp, J.R. Muscle satellite cells adopt divergent fates. J. Cell Biol. 2004, 166, 347–357. [Google Scholar] [CrossRef]
- Kokabu, S.; Nakatomi, C.; Matsubara, T.; Ono, Y.; Addison, W.N.; Lowery, J.W.; Urata, M.; Hudnall, A.M.; Hitomi, S.; Nakatomi, M.; et al. The transcriptional co-repressor TLE3 regulates myogenic differentiation by repressing the activity of the MyoD transcription factor. J. Biol. Chem. 2017, 292, 12885–12894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Campos, M.D.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khazaei, M.; Ansarian, A.; Ghanbari, E. New Findings on Biological Actions and Clinical Applications of Royal Jelly: A Review. J. Diet. Suppl. 2017, 15, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.-I.; Koya-Miyata, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly prolongs the life span of C3H/HeJ mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969. [Google Scholar] [CrossRef]
- Honda, Y.; Fujita, Y.; Maruyama, H.; Araki, Y.; Ichihara, K.; Sato, A.; Kojima, T.; Tanaka, M.; Nozawa, Y.; Ito, M.; et al. Lifespan-Extending Effects of Royal Jelly and Its Related Substances on the Nematode Caenorhabditis elegans. PLoS ONE 2011, 6, e23527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamakura, M.; Mitani, N.; Fukuda, T.; Fukushima, M. Antifatigue Effect of Fresh Royal Jelly in Mice. J. Nutr. Sci. Vitaminol. 2001, 47, 394–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Liu, J.-R.; Yang, Y.-C.; Shi, L.-S.; Peng, C.-C. Antioxidant Properties of Royal Jelly Associated with Larval Age and Time of Harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly Inhibits the Production of Proinflammatory Cytokines by Activated Macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Vittek, J. Effect of Royal Jelly on serum lipids in experimental animals and humans with atherosclerosis. Cell. Mol. Life Sci. 1995, 51, 927–935. [Google Scholar] [CrossRef]
- Isidorov, V.; Bakier, S.; Grzech, I. Gas chromatographic–mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J. Chromatogr. B 2012, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Townsend, G.F.; Brown, W.H.; Felauer, E.E.; Hazlett, B. Studies on the In Vitro antitumor activity of fatty acids: IV. The esters of acids closely related to 10-Hydroxy- 2-decenoic acid from royal jelly against transplantable mouse leukemia. Can. J. Biochem. Physiol. 1961, 39, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Kuroda, H.; Motoyoshi, K. Effects of royal jelly and 10-hydroxy decenoic acid on the sebaceous glands of hamster ear. Nihon Hifuka Gakkai Zasshi. Jpn. J. Dermatol. 1988, 98, 469–475. [Google Scholar]
- Koya-Miyata, S.; Okamoto, I.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci. Biotechnol. Biochem. 2004, 68, 767–773. [Google Scholar] [CrossRef]
- Blum, M.S.; Novak, A.F.; Taber, S. 3rd, 10-Hydroxy-delta 2-decenoic acid, an antibiotic found in royal jelly. Science 1959, 130, 452–453. [Google Scholar] [CrossRef]
- Ito, S.; Nitta, Y.; Fukumitsu, H.; Soumiya, H.; Ikeno, K.; Nakamura, T.; Furukawa, S. Antidepressant-Like Activity of 10-Hydroxy-Trans-2-Decenoic Acid, a Unique Unsaturated Fatty Acid of Royal Jelly, in Stress-Inducible Depression-Like Mouse Model. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rosmilah, M.; Shahnaz, M.; Patel, G.; Lock, J.; Rahman, D.; Masita, A.; Noormalin, A. Characterization of major allergens of royal jelly Apis mellifera. Trop. Biomed. 2008, 25, 243–251. [Google Scholar]
- Mizutani, Y.; Shibuya, Y.; Takahashi, T.; Tsunoda, T.; Moriyama, T.; Seishima, M. Major royal jelly protein 3 as a possible allergen in royal jelly-induced anaphylaxis. J. Dermatol. 2011, 38, 1079–1081. [Google Scholar] [CrossRef]
- Blank, S.; Bantleon, F.I.; McIntyre, M.; Ollert, M.; Spillner, E. The major royal jelly proteins 8 and 9 (Api m 11) are glycosylated components ofApis melliferavenom with allergenic potential beyond carbohydrate-based reactivity. Clin. Exp. Allergy 2012, 42, 976–985. [Google Scholar] [CrossRef]
- Hayashi, T.; Takamatsu, N.; Nakashima, T.; Arita, T. Immunological Characterization of Honey Proteins and Identification of MRJP 1 as an IgE-Binding Protein. Biosci. Biotechnol. Biochem. 2011, 75, 556–560. [Google Scholar] [CrossRef]
- Moriyama, T.; Yanagihara, M.; Yano, E.; Kimura, G.; Seishima, M.; Tani, H.; Kanno, T.; Nakamura-Hirota, T.; Hashimoto, K.; Tatefuji, T.; et al. Hypoallergenicity and Immunological Characterization of Enzyme-Treated Royal Jelly fromApis mellifera. Biosci. Biotechnol. Biochem. 2013, 77, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Guo, H.; Guo, Y.; Ebihara, S.; Asada, M.; Ohrui, T.; Furukawa, K.; Ichinose, M.; Yanai, K.; Kudo, Y.; et al. Royal Jelly Prevents the Progression of Sarcopenia in Aged Mice In Vivo and In Vitro. Journals Gerontol. Ser. A: Boil. Sci. Med Sci. 2013, 68, 1482–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Hijikata, K.; Seike, K.; Nakano, S.; Banjo, M.; Sato, Y.; Takahashi, K.; Hatta, H. Effects of Royal Jelly Administration on Endurance Training-Induced Mitochondrial Adaptations in Skeletal Muscle. Nutrients 2018, 10, 1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, G.; Wang, H.; Pei, Y.; Li, Y.; Wu, H.; Song, K.; Guo, Q.; Guo, H.; Fukushima, S.; Tatefuji, T.; et al. Effects of protease-treated royal jelly on muscle strength in elderly nursing home residents: A randomized, double-blind, placebo-controlled, dose-response study. Sci. Rep. 2017, 7, 11416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Kohno, S.; Yama, T.; Ochi, A.; Suto, T.; Hirasaka, K.; Ohno, A.; Teshima-Kondo, S.; Okumura, Y.; Oarada, M.; et al. Soy Glycinin Contains a Functional Inhibitory Sequence against Muscle-Atrophy-Associated Ubiquitin Ligase Cbl-b. Int. J. Endocrinol. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usui, S.; Soda, M.; Iguchi, K.; Abe, N.; Oyama, M.; Nakayama, T.; Kitaichi, K. Down-regulation of aquaporin 9 gene transcription by 10-hydroxy-2-decenoic acid: A major fatty acid in royal jelly. Food Sci. Nutr. 2019, 7, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ogawa, M.; Yaginuma, T.; Nakatomi, C.; Nakajima, T.; Tada-Shigeyama, Y.; Addison, W.N.; Urata, M.; Matsubara, T.; Watanabe, K.; Matsuo, K.; et al. Transducin-like enhancer of split 3 regulates proliferation of melanoma cells via histone deacetylase activity. Oncotarget 2019, 10, 404–414. [Google Scholar] [CrossRef]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nat. Cell Biol. 1977, 270, 725–727. [Google Scholar] [CrossRef]
- Yamaga, M.; Tani, H.; Yamaki, A.; Tatefuji, T.; Hashimoto, K. Metabolism and pharmacokinetics of medium chain fatty acids after oral administration of royal jelly to healthy subjects. RSC Adv. 2019, 9, 15392–15401. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.L.; Weintraub, H.; Lassar, A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 1987, 51, 987–1000. [Google Scholar] [CrossRef]
- Sunadome, K.; Suzuki, T.; Usui, M.; Ashida, Y.; Nishida, E. Antagonism between the Master Regulators of Differentiation Ensures the Discreteness and Robustness of Cell Fates. Mol. Cell 2014, 54, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleszka, R. Epigenetic integration of environmental and genomic signals in honey bees: The critical interplay of nutritional, brain and reproductive networks. Epigenetics 2008, 3, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Zheng, L.; A Almeida, F. Epigenetic reprogramming in metabolic disorders: Nutritional factors and beyond. J. Nutr. Biochem. 2017, 54, 1–10. [Google Scholar] [CrossRef]
- Spannhoff, A.; Kim, Y.K.; Raynal, N.J.-M.; Gharibyan, V.; Su, M.-B.; Zhou, Y.-Y.; Li, J.; Castellano, S.; Sbardella, G.; Issa, J.-P.; et al. Histone deacetylase inhibitor activity in royal jelly might facilitate caste switching in bees. EMBO Rep. 2011, 12, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Hrebackova, J.; Hrabeta, J.; Eckschlager, T. Valproic Acid in the Complex Therapy of Malignant Tumors. Curr. Drug Targets 2010, 11, 361–379. [Google Scholar] [CrossRef]
- Beumer, J.H.; Tawbi, H. Role of histone deacetylases and their inhibitors in cancer biology and treatment. Curr. Clin. Pharmacol. 2010, 5, 196–208. [Google Scholar] [CrossRef]
- Montesano, A.; Luzi, L.; Senesi, P.; Terruzzi, I. Modulation of Cell Cycle Progression by 5-Azacytidine Is Associated with Early Myogenesis Induction in Murine Myoblasts. Int. J. Biol. Sci. 2013, 9, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Hupkes, M.; Jonsson, M.K.B.; Scheenen, W.J.; Van Rotterdam, W.; Sotoca, A.M.; Van Someren, E.P.; Van Der Heyden, M.A.G.; Van Veen, T.A.; Os, R.I.V.R.-V.; Bauerschmidt, S.; et al. Epigenetics: DNA demethylation promotes skeletal myotube maturation. FASEB J. 2011, 25, 3861–3872. [Google Scholar] [CrossRef]
- Murray, R.L.; Zhang, W.; Iwaniuk, M.; Grilli, E.; Stahl, C.H. Dietary tributyrin, an HDAC inhibitor, promotes muscle growth through enhanced terminal differentiation of satellite cells. Physiol. Rep. 2018, 6, e13706. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, R.; Tesfaye, D.; Tholen, E.; Looft, C.; Hoelker, M.; Schellander, K.; Cinar, U. Sulforaphane causes a major epigenetic repression of myostatin in porcine satellite cells. Epigenetics 2012, 7, 1379–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagiwara, H.; Saito, F.; Masaki, T.; Ikeda, M.; Nakamura-Ohkuma, A.; Shimizu, T.; Matsumura, K. Histone deacetylase inhibitor trichostatin A enhances myogenesis by coordinating muscle regulatory factors and myogenic repressors. Biochem. Biophys. Res. Commun. 2011, 414, 826–831. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirakawa, T.; Miyawaki, A.; Matsubara, T.; Okumura, N.; Okamoto, H.; Nakai, N.; Rojasawasthien, T.; Morikawa, K.; Inoue, A.; Goto, A.; et al. Daily Oral Administration of Protease-Treated Royal Jelly Protects Against Denervation-Induced Skeletal Muscle Atrophy. Nutrients 2020, 12, 3089. https://doi.org/10.3390/nu12103089
Shirakawa T, Miyawaki A, Matsubara T, Okumura N, Okamoto H, Nakai N, Rojasawasthien T, Morikawa K, Inoue A, Goto A, et al. Daily Oral Administration of Protease-Treated Royal Jelly Protects Against Denervation-Induced Skeletal Muscle Atrophy. Nutrients. 2020; 12(10):3089. https://doi.org/10.3390/nu12103089
Chicago/Turabian StyleShirakawa, Tomohiko, Aki Miyawaki, Takuma Matsubara, Nobuaki Okumura, Hideto Okamoto, Naoya Nakai, Thira Rojasawasthien, Kazumasa Morikawa, Asako Inoue, Akino Goto, and et al. 2020. "Daily Oral Administration of Protease-Treated Royal Jelly Protects Against Denervation-Induced Skeletal Muscle Atrophy" Nutrients 12, no. 10: 3089. https://doi.org/10.3390/nu12103089
APA StyleShirakawa, T., Miyawaki, A., Matsubara, T., Okumura, N., Okamoto, H., Nakai, N., Rojasawasthien, T., Morikawa, K., Inoue, A., Goto, A., Washio, A., Tsujisawa, T., Kawamoto, T., & Kokabu, S. (2020). Daily Oral Administration of Protease-Treated Royal Jelly Protects Against Denervation-Induced Skeletal Muscle Atrophy. Nutrients, 12(10), 3089. https://doi.org/10.3390/nu12103089




