Therapeutic Effects of Morinda citrifolia Linn. (Noni) Aqueous Fruit Extract on the Glucose and Lipid Metabolism in High-Fat/High-Fructose-Fed Swiss Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of M. citrifolia Fruit Aqueous Extract (AE)
2.3. Ultra-Fast Liquid Chromatography–Diode Array Detector–Tandem Mass Spectrometry (UFLC–DAD–MS) Analyses
2.4. Ethical Statement
2.5. Acute Oral Toxicity
2.6. Animals and Experiment Design
2.7. Assessment of Body Fat and Liver Weight
2.8. Body Weight and Diet Intake
2.9. Biochemical Analysis
2.10. Histopathological Analysis
2.11. Oral Glucose Tolerance Test (OGTT)
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Quantitative Real-Time PCR (qRT-PCR)
2.14. Statistical Analysis
3. Results
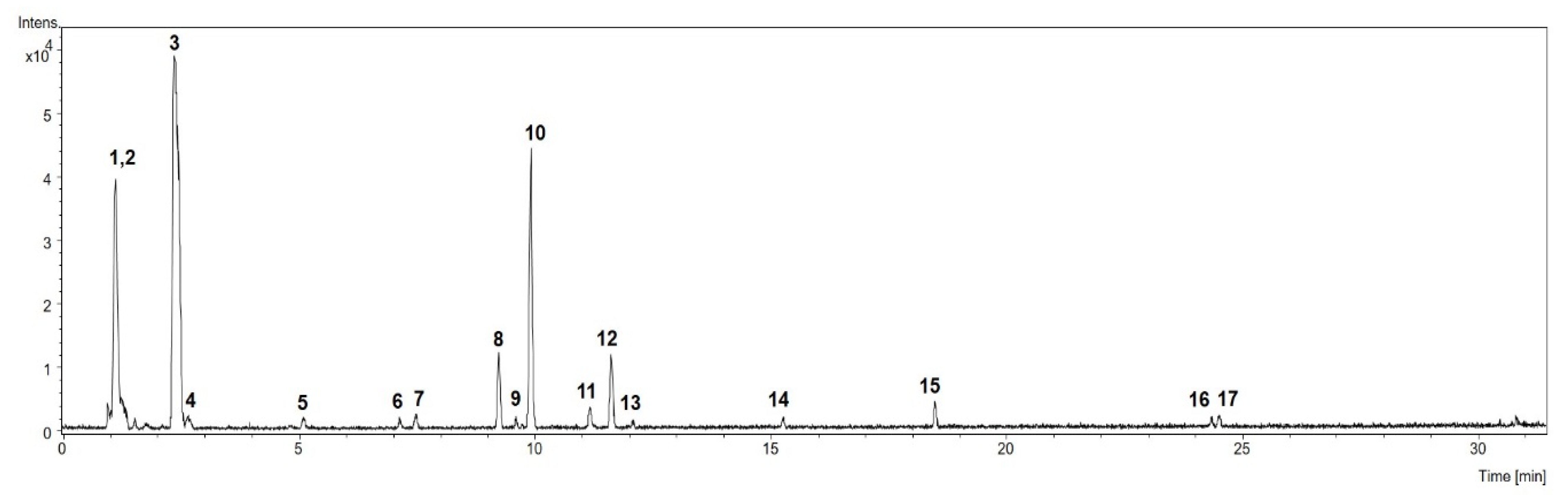
3.1. Chemical Profile of M. citrifolia Linn. (noni) Fruit Aqueous Extract (AE)
3.2. Acute Oral Toxicity of M. citrifolia (noni) Fruit Aqueous Extract (AE)
