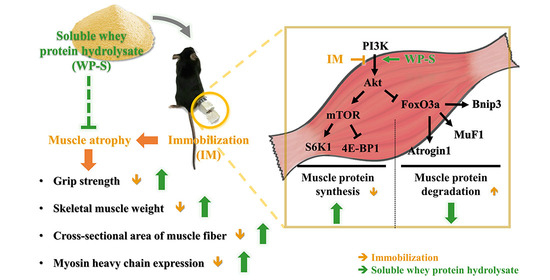
Soluble Whey Protein Hydrolysate Ameliorates Muscle Atrophy Induced by Immobilization via Regulating the PI3K/Akt Pathway in C57BL/6 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Whey Protein Hydrolysates
2.2. Amino Acid Composition Analysis
2.3. Animals and Experimental Design
2.4. Dosage Information
2.5. Measurement of Grip Strength
2.6. The Toxicity Test of WP-S
2.7. Measurement of Muscle Weight and Protein Content
2.8. Histological Analysis of Muscle Cross-Sectional Area (CSA)
2.9. Quantitative Real Time-PCR (qRT-PCR) Assay
2.10. Western Blot Assay
2.11. Statistical Analysis
3. Results
3.1. Comparison of Four Different Whey Protein Hydrolysates (AW-H, AW-S, WP-H, and WP-S) in Immobilization-Induced Muscle Atrophy Model
3.2. Comparison of the Efficacy of Four Different Whey Protein Hydrolysates on Muscle Protein Synthesis and Degradation
3.3. WP-S Increased Grip Strength, Muscle Mass, and Cross-Sectional Area of Muscle Fiber
3.4. WP-S Recovered the Imbalance between Muscle Protein Synthesis and Degradation
3.5. WP-S Changed Total Myosin Heavy Chain and Its Isoform Expressions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lopes, J.; Russell, D.; Whitwell, J.; Jeejeebhoy, K.N. Skeletal muscle function in malnutrition. Am. J. Clin. Nutr. 1982, 36, 602–610. [Google Scholar] [CrossRef] [PubMed]
- English, C.; McLennan, H.; Thoirs, K.; Coates, A.; Bernhardt, J. Loss of skeletal muscle mass after stroke: A systematic review. Int. J. Stroke 2010, 5, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. CachexiaSarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Baehr, L.M.; West, D.W.; Marshall, A.G.; Marcotte, G.R.; Baar, K.; Bodine, S.C. Muscle-specific and age-related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J. Appl. Physiol. 2017, 122, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80, A8–A15. [Google Scholar] [CrossRef]
- Keri Marshall, N. Therapeutic applications of whey protein. Altern. Med. Rev. 2004, 9, 136–156. [Google Scholar]
- Manninen, A.H. Protein hydrolysates in sports nutrition. Nutr. Metab. 2009, 6, 38. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Somoto, Y.; Mitsuyama, E.; Tanaka, A.; Yuda, N.; Nakada, H.; Yamada, A.; Yamauchi, K.; Abe, F.; Nagasawa, T. Supplementation of protein-free diet with whey protein hydrolysates prevents skeletal muscle mass loss in rats. J. Nutr. Intermed. Metab. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Morifuji, M.; Ishizaka, M.; Baba, S.; Fukuda, K.; Matsumoto, H.; Koga, J.; Kanegae, M.; Higuchi, M. Comparison of different sources and degrees of hydrolysis of dietary protein: Effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J. Agric. Food Chem. 2010, 58, 8788–8797. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kato, H.; Hirabayashi, Y.; Murakami, H.; Suzuki, H. Modulations of muscle protein metabolism by branched-chain amino acids in normal and muscle-atrophying rats. J. Nutr. 2006, 136, 234S–236S. [Google Scholar] [CrossRef]
- Yamamoto, D.; Maki, T.; Herningtyas, E.H.; Ikeshita, N.; Shibahara, H.; Sugiyama, Y.; Nakanishi, S.; Iida, K.; Iguchi, G.; Takahashi, Y. Branched-chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2010, 41, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.T.; Herda, T.J.; Fry, A.C.; Cooper, M.A.; Andre, M.J.; Gallagher, P.M. Endocrine responses and acute mTOR pathway phosphorylation to resistance exercise with leucine and whey. Biol. Sport 2017, 34, 197. [Google Scholar] [CrossRef]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. In Phosphoinositide 3-Kinase in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2010; pp. 267–278. [Google Scholar]
- Yoon, M.-S. mTOR as a key regulator in maintaining skeletal muscle mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Zhang, Y.; Aguilar, O.A.; Storey, K.B. Transcriptional activation of muscle atrophy promotes cardiac muscle remodeling during mammalian hibernation. PeerJ 2016, 4, e2317. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Autophagy in skeletal muscle. FEBS Lett. 2010, 584, 1411–1416. [Google Scholar] [CrossRef]
- Pérez-Baos, S.; Prieto-Potin, I.; Román-Blas, J.A.; Sánchez-Pernaute, O.; Largo, R.; Herrero-Beaumont, G. Mediators and patterns of muscle loss in chronic systemic inflammation. Front. Physiol. 2018, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Foletta, V.C.; White, L.J.; Larsen, A.E.; Léger, B.; Russell, A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflügers Arch.-Eur. J. Physiol. 2011, 461, 325–335. [Google Scholar]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Tagawa, R.; Saito, Y.; Sanbongi, C. Effects of whey protein hydrolysate ingestion on post-exercise muscle protein synthesis compared with intact whey protein in rats. Nutr. Metab. 2019, 16, 1–7. [Google Scholar] [CrossRef]
- Kakigi, R.; Yoshihara, T.; Ozaki, H.; Ogura, Y.; Ichinoseki-Sekine, N.; Kobayashi, H.; Naito, H. Whey protein intake after resistance exercise activates mTOR signaling in a dose-dependent manner in human skeletal muscle. Eur. J. Appl. Physiol. 2014, 114, 735–742. [Google Scholar] [CrossRef]
- Moro, T.; Brightwell, C.R.; Velarde, B.; Fry, C.S.; Nakayama, K.; Sanbongi, C.; Volpi, E.; Rasmussen, B.B. Whey protein Hydrolysate increases amino acid uptake, mTORC1 signaling, and protein synthesis in skeletal muscle of healthy young men in a randomized crossover trial. J. Nutr. 2019, 149, 1149–1158. [Google Scholar] [CrossRef]
- You, J.-S.; Anderson, G.B.; Dooley, M.S.; Hornberger, T.A. The role of mTOR signaling in the regulation of protein synthesis and muscle mass during immobilization in mice. Dis. Models Mech. 2015, 8, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.D.; Thomson, R.L.; Coates, A.M.; Howe, P.R.; DeNichilo, M.O.; Rowney, M.K. Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. J. Sci. Med. Sport 2010, 13, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Whey protein hydrolysate supplementation accelerates recovery from exercise-induced muscle damage in females. Appl. Physiol. Nutr. Metab. 2018, 43, 324–330. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Chacon-Cabrera, A.; Lund-Palau, H.; Gea, J.; Barreiro, E. Time-course of muscle mass loss, damage, and proteolysis in gastrocnemius following unloading and reloading: Implications in chronic diseases. PLoS ONE 2016, 11, e0164951. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, E.; Hoogland, I.C.; Wieske, L.; Weber, N.C.; Verhamme, C.; Schultz, M.J.; Van Schaik, I.N.; Horn, J. Assessment of intensive care unit-acquired weakness in young and old mice: An E. coli septic peritonitis model. Muscle Nerve 2016, 53, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Landolt, L.; Marti, H.-P.; Beisland, C.; Flatberg, A.; Eikrem, O.S. RNA extraction for RNA sequencing of archival renal tissues. Scand. J. Clin. Lab. Investig. 2016, 76, 426–434. [Google Scholar] [CrossRef]
- Augusto, V.; Padovani, C.R.; Campos, G.E.R. Skeletal muscle fiber types in C57BL6J mice. J. Morphol. Sci. 2017, 21. [Google Scholar]
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Steffl, M.; Bohannon, R.W.; Sontakova, L.; Tufano, J.J.; Shiells, K.; Holmerova, I. Relationship between sarcopenia and physical activity in older people: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 835. [Google Scholar] [CrossRef]
- Palus, S.; Springer, J.; Doehner, W.; von Haehling, S.; Anker, M.; Anker, S.; Springer, J. Models of sarcopenia: Short review. Int. J. Cardiol. 2017, 238, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.; Artioli, G.G.; dos Santos Costa, A.; Solis, M.Y.; Da Luz, C.R.; Blachier, F.; Lancha, A.H. An overview of the therapeutic effects of leucine supplementation on skeletal muscle under atrophic conditions. Amino Acids 2011, 40, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Columbus, D.A.; Fiorotto, M.L.; Davis, T.A. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 2015, 47, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yoshikawa, Y.; Tsujimoto, H.; Kitaura, T.; Muraoka, I. Clenbuterol accelerates recovery after immobilization-induced atrophy of rat hindlimb muscle. Acta Histochem. 2020, 122, 151453. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Arfat, Y.; Wang, H.; Goswami, N. Muscle atrophy induced by mechanical unloading: Mechanisms and potential countermeasures. Front. Physiol. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; McGlory, C. CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J. Physiol. 2014, 592, 5341. [Google Scholar] [CrossRef]
- Kim, C.; Kim, M.-B.; Hwang, J.-K. Red Bean Extract Inhibits Immobilization-Induced Muscle Atrophy in C57BL/6N Mice. J. Med. Food 2019, 23, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Horikawa, H.; Lin, P.-Y.; Tsukumo, N.; Nikawa, T.; Kawamura, T.; Nemoto, H.; Terao, J. 8-Prenylnaringenin promotes recovery from immobilization-induced disuse muscle atrophy through activation of the Akt phosphorylation pathway in mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 311, R1022–R1031. [Google Scholar] [CrossRef] [PubMed]
- Canepari, M.; Pellegrino, M.; D’antona, G.; Bottinelli, R. Skeletal muscle fibre diversity and the underlying mechanisms. Acta Physiol. 2010, 199, 465–476. [Google Scholar] [CrossRef]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef]
- Pierno, S.; Camerino, G.M.; Cannone, M.; Liantonio, A.; De Bellis, M.; Digennaro, C.; Gramegna, G.; De Luca, A.; Germinario, E.; Danieli-Betto, D. Paracrine effects of IGF-1 overexpression on the functional decline due to skeletal muscle disuse: Molecular and functional evaluation in hindlimb unloaded MLC/mIgf-1 transgenic mice. PLoS ONE 2013, 8, e65167. [Google Scholar] [CrossRef]





| Amino Acids | Contents (mg/g) | |||
|---|---|---|---|---|
| AW-H | AW-S | WP-H | WP-S | |
| Threonine | 35.6 | 36.5 | 54.0 | 54.8 |
| Tyrosine | 24.9 | 25.4 | 22.3 | 23.0 |
| Arginine | 18.4 | 18.7 | 18.9 | 19.4 |
| Alanine | 39.1 | 38.6 | 39.3 | 39.6 |
| Proline | 36.8 | 35.3 | 39.2 | 41.7 |
| Lysine | 73.2 | 74.4 | 69.9 | 70.9 |
| Histidine | 16.5 | 17.3 | 16.2 | 16.1 |
| Isoleucine | 36.4 | 36.5 | 44.2 | 45.6 |
| leucine | 88.9 | 88.8 | 81.3 | 81.9 |
| Methionine | 17.5 | 17.7 | 17.4 | 16.8 |
| Phenylalanine | 27.3 | 28.4 | 25.8 | 27.4 |
| Valine | 31.1 | 31.3 | 40.2 | 41.9 |
| Glutamine | 107.5 | 111.9 | 132.8 | 135.0 |
| Aspartic acid | 79.4 | 81.9 | 58.9 | 86.4 |
| Serine | 23.5 | 25.7 | 42.2 | 40.9 |
| Glycine | 13.4 | 13.5 | 12.5 | 15.8 |
| BCAAs | 156.4 | 156.5 | 165.7 | 169.4 |
| Total AA | 669.4 | 681.8 | 715.0 | 757.1 |
| Component | ||
| Energy | kcal/100 g | 375.44 |
| Carbohydrate | g/100 g | 14.64 |
| Crude Fat | g/100 g | 3.72 |
| Crude Protein | g/100 g | 70.85 |
| Moisture | % | 4.02 |
| Ash | % | 6.77 |
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| atrogin-1 | AGA AAG AAA GAC ATT CAG AAC A | GCT CCT TCG TAC TTC CTT |
| murf1 | AAG ACT GAG CTG AGT AAC TG | TAG AGG GTG TCA AAC TTC TG |
| bnip3 | TTC CAC TAG CAC CTT CTG ATG A | GAA CAC CGC ATT TAC AGA ACA A |
| myhc I | CTC AAG CTG CTC AGC AAT CTA TTT | GGA GCG CAA GTT TGT CAT AAG T |
| myhc IIa | AGG CGG CTG AGG AGC ACG TA | GCG GCA CAA GCA GCG TTG G |
| myhc IIx | GAG GGA CAG TTC ATC GAT AGC AA | GGG CCA ACT TGT CAT CTC TCA T |
| myhc IIb | CAA TCA GGA ACC TTC GGA ACA C | GTC CTG GCC TCT GAG AGC AT |
| gadph | TCG GTG TGA ACG GAT TTG | GGT CTC GCT CCT GGA AGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.E.; Park, S.J.; Ahn, S.I.; Choung, S.-Y. Soluble Whey Protein Hydrolysate Ameliorates Muscle Atrophy Induced by Immobilization via Regulating the PI3K/Akt Pathway in C57BL/6 Mice. Nutrients 2020, 12, 3362. https://doi.org/10.3390/nu12113362
Shin JE, Park SJ, Ahn SI, Choung S-Y. Soluble Whey Protein Hydrolysate Ameliorates Muscle Atrophy Induced by Immobilization via Regulating the PI3K/Akt Pathway in C57BL/6 Mice. Nutrients. 2020; 12(11):3362. https://doi.org/10.3390/nu12113362
Chicago/Turabian StyleShin, Ji Eun, Seok Jun Park, Seung Il Ahn, and Se-Young Choung. 2020. "Soluble Whey Protein Hydrolysate Ameliorates Muscle Atrophy Induced by Immobilization via Regulating the PI3K/Akt Pathway in C57BL/6 Mice" Nutrients 12, no. 11: 3362. https://doi.org/10.3390/nu12113362
APA StyleShin, J. E., Park, S. J., Ahn, S. I., & Choung, S.-Y. (2020). Soluble Whey Protein Hydrolysate Ameliorates Muscle Atrophy Induced by Immobilization via Regulating the PI3K/Akt Pathway in C57BL/6 Mice. Nutrients, 12(11), 3362. https://doi.org/10.3390/nu12113362