l-Cysteine and Vitamin D Co-Supplementation Alleviates Markers of Musculoskeletal Disorders in Vitamin D-Deficient High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experimental Design and Treatment
2.2. Cell Culture, Treatments, and RNA Interference of GCLC and CSE
2.3. Relative Gene Expression
2.4. Statistical Analyses
3. Results
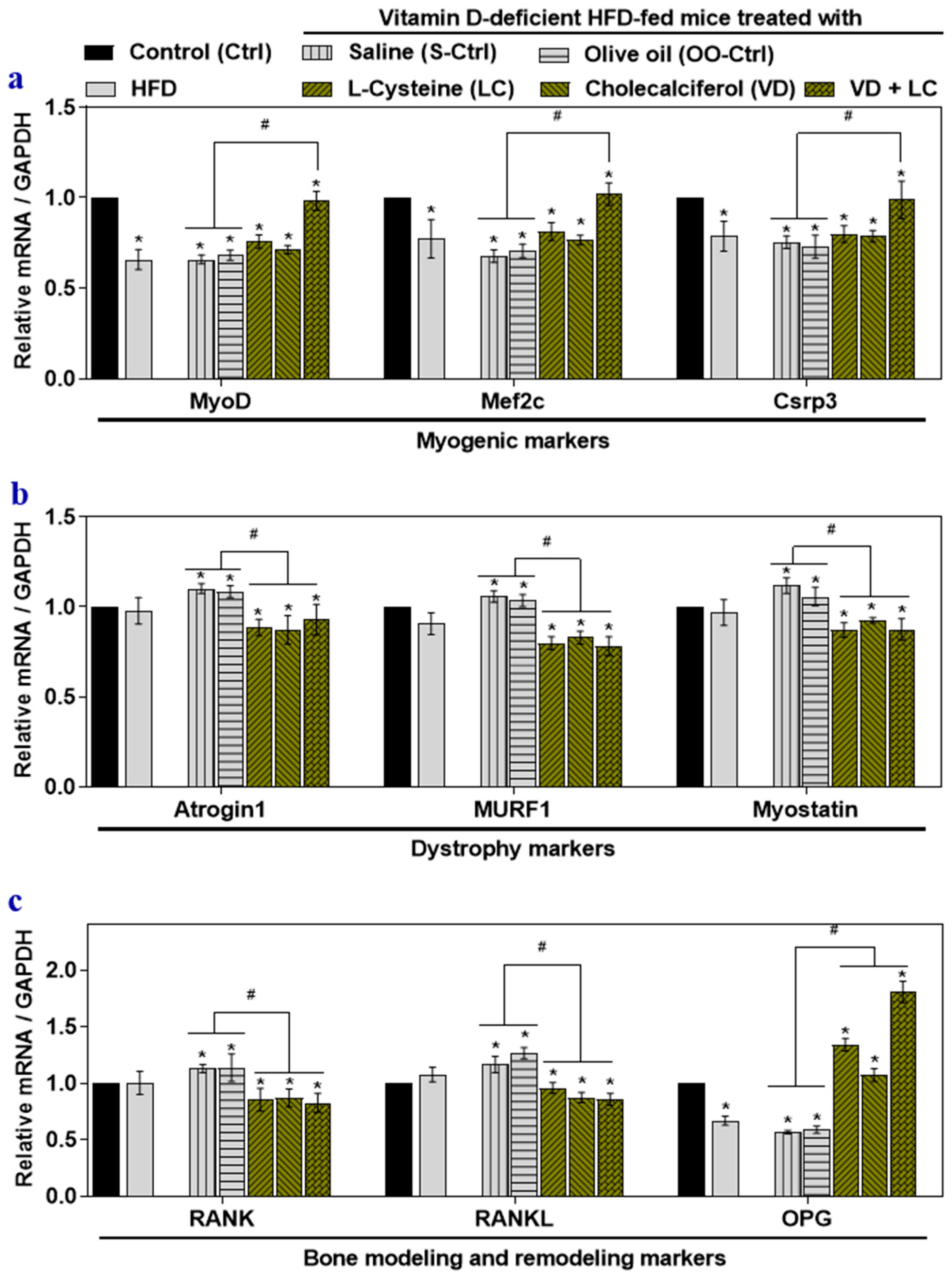
3.1. Effect of HFD, VD-Deficient HFD, and l-Cysteine and Vitamin D Co-Supplementation on Gene Expression of Musculoskeletal Markers in Mouse Skeletal Muscle
3.2. Impact of High Glucose, Palmitate, and Inflammatory Cytokines on Musculoskeletal Markers
3.3. The Deficiency of Transsulfuration Pathway Key Genes GCLC and CSE (Knockdown) in Myotubes Affects Musculoskeletal Markers
3.4. GSH and H2S Inhibit Muscle Dystrophy Markers and Positively Induce Myogenic Markers Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Atrogin1/Fbxo32 | skeletal muscle-specific F-box protein |
| CSE/CTH | Cystathionine Gamma-Lyase |
| GCLC/ γ-GCSc | Glutamate-Cysteine Ligase Catalytic Subunit |
| GSH | Glutathione |
| H2S | Hydrogen sulfide |
| LC | l-cysteine |
| HFD-VD- | VD-deficient high-fat diet-fed |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| TNF | Tumor Necrosis Factor |
| MuRF1/Trim63 | Muscle RING-finger protein-1 |
| Mstn | Myostatin |
| MyoD | Myoblast determination protein 1 |
| Mef2c | Myocyte enhancer factor 2C |
| Csrp3 | Cysteine and glycine-rich protein 3 |
| Rank/Tnfrsf11a | Receptor activator of nuclear factor-kB |
| RANKL | Receptor activator of nuclear factor-kB ligand |
| OPG | Osteoprotegerin |
| VD | Vitamin D |
References
- Holick, M.F. Sunlight "D"ilemma: Risk of skin cancer or bone disease and muscle weakness. Lancet 2001, 357, 4–6. [Google Scholar] [CrossRef]
- Holick, M.F.; Grant, W.B. Vitamin D status and ill health. Lancet Diabetes Endocrinol. 2014, 2, 273–274. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.K.; Parsanathan, R.; Achari, A.E.; Kanikarla-Marie, P.; Bocchini, J.A., Jr. Glutathione stimulates vitamin D regulatory and glucose-metabolism genes, lowers oxidative stress and inflammation, and increases 25-hydroxy-vitamin D levels in blood: A novel approach to treat 25-hydroxyvitamin D deficiency. Antioxid. Redox Signal. 2018, 29, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Glutathione deficiency induces epigenetic alterations of vitamin D metabolism genes in the livers of high-fat diet-fed obese mice. Sci. Rep. 2019, 9, 14784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsanathan, R.; Jain, S.K. Glutathione deficiency alters the vitamin D-metabolizing enzymes CYP27B1 and CYP24A1 in human renal proximal tubule epithelial cells and kidney of HFD-fed mice. Free Radic. Biol. Med. 2019, 131, 376–381. [Google Scholar] [CrossRef]
- Jain, S.K.; Parsanathan, R. Can vitamin D and l-Cysteine co-supplementation reduce 25(OH)-vitamin D deficiency and the mortality associated with COVID-19 in African Americans? J. Am. Coll. Nutr. 2020. [Google Scholar] [CrossRef]
- Jain, S.K.; Kanikarla-Marie, P.; Warden, C.; Micinski, D. l-Cysteine supplementation upregulates glutathione (GSH) and vitamin D binding protein (VDBP) in hepatocytes cultured in high glucose and in vivo in liver, and increases blood levels of GSH, VDBP, and 25-hydroxy-vitamin D in Zucker diabetic fatty rats. Mol. Nutr. Food Res. 2016, 60, 1090–1098. [Google Scholar] [CrossRef]
- Dunn, A.; Talovic, M.; Patel, K.; Patel, A.; Marcinczyk, M.; Garg, K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J. Orthop. Res. 2019, 37, 1246–1262. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Kong, Y.; Flick, M.J.; Kudla, A.J.; Konieczny, S.F. Muscle LIM protein promotes myogenesis by enhancing the activity of MyoD. Mol. Cell Biol. 1997, 17, 4750–4760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [Green Version]
- Lokireddy, S.; McFarlane, C.; Ge, X.; Zhang, H.; Sze, S.K.; Sharma, M.; Kambadur, R. Myostatin induces degradation of sarcomeric proteins through a Smad3 signaling mechanism during skeletal muscle wasting. Mol. Endocrinol. 2011, 25, 1936–1949. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Lynch, G.S.; Murphy, K.T.; Reid, M.B.; Zijdewind, I. Disease-induced skeletal muscle atrophy and fatigue. Med. Sci. Sports Exerc. 2016, 48, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, S.S.; Boulanger-Piette, A.; Bosse, S.; Frenette, J. Physiological role of receptor activator nuclear factor-kB (RANK) in denervation-induced muscle atrophy and dysfunction. Receptors Clin. Investig. 2016, 3, e13231–e13236. [Google Scholar] [CrossRef]
- Grimaud, E.; Soubigou, L.; Couillaud, S.; Coipeau, P.; Moreau, A.; Passuti, N.; Gouin, F.; Redini, F.; Heymann, D. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am. J. Pathol. 2003, 163, 2021–2031. [Google Scholar] [CrossRef]
- Collins, K.H.; Paul, H.A.; Hart, D.A.; Reimer, R.A.; Smith, I.C.; Rios, J.L.; Seerattan, R.A.; Herzog, W. A high-fat high-sucrose diet rapidly alters muscle integrity, inflammation and gut microbiota in male rats. Sci. Rep. 2016, 6, 37278. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, Z.; Torigoe, D.; Zhao, J.; Xie, P.; Sugizaki, T.; Sato, M.; Horiguchi, H.; Terada, K.; Kadomatsu, T.; et al. Aging- and obesity-related peri-muscular adipose tissue accelerates muscle atrophy. PLoS ONE 2019, 14, e0221366. [Google Scholar] [CrossRef] [Green Version]
- Peris-Moreno, D.; Taillandier, D.; Polge, C. MuRF1/TRIM63, master regulator of muscle mass. Int. J. Mol. Sci. 2020, 21, 6663. [Google Scholar] [CrossRef]
- McLeay, Y.; Stannard, S.; Houltham, S.; Starck, C. Dietary thiols in exercise: Oxidative stress defence, exercise performance, and adaptation. J. Int. Soc. Sports Nutr. 2017, 14, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michailidis, Y.; Karagounis, L.G.; Terzis, G.; Jamurtas, A.Z.; Spengos, K.; Tsoukas, D.; Chatzinikolaou, A.; Mandalidis, D.; Stefanetti, R.J.; Papassotiriou, I.; et al. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am. J. Clin. Nutr. 2013, 98, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Lands, L.C.; Grey, V.L.; Smountas, A.A. Effect of supplementation with a cysteine donor on muscular performance. J. Appl. Physiol. (1985) 1999, 87, 1381–1385. [Google Scholar] [CrossRef]
- Cao, J.J.; Picklo, M.J. N-acetylcysteine supplementation decreases osteoclast differentiation and increases bone mass in mice fed a high-fat diet. J. Nutr. 2014, 144, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Udagawa, N.; Suda, T. Vitamin D endocrine system and osteoclasts. Bonekey Rep. 2014, 3, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunton, J.E.; Girgis, C.M. Vitamin D and muscle. Bone Rep. 2018, 8, 163–167. [Google Scholar] [CrossRef]
- Jain, S.K.; Parsanathan, R.; Levine, S.N.; Bocchini, J.A.; Holick, M.F.; Vanchiere, J.A. The potential link between inherited G6PD deficiency, oxidative stress, and vitamin D deficiency and the racial inequities in mortality associated with COVID-19. Free Radical Biol. Med. 2020, 161, 84–91. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Speck, A.E.; Amaral, I.M.; Canas, P.M.; Cunha, R.A. The exercise sex gap and the impact of the estrous cycle on exercise performance in mice. Sci. Rep. 2018, 8, 10742. [Google Scholar] [CrossRef]
- Carson, J.A.; Manolagas, S.C. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Vanderschueren, D.; Laurent, M.R.; Claessens, F.; Gielen, E.; Lagerquist, M.K.; Vandenput, L.; Borjesson, A.E.; Ohlsson, C. Sex steroid actions in male bone. Endocr. Rev. 2014, 35, 906–960. [Google Scholar] [CrossRef]
- Galhardo, A.P.M.; Mukai, M.K.; Mori, M.; Carvalho, K.C.; Baracat, M.C.P.; Simoes, M.J.; Soares, J.M., Jr.; Baracat, E.C. Influence of age and gender on sex steroid receptors in rat masticatory muscles. Sci. Rep. 2019, 9, 18403. [Google Scholar] [CrossRef]
- Brown, M. Skeletal muscle and bone: Effect of sex steroids and aging. Adv. Physiol. Educ. 2008, 32, 120–126. [Google Scholar] [CrossRef]
- Keijer, J.; Li, M.; Speakman, J.R. What is the best housing temperature to translate mouse experiments to humans? Mol. Metab. 2019, 25, 168–176. [Google Scholar] [CrossRef]
- Fischer, A.W.; Cannon, B.; Nedergaard, J. The answer to the question "What is the best housing temperature to translate mouse experiments to humans?" is: Thermoneutrality. Mol. Metab. 2019, 26, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Della Vedova, M.C.; Munoz, M.D.; Santillan, L.D.; Plateo-Pignatari, M.G.; Germano, M.J.; Rinaldi Tosi, M.E.; Garcia, S.; Gomez, N.N.; Fornes, M.W.; Gomez Mejiba, S.E.; et al. A mouse model of diet-induced obesity resembling most features of human metabolic syndrome. Nutr. Metab. Insights 2016, 9, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Parsanathan, R.; Jain, S.K. Hydrogen sulfide increases glutathione biosynthesis, and glucose uptake and utilisation in C2C12 mouse myotubes. Free Radic. Res. 2018, 52, 288–303. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Hydrogen sulfide regulates circadian-clock genes in C2C12 myotubes and the muscle of high-fat-diet-fed mice. Arch. Biochem. Biophys. 2019, 672, 108054. [Google Scholar] [CrossRef]
- Grober, U.; Spitz, J.; Reichrath, J.; Kisters, K.; Holick, M.F. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Dermatoendocrinol. 2013, 5, 331–347. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic and consequences for nonskeletal health: Mechanisms of action. Mol. Aspects Med. 2008, 29, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [Green Version]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [Green Version]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stockle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial role of vitamin D in the musculoskeletal system. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef]
- National Institutes of Health, O.o.D.S. Vitamin D: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 30 October 2020).
- National Institutes of Health, O.o.D.S. Vitamin D: Fact Sheet for Consumers. Available online: https://ods.od.nih.gov/factsheets/VitaminD-Consumer/ (accessed on 30 October 2020).
- National Institutes of Health, O.o.D.S. Multivitamin/mineral Supplements: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/MVMS-HealthProfessional/ (accessed on 30 October 2020).
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Ryan, K.J.; Daniel, Z.C.; Craggs, L.J.; Parr, T.; Brameld, J.M. Dose-dependent effects of vitamin D on transdifferentiation of skeletal muscle cells to adipose cells. J. Endocrinol. 2013, 217, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Hosoyama, T.; Iida, H.; Kawai-Takaishi, M.; Watanabe, K. Vitamin D inhibits myogenic cell fusion and expression of fusogenic genes. Nutrients 2020, 12, 2192. [Google Scholar] [CrossRef]
- Chang, E.; Kim, Y. Vitamin D ameliorates fat accumulation with AMPK/SIRT1 activity in C2C12 skeletal muscle cells. Nutrients 2019, 11, 2806. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.; Achari, A.E.; Jain, S.K. 1,25(OH)2-vitamin D3 upregulates glucose uptake mediated by SIRT1/IRS1/GLUT4 signaling cascade in C2C12 myotubes. Mol. Cell Biochem. 2018, 444, 103–108. [Google Scholar] [CrossRef]
- Ardite, E.; Barbera, J.A.; Roca, J.; Fernandez-Checa, J.C. Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-kappaB activation. Am. J. Pathol. 2004, 165, 719–728. [Google Scholar] [CrossRef]
- Dzik, K.P.; Skrobot, W.; Kaczor, K.B.; Flis, D.J.; Karnia, M.J.; Libionka, W.; Antosiewicz, J.; Kloc, W.; Kaczor, J.J. Vitamin D deficiency is associated with muscle atrophy and reduced mitochondrial function in patients with chronic low back pain. Oxid. Med. Cell Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Pinniger, G.J.; Terrill, J.R.; Assan, E.B.; Grounds, M.D.; Arthur, P.G. Preclinical evaluation of N-acetylcysteine reveals side effects in the mdx mouse model of Duchenne muscular dystrophy. J. Physiol. 2017, 595, 7093–7107. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Guo, Y.; Guo, J.; Wang, Q.; Wang, C.; Wang, X. N-Acetylcysteine attenuates lipopolysaccharide-induced osteolysis by restoring bone remodeling balance via reduction of reactive oxygen species formation during osteoclastogenesis. Inflammation 2020. [Google Scholar] [CrossRef]
- Shymanskyi, I.; Lisakovska, O.; Mazanova, A.; Labudzynskyi, D.; Veliky, M. Vitamin D3 modulates impaired crosstalk between RANK and glucocorticoid receptor signaling in bone marrow cells after chronic prednisolone administration. Front. Endocrinol. (Lausanne) 2018, 9, 303. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parsanathan, R.; Achari, A.E.; Manna, P.; Jain, S.K. l-Cysteine and Vitamin D Co-Supplementation Alleviates Markers of Musculoskeletal Disorders in Vitamin D-Deficient High-Fat Diet-Fed Mice. Nutrients 2020, 12, 3406. https://doi.org/10.3390/nu12113406
Parsanathan R, Achari AE, Manna P, Jain SK. l-Cysteine and Vitamin D Co-Supplementation Alleviates Markers of Musculoskeletal Disorders in Vitamin D-Deficient High-Fat Diet-Fed Mice. Nutrients. 2020; 12(11):3406. https://doi.org/10.3390/nu12113406
Chicago/Turabian StyleParsanathan, Rajesh, Arunkumar E. Achari, Prasenjit Manna, and Sushil K. Jain. 2020. "l-Cysteine and Vitamin D Co-Supplementation Alleviates Markers of Musculoskeletal Disorders in Vitamin D-Deficient High-Fat Diet-Fed Mice" Nutrients 12, no. 11: 3406. https://doi.org/10.3390/nu12113406