3.3. Effects of M. citrifolia (noni) Fruit Aqueous Extract (AE) on Body Weight and Food Intake
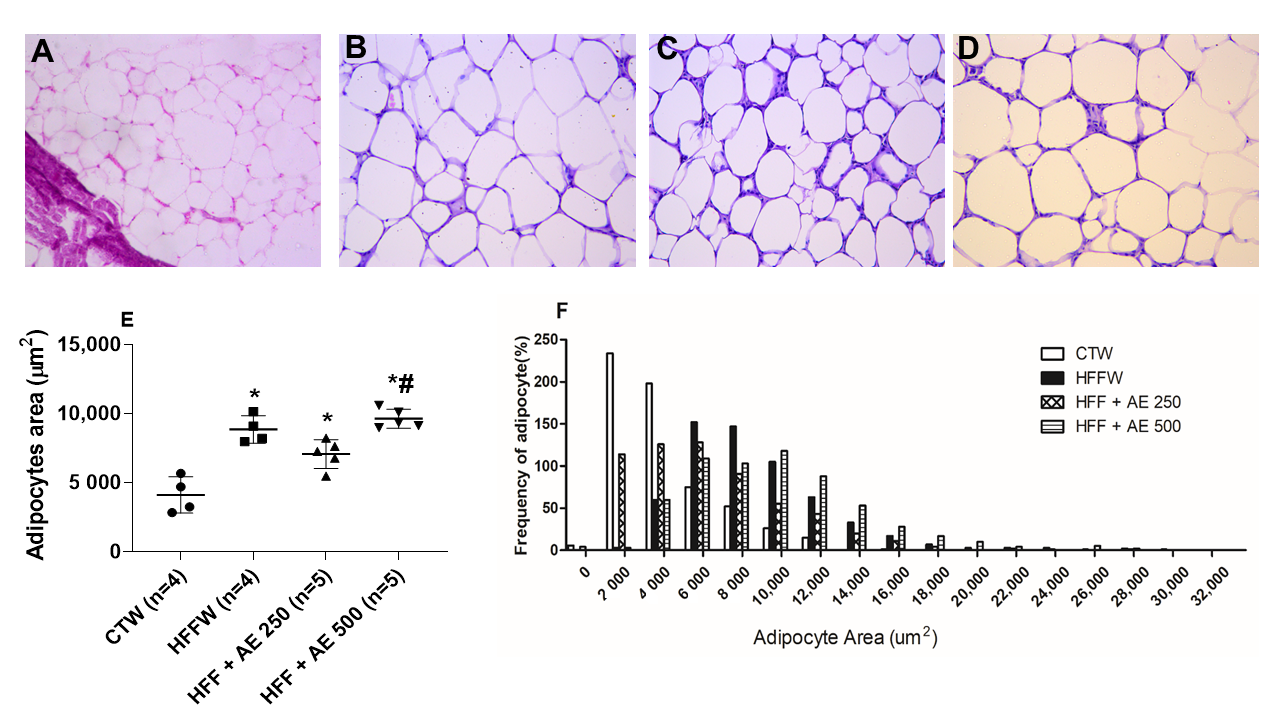
3.4. Effects of M. citrifolia (noni) Aqueous Extract (AE) on the Visceral Adiposity
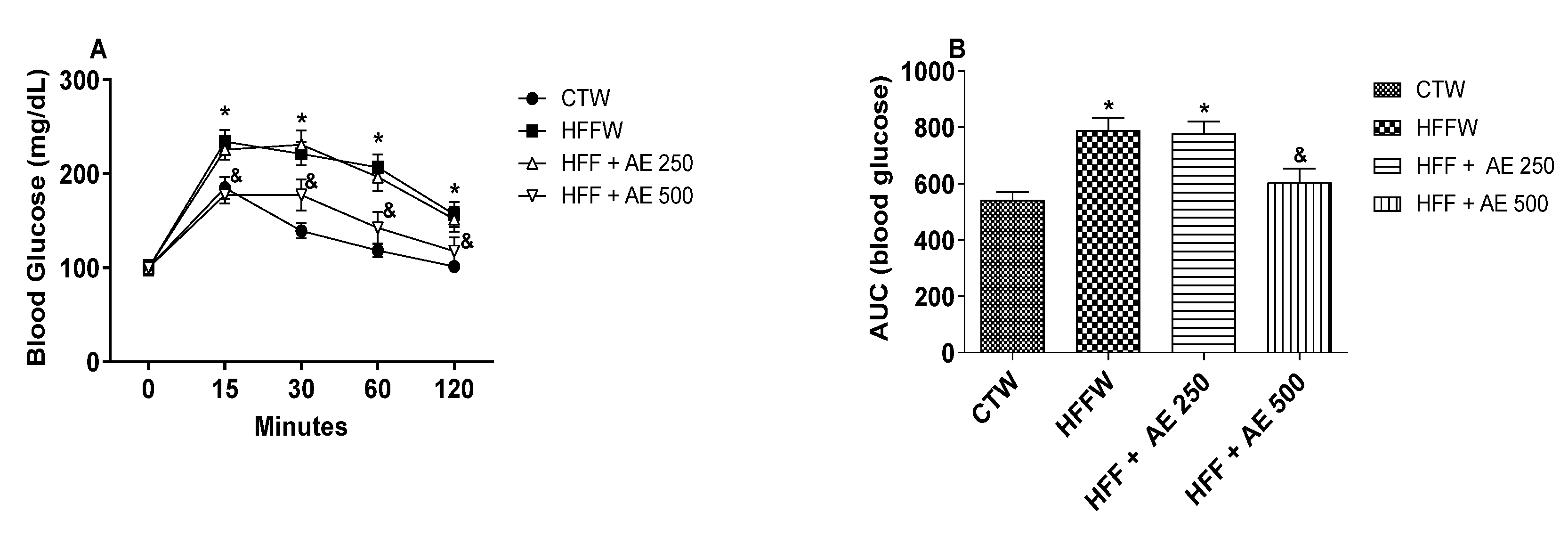
3.5. Effects of M. citrifolia (noni) Aqueous Extract (AE) on Glucose Tolerance and Systemic Insulin Sensitivity
3.6. Effects of M. citrifolia (noni) Aqueous Extract on Serum Biochemical Parameters
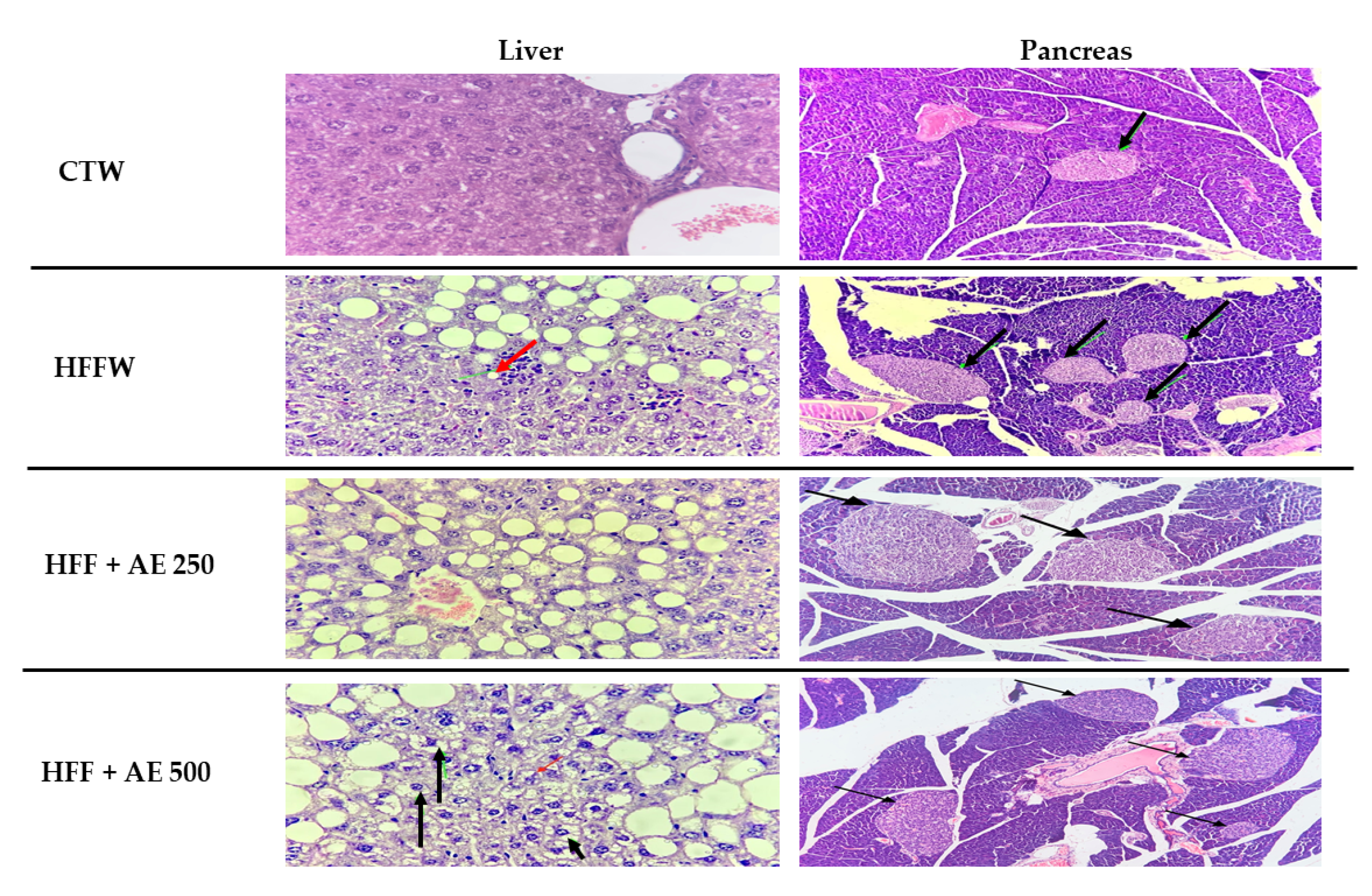
3.7. Histopathological Analysis of the Epididymal Adipose Tissue, Pancreas, and Liver
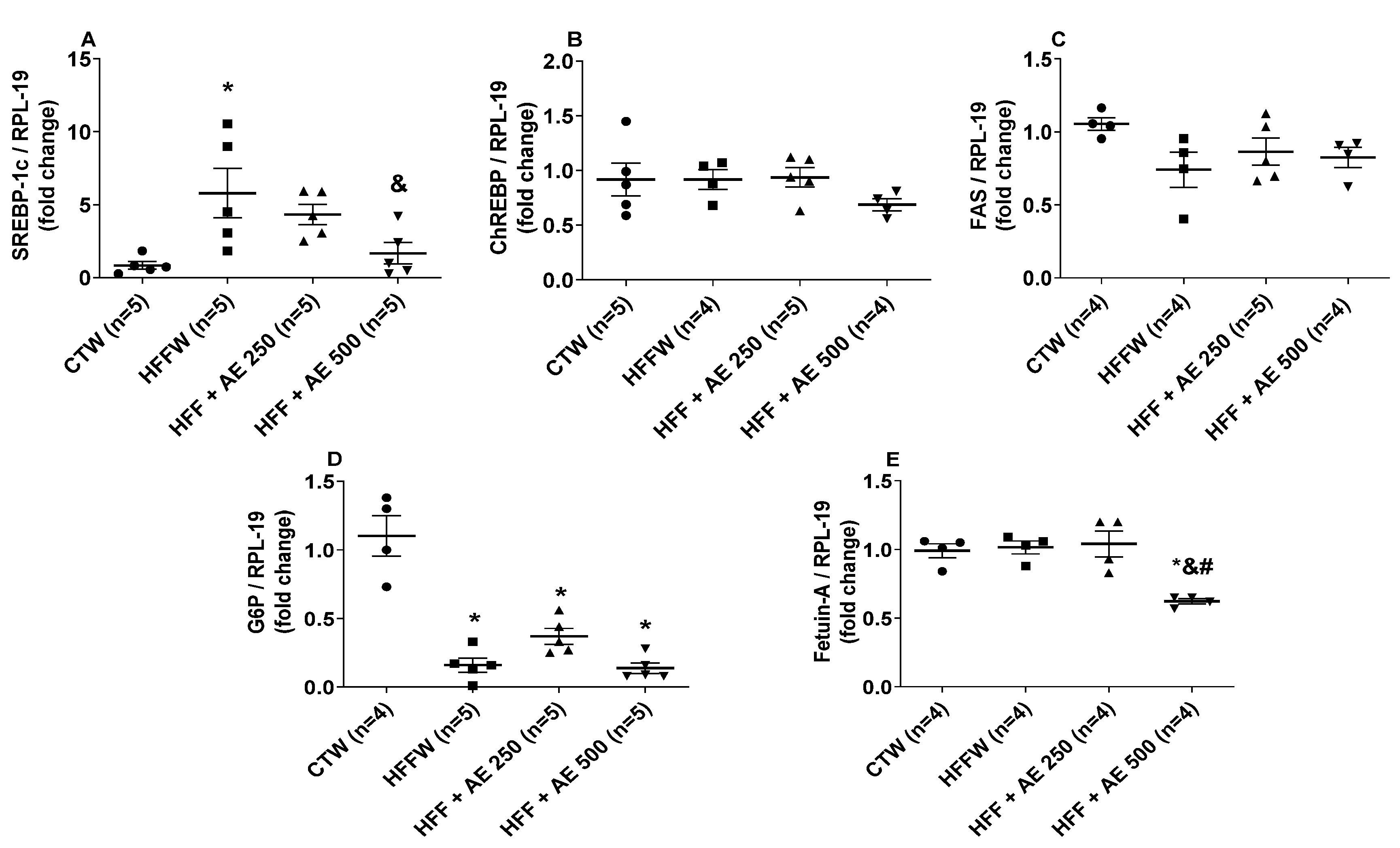
3.8. Effects of M. citrifolia Fruit Aqueous Extract (AE) in the Expression of Genes Involved in Adipocyte Differentiation in White Adipose Tissue and the Lipid and Glycemic Metabolism in the Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, O.; Kim, H.-S. Dietary chokeberry and dried jujube fruit attenuates high-fat and high-fructose dietinduced dyslipidemia and insulin resistance via activation of the IRS-1PI3KAkt pathway in C57BL6 J mice. Nutr. Metab. 2019, 16, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, A.J.; Thomas, E.L.; Lessan, N.; Trovato, F.M.; Trovato, G.M.; Taylor-Robinson, S.D. Non-alcoholic fatty liver disease: Relationship with cardiovascular risk markers and clinical endpoints. Diabetes Res. Clin. Pract. 2018, 144, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Lê, K.A.; Tran, C.; Paquot, N. Fructose and metabolic diseases: New findings, new questions. Nutrition 2010, 26, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Packard, C.J.; Borén, J. Dietary fructose and the metabolic syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usharani, P.; Merugu, P.L.; Nutalapati, C. Evaluation of the effects of a standardized aqueous extract of Phyllanthus emblica fruits on endothelial dysfunction, oxidative stress, systemic inflammation and lipid profile in subjects with metabolic syndrome: A randomised, double blind, placebo controlled clinical study. BMC Complem. Altern. Med. 2019, 19, 97. [Google Scholar] [CrossRef]
- Simpson, E.J.; Mendis, B.; Macdonald, I.A. Orange juice consumption and its effect on blood lipid profile and indices of the metabolic syndrome; a randomised, controlled trial in an at-risk population. Food Funct. 2016, 7, 1884–1891. [Google Scholar] [CrossRef] [Green Version]
- Moazzen, H.; Alizadeh, M. Effects of pomegranate juice on cardiovascular risk factors in patients with metabolic syndrome: A double-blinded, randomized crossover controlled trial. Plant Foods Hum. Nutr. 2017, 72, 126–133. [Google Scholar] [CrossRef]
- Chan-Blanco, Y.; Vaillant, F.; Mercedes Perez, A.; Reynes, M.; Brillouet, J.-M.; Brat, P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J. Food Compos. Anal. 2006, 19, 645–654. [Google Scholar] [CrossRef]
- Dixon, A.R.; Mcmillen, H.; Etkin, N.L. Ferment this: The transformation of noni, a traditional Polynesian medicine (Morinda citrifolia, Rubiaceae). Econ. Bot. 1999, 53, 51–68. [Google Scholar] [CrossRef]
- Singh, R. Morinda citrifolia L. (Noni): A review of the scientific validation for its nutritional and therapeutic properties. J. Diabetes Endocrinol. 2012, 3, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Motshakeri, M.; Ghazali, H.M. Nutritional, phytochemical and commercial quality of Noni fruit: A multi-beneficial gift from nature. Trends Food Sci. Technol. 2015, 45, 118–129. [Google Scholar] [CrossRef]
- ANVISA—Agência Nacional de Vigilância Sanitária. Informe Técnico: Esclarecimentos Sobre as Avaliações de Segurança Realizadas de Produtos Contendo Morinda citrifolia, Também Conhecida Como noni. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-vegetal/legislacao-1/biblioteca-de-normas-vinhos-e-bebidas/informe-tecnico-no-25-de-maio-de-2007_anvisa.pdf/view (accessed on 13 March 2020).
- Stadlbauer, V. Hepatotoxicity of NONI juice: Report of two cases. World J. Gastroenterol. 2005, 11, 4758. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Shalan, N.A.A.; Mustapha, N.M.; Mohamed, S. Chronic toxicity evaluation of Morinda citrifolia fruit and leaf in mice. Regul. Toxicol. Pharmacol. 2017, 83, 46–53. [Google Scholar] [CrossRef] [PubMed]
- West, B.J. Noni juice is not hepatotoxic. World J. Gastroenterol. 2006, 12, 3616. [Google Scholar] [CrossRef]
- Westendorf, J.; Effenberger, K.; Iznaguen, H.; Basar, S. Toxicological and analytical investigations of Noni (Morinda citrifolia) fruit juice. J. Agric. Food Chem. 2007, 55, 529–537. [Google Scholar] [CrossRef]
- Bramorski, A.; Cherem, A.D.R.; Marmentini, C.P.; Torresani, J.; Mezadri, T.; Costa, A.D.A.S. Total polyphenol content and antioxidant activity of commercial Noni (Morinda citrifolia L.) juice and its components. Braz. J. Pharm. Sci. 2010, 46, 651–656. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Shimoda, M.; Suzuki, J.; Kawaraya, A.; Igura, N.; Hayakawa, I. Changes in the odors of squeezed apple juice during thermal processing. Food Res. Int. 2003, 36, 777–785. [Google Scholar] [CrossRef]
- Joshi, A.A.; Chikawar, P.M.; Jadhav, B.A. Studies on physico-chemical properties of noni fruit (Morinda citrifolia) and preparation of noni beverages. Int. J. Food Sci. Nutr. Diet. 2012, 1, 3–8. [Google Scholar] [CrossRef]
- Daud, M.; Jalil, J.A.; Azmi, I.M.A.G.; Ismail, S.F.; Safuan, S. ‘Unsafe’ nutraceuticals products on the Internet: The need for stricter regulation in Malaysia. In Proceedings of the 2017 5th International Conference on Cyber and IT Service Management (CITSM), IEEE, Denpasar, Bali, Indonesia, 8–10 August 2017; pp. 1–5. [Google Scholar]
- Mohd Zin, Z.; Abdul Hamid, A.; Osman, A.; Saari, N.; Misran, A. Isolation and identification of antioxidative compound from fruit of mengkudu (Morinda citrifolia L.). Int. J. Food Prop. 2007, 10, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Mahattanadul, S.; Ridtitid, W.; Nima, S.; Phdoongsombut, N.; Ratanasuwon, P.; Kasiwong, S. Effects of Morinda citrifolia aqueous fruit extract and its biomarker scopoletin on reflux esophagitis and gastric ulcer in rats. J. Ethnopharmacol. 2011, 134, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Seino, K.; Kaneko, E.; Watanabe, K.; Tochizawa, S.; Fukatsu, M.; Banno, N.; Metori, K.; Kimura, Y. Melanogenesis Inhibitory Activities of iridoid-, hemiterpene-, and fatty acid-glycosides from the fruits of Morinda citrifolia (Noni). J. Oleo Sci. 2010, 59, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.C. Anticancer activity of Morinda citrifolia (Noni) Fruit: A review. Phytother. Res. 2012, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Mandukhail, S.; Aziz, N.; Gilani, A.-H. Studies on antidyslipidemic effects of Morinda citrifolia (Noni) fruit, leaves and root extracts. Lipids Health Dis. 2010, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoeb, A. Effect of Morinda citrifolia (Noni) fruit juice on high fat diet induced dyslipidemia in rats. J. Clin. Diagn. Res. 2016, 10, FF06–FF10. [Google Scholar] [CrossRef]
