Quercetin Intervention Alleviates Offspring’s Oxidative Stress, Inflammation, and Tight Junction Damage in the Colon Induced by Maternal Fine Particulate Matter (PM2.5) Exposure through the Reduction of Bacteroides
Abstract
1. Introduction
2. Materials and Methods
2.1. PM2.5 and Quercetin
2.2. Animals and Treatment
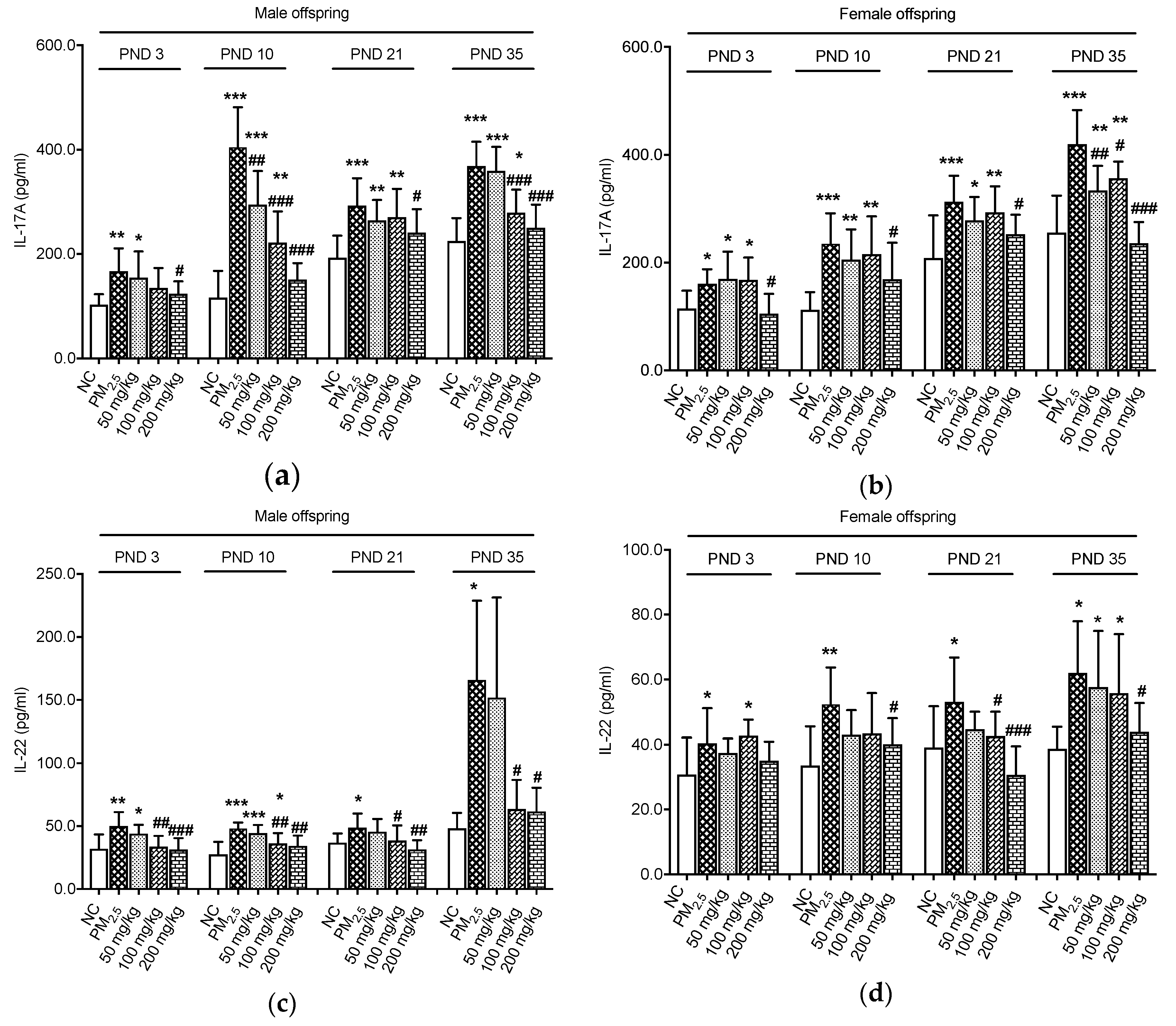
2.3. Enzyme-Linked Immunosorbent Assays (ELISA) for Interleukin-17A (IL-17A) and Interleukin-22 (IL-22) in the Colon
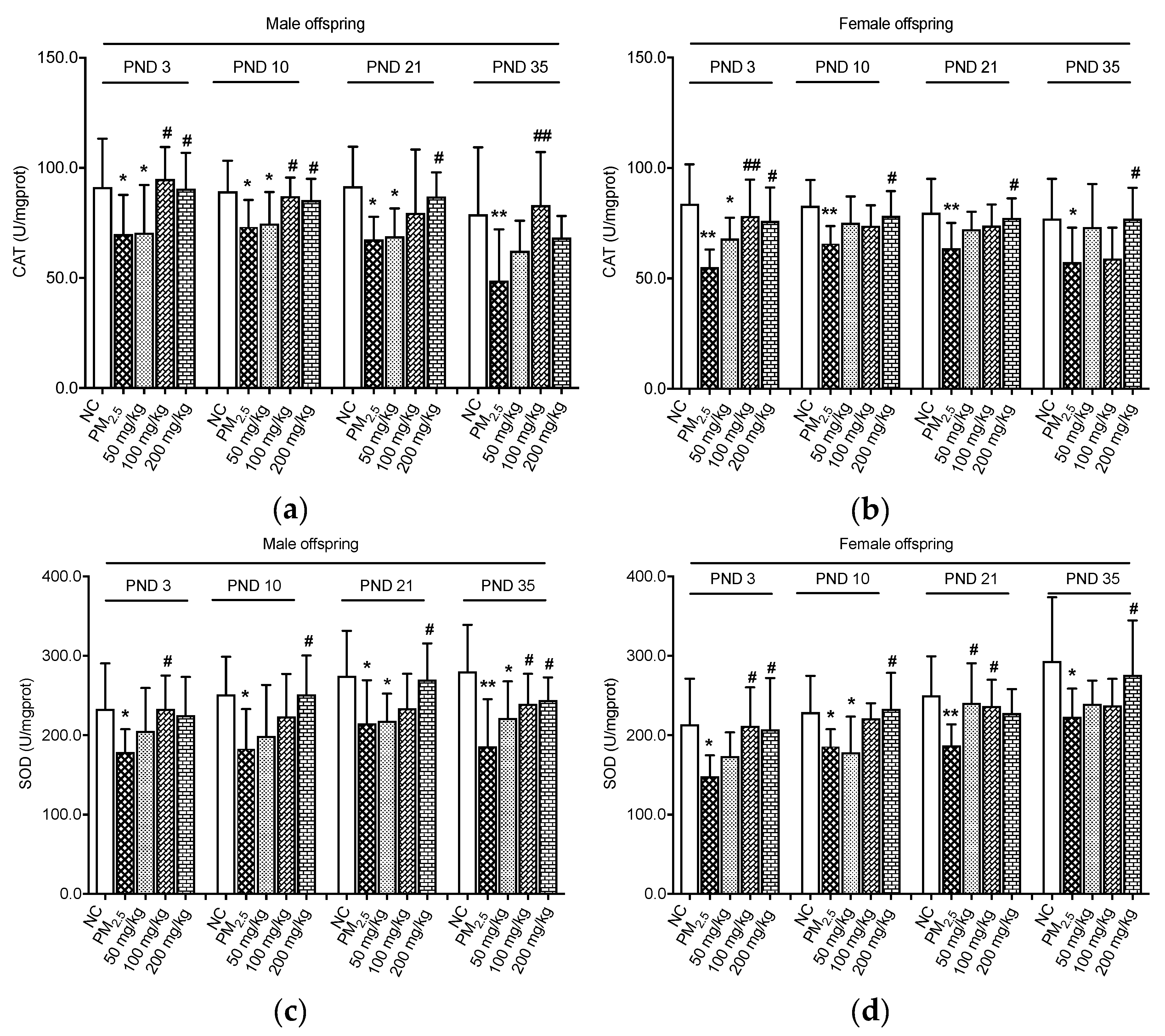
2.4. Biochemical Analysis for Catalase (CAT) and Superoxide Dismutase (SOD) in the Colon
2.5. ZO-1 and Occludin in the Colon
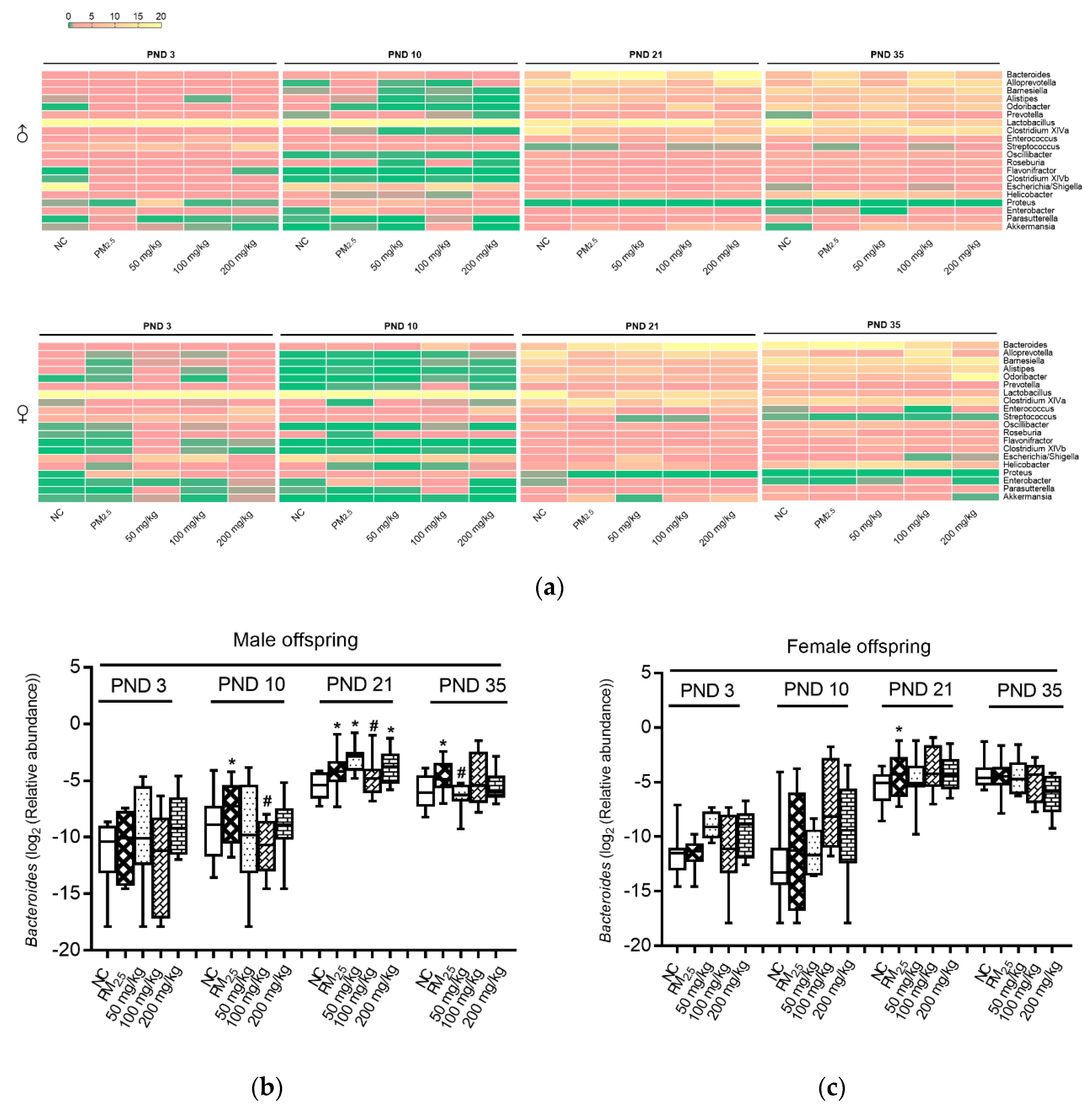
2.6. Colonic Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Effects on Antioxidant Enzymes
3.2. Effects on Inflammation Indicators
3.3. Effects on Colonic Proteins
3.4. Axonomic Composition of Gut Microbiota
3.5. Association between Bacteroides and Inflammation and Oxidative Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Opstelten, J.L.; Beelen, R.J.; Leenders, M.; Hoek, G.; Brunekreef, B.; Van Schaik, F.D.M.; Siersema, P.D.; Eriksen, K.T.; Raaschou-Nielsen, O.; Tjønneland, A.; et al. Exposure to ambient air pollution and the risk of inflammatory bowel disease: A European nested case—Control study. Dig. Dis. Sci. 2016, 61, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cavallero, S.; Hsiai, T.; Li, R. Impact of air pollution on intestinal redox lipidome and microbiome. Free Radic. Biol. Med. 2020, 151, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Şengul, N.; Işik, S.; Aslim, B.; Uçar, G.; Demirbağ, A.E. The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Dig. Dis. Sci. 2011, 56, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, H.; Meng, Z. Oxidative damage of PM2.5 on hearts, lungs and testicles of rats. Zhongguo Huanjing Kexue 2005, 25, 160–164. Available online: https://europepmc.org/article/cba/515190 (accessed on 10 October 2019).
- Al Hanai, A.H.; Antkiewicz, D.S.; Hemming, J.D.C.; Shafer, M.M.; Lai, A.M.; Arhami, M.; Hosseini, V.; Schauer, J.J. Seasonal variations in the oxidative stress and inflammatory potential of PM2.5 in Tehran using an alveolar macrophage model; The role of chemical composition and sources. Environ. Int. 2019, 123, 417–427. [Google Scholar] [CrossRef]
- Riva, D.R.; Magalhaes, C.B.; Lopes, A.A.; Lanças, T.; Mauad, T.; Malm, O.; Valenca, S.S.; Saldiva, P.; Faffe, D.; Zin, W.A. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal. Toxicol. 2011, 23, 257–267. [Google Scholar] [CrossRef]
- Aijaz, S.; Balda, M.S.; Matter, K. Tight junctions: Molecular architecture and function. Int. Rev. Cytol. 2006, 248, 261–298. [Google Scholar] [CrossRef]
- Quiros, M.; Nusrat, A. RhoGTPases, actomyosin signaling and regulation of the epithelial apical junctional complex. Semin. Cell Dev. Biol. 2014, 36, 194. [Google Scholar] [CrossRef]
- Itallie, C.M.V.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 2009, 20, 3930. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cao, X.N.; Tang, X.L.; Lian-Ju, S.; Lin, T.; He, D.-W.; Wu, S.; Wei, G. Urban fine particulate matter (PM2.5) exposure destroys blood-testis barrier (BTB) integrity through excessive ROS-mediated autophagy. Toxicol. Mech. Methods 2018, 28, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.A.; Engen, P.A.; Soberanes, S.; Urich, D.; Forsyth, C.B.; Nigdelioglu, R.; Chiarella, S.E.; Radigan, K.A.; Gonzalez, A.; Jakate, S.; et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part. Fibre Toxicol. 2011, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.J.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Salim, S.Y.; Kaplan, G.G.; Madsen, K.L. Air pollution effects on the gut microbiota: A link between exposure and inflammatory disease. Gut Microbes 2014, 5, 215–219. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.; Yazıcı, C.; Soberanes, S.; Hamanaka, R.B.; Nigdelioglu, R.; Meliton, A.Y.; Ghio, A.J.; et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018, 240, 817–830. [Google Scholar] [CrossRef]
- Santangelo, R.; Silvestrini, A.; Mancuso, C. Ginsenosides, catechins, quercetin and gut microbiota: Current evidence of challenging interactions. Food Chem. Toxicol. 2019, 123, 42–49. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, X.; Li, S.; Zhang, N.; Wang, Y.; Wei, H. Isolation and identification of quercetin degrading bacteria from human fecal microbes. PLoS ONE 2014, 9, e90531. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Gotteland, M.; Castillo, R.L.; Chen, C. 3,4-dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, protects against pancreatic β-cells dysfunction induced by high cholesterol. Exp. Cell Res. 2015, 334, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, Y.; Li, Y.; Qin, Y.; Yu, L.; Li, R.; Chen, Y.; Xu, Y. Effects of PM2.5 exposure during gestation on maternal gut microbiota and pregnancy outcomes. Chemosphere 2020, 247, 125879. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, M.; Feng, J.; Fan, A.; Zhou, Y.; Xu, Y. The influence of quercetin on maternal immunity, oxidative stress, and inflammation in mice with exposure of fine particulate matter during gestation. Int. J. Environ. Res. Public Health 2017, 14, 592. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Y.; Qin, Y.; Li, Y.; Yu, L.; Li, R.; Chen, Y.; Xu, Y. Sex-dependent effects of PM2.5 maternal exposure and quercetin intervention on offspring’s short chain fatty acids. Int. J. Environ. Res. Public Health 2019, 16, 4371. [Google Scholar] [CrossRef]
- Tom, V.D.W.; Vanhaecke, L.; Boeckaert, C.; Peru, K.; Headley, J.; Verstraete, W.; Siciliano, S. Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environ. Health. Perspect. 2004, 113, 6–10. [Google Scholar] [CrossRef]
- Breton, J.; Massart, S.; Vandamme, P.; De Brandt, E.; Pot, B.; Foligné, B. Ecotoxicology inside the gut: Impact of heavy metals on the mouse microbiome. BMC Pharmacol. Toxicol. 2013, 14. [Google Scholar] [CrossRef]
- Zhang, L.; Nichols, R.G.; Correll, J.; Murray, I.A.; Tanaka, N.; Smith, P.B.; Hubbard, T.D.; Sebastian, A.; Albert, I.; Hatzakis, E.; et al. Persistent organic pollutants modify gut microbiota-host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environ. Health. Perspect. 2015, 123, 679–688. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from different geographic origins decreases intestinal inflammation and Bacteroides spp. populations in a model of DSS-induced colitis. Mol. Nutr. Food Res. 2018, 62, e1800080. [Google Scholar] [CrossRef]
- Taira, T.; Yamaguchi, S.; Takahashi, A.; Okazaki, Y.; Yamaguchi, A.; Sakaguchi, H.; Chiji, H. Dietary polyphenols increase fecal mucin and immunoglobulin A and ameliorate the disturbance in gut microbiota caused by a high fat diet. J. Clin. Biochem. Nutr. 2015, 57, 212–216. [Google Scholar] [CrossRef]
- Xue, B.; Xie, J.; Huang, J.; Chen, L.; Gao, L.; Ou, S.; Wang, Y.; Peng, X. Plant polyphenols altering a pathway of energy metabolism by inhibiting fecal Bacteriodetes and Firmicutes in vitro. Food Funct. 2016, 7, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.; Portillo, M.P.; Martinez, J.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]



| Group | N | PM2.5 (mg/kg) | Quercetin (mg/kg) |
|---|---|---|---|
| NC group | 8 | - | - |
| PM2.5 group | 8 | 15 | - |
| 50 mg/kg quercetin group | 8 | 15 | 50 |
| 100 mg/kg quercetin group | 8 | 15 | 100 |
| 200 mg/kg quercetin group | 8 | 15 | 200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhou, Y.; Qin, Y.; Yu, L.; Li, R.; Chen, Y.; Xu, Y. Quercetin Intervention Alleviates Offspring’s Oxidative Stress, Inflammation, and Tight Junction Damage in the Colon Induced by Maternal Fine Particulate Matter (PM2.5) Exposure through the Reduction of Bacteroides. Nutrients 2020, 12, 3095. https://doi.org/10.3390/nu12103095
Liu W, Zhou Y, Qin Y, Yu L, Li R, Chen Y, Xu Y. Quercetin Intervention Alleviates Offspring’s Oxidative Stress, Inflammation, and Tight Junction Damage in the Colon Induced by Maternal Fine Particulate Matter (PM2.5) Exposure through the Reduction of Bacteroides. Nutrients. 2020; 12(10):3095. https://doi.org/10.3390/nu12103095
Chicago/Turabian StyleLiu, Wei, Yalin Zhou, Yong Qin, Lanlan Yu, Ruijun Li, Yuhan Chen, and Yajun Xu. 2020. "Quercetin Intervention Alleviates Offspring’s Oxidative Stress, Inflammation, and Tight Junction Damage in the Colon Induced by Maternal Fine Particulate Matter (PM2.5) Exposure through the Reduction of Bacteroides" Nutrients 12, no. 10: 3095. https://doi.org/10.3390/nu12103095
APA StyleLiu, W., Zhou, Y., Qin, Y., Yu, L., Li, R., Chen, Y., & Xu, Y. (2020). Quercetin Intervention Alleviates Offspring’s Oxidative Stress, Inflammation, and Tight Junction Damage in the Colon Induced by Maternal Fine Particulate Matter (PM2.5) Exposure through the Reduction of Bacteroides. Nutrients, 12(10), 3095. https://doi.org/10.3390/nu12103095