- Nerurkar, P.V.; Nishioka, A.; Eck, P.O.; Johns, L.M.; Volper, E.; Nerurkar, V.R. Regulation of glucose metabolism via hepatic forkhead transcription factor 1 (FoxO1) by Morinda citrifolia (Noni) in high-fat diet-induced obese mice. Br. J. Nutr. 2012, 108, 218–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-L.; Chang, Y.-Y.; Yang, D.-J.; Tzang, B.-S.; Chen, Y.-C. Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters. Food Chem. 2013, 140, 31–38. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-L.; Hwang, J.-T.; Yi, S.-H.; Nam, Y.-D.; Lim, S.-I. Antidiabetic effect of Morinda citrifolia (Noni) fermented by Cheonggukjang in KK-Ay diabetic mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Wigati, D.; Anwar, K.; Sudarsono; Nugroho, A.E. Hypotensive activity of ethanolic extracts of Morinda citrifolia L. Leaves and fruit in dexamethasone-induced hypertensive rat. J. Evid. Based Complem. Altern. Med. 2017, 22, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Inada, A.C.; Figueiredo, P.S.; Dos Santos-Eichler, R.A.; Freitas, K.C.; Hiane, P.A.; De Castro, A.P.; Guimarães, R.C.A. Morinda citrifolia Lin (Noni) and its potential in obesity-related metabolic dysfunction. Nutrients 2017, 9, 540. [Google Scholar] [CrossRef]
- Algenstaedt, P.; Stumpenhagen, A.; Westendorf, J. The effect of Morinda citrifolia L. fruit juice on the blood sugar level and other serum parameters in patients with diabetes type 2. Evid. Based Complement. Alternat. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, W.C.S.; Bett, S.C.; Santos, C.G.B.; Brasil, A.S.; Gauto, R.F.; Beserra, A.M.S.S.; Oliveira, A.P. Caracterização físico-química e análise fitoquímica preliminar do fruto Noni (Morinda citrifolia L.) produzido na cidade de cuiabá-MT. Rev. Bras. Tecnol. Agroind. 2014, 8, 1208–1215. [Google Scholar] [CrossRef] [Green Version]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure; OECD Publishing: Paris, France, 2008. [Google Scholar] [CrossRef]
- Malone, M.H.; Robichaud, R.C.A. Hippocratic screen for pure or crude drug materials. Lloydia 1962, 25, 320–322. [Google Scholar]
- Da Silva, É.R.S.; Salmazzo, G.R.; Da Silva Arrigo, J.; Oliveira, R.J.; Kassuya, C.A.L.; Cardoso, C.A.L. Anti-inflammatory evaluation and toxicological analysis of Campomanesia xanthocarpa Berg. Inflammation 2016, 39, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.G.; Carvalho, M.H.C.D.; Akamine, E. Obesity induces artery-specific alterations: Evaluation of vascular function and inflammatory and smooth muscle phenotypic markers. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lenquiste, S.A.; De Almeida Lamas, C.; Da Silva Marineli, R.; Moraes, É.A.; Borck, P.C.; Camargo, R.L.; Quitete, V.H.A.C.; Carneiro, E.M.; Junior, M.R.M. Jaboticaba peel powder and jaboticaba peel aqueous extract reduces obesity, insulin resistance and hepatic fat accumulation in rats. Food Res. Int. 2019, 120, 880–887. [Google Scholar] [CrossRef]
- Gooda Sahib Jambocus, N.; Saari, N.; Ismail, A.; Khatib, A.; Mahomoodally, M.F.; Abdul Hamid, A. An Investigation into the antiobesity effects of Morinda citrifolia L. leaf extract in high fat diet induced obese rats using a 1H NMR metabolomics approach. J. Diabetes Res. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cardozo, C.M.L.; Inada, A.C.; Cardoso, C.A.L.; Filiú, W.F.O.; De Farias, B.B.; Alves, F.M.; Tatara, M.B.; Croda, J.H.R.; Guimarães, R.C.A.; Hiane, P.A.; et al. Effect of supplementation with hydroethanolic extract of Campomanesia xanthocarpa (Berg.) leaves and two isolated substances from the extract on metabolic parameters of mice fed a high-fat diet. Molecules 2020, 25, 2693. [Google Scholar] [CrossRef]
- White, P.A.S.; Cercato, L.M.; Batista, V.S.; Camargo, E.A.; DE Lucca, W.; Oliveira, A.S.; Silva, F.T.; Goes, T.C.; Oliveira, E.R.A.; Moraes, V.R.S.; et al. Aqueous extract of Chrysobalanus icaco leaves, in lower doses, prevent fat gain in obese high-fat fed mice. J. Ethnopharmacol. 2016, 179, 92–100. [Google Scholar] [CrossRef]
- Sasso, S.; Sampaio e Souza, P.C.; Santana, L.F.; Cardoso, C.A.L.; Alves, F.M.; Portugal, L.C.; De Farias, B.B.; Da Silva, A.F.; Motta-Castro, A.R.C.; Soares, L.S.; et al. Use of an extract of Annona muricata Lin to prevent high-fat diet induced metabolic disorders in C57BL/6 mice. Nutrients 2019, 11, 1509. [Google Scholar] [CrossRef] [Green Version]
- Nery, C.S.; Pinheiro, I.L.; Muniz, G.S.; Vasconcelos, D.A.A.; França, S.P.; Nascimento, E. Murinometric evaluations and feed efficiency in rats from reduced litter during lactation and submitted or not to swimming exercise. Revista Brasileira de Medicina do Esporte 2011, 17, 49–55. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Urida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Coelho, C.F.F.; França, L.M.; Nascimento, J.R.; Dos Santos, A.M.; Azevedo-Santos, A.P.S.; Nascimento, F.R.F.; Paes, A.M.A. Early onset and progression of non-alcoholic fatty liver disease in young monosodium L-glutamate-induced obese mice. J. Dev. Orig. Health Dis. 2018, 10, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Arimelazhagan, T.; Shanmugam, S.; Thankarajan, S. Antidiabetic activity of Syzygium calophyllifolium in streptozotocin-nicotinamide induced type-2 diabetic rats. Biomed. Pharmacother. 2016, 82, 547–554. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Wang, B.; Zhao, J.; Yan, X.; Huang, Y.; Du, M.; Zhu, M.J. Maternal obesity exacerbates insulitis and type 1 diabetes in non-obese diabetic mice. Reproduction 2014, 148, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, M.C.; Steffens, R.S.; Jablonski, A.; Hertz, P.F.; De, O.; Rios, A.; Vizzotto, M.; Flôres, S.H. Characterization and antioxidant potential of Brazilian fruits from the Myrtaceae family. J. Agric. Food Chem. 2012, 60, 3061–3067. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.H.S.; Fernandes, L.R.; Mario, E.G.; Ferreira, A.V.M.; Porto, L.C.J.; Alvarez-Leite, J.I.; Botion, L.M.; Bader, M.; Alenina, N.; Santos, R.A.S.; et al. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 2008, 57, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J. Fragmentation of negative ions from carbohydrates: Part 2. Fragmentation of high-mannose N-linked glycans. J. Am. Soc. Mass Spectrom. 2005, 16, 631–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Xue, R.; Li, Z.; Chen, M.; Sun, Z.; Hu, Y.; Huang, C. Fragmentation patterns study of iridoid glycosides in fructus Gardeniae by HPLC-Q/TOF-MS/MS. Biomed. Chromatogr. 2014, 28, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Wang, M.; Yohei, E.; Hanani, U.; Umar, M.; Satake, T. New anthraquinone and iridoid from the fruits of Morinda citrifolia. Chem. Pharm. Bull. 2005, 53, 1597–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, S.; Wang, M.; He, K.; Liu, G.; Dong, Z.; Badmaev, V.; Zheng, Q.I.; Ghai, G.; Rosen, R.; Robert, T.; et al. Chemical components in noni fruits and leaves (Morinda citrifolia L.). ACS Symp. Ser. 2002, 803, 134–150. [Google Scholar] [CrossRef]
- Tang, J.; Liu, H.; Zhou, Z.; Liu, J. Iridoids from Morinda citrifolia. Zhongguo Yaowu Huaxue Zazhi 2009, 19, 379–381. [Google Scholar]
- Friščić, M.; Bucar, F.; Pilepić, K.H. LC-PDA-ESI-MSn analysis of phenolic and iridoid compounds from Globularia spp. J. Mass Spectrom. 2009, 51, 1211–1236. [Google Scholar] [CrossRef]
- Dalsgaard, P.W.; Potterat, O.; Dieterle, F.; Paululat, T.; Kühn, T.; Hamburger, M. New trisaccharide fatty acid esters from the fruit of Morinda citrifolia (Noni). Planta Med. 2006, 72, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-X.; Zhang, H.-C.; Wang, Y.; Liu, S.-M.; Liu, L. Two new glycosides from the fruits of Morinda citrifolia L. Molecules 2012, 17, 12651–12656. [Google Scholar] [CrossRef] [Green Version]
- Youn, U.J.; Park, E.-J.; Kondratyuk, T.; Sang-Ngern, M.; Wall, M.W.; Wei, Y.; Pezzuto, J.M.; Chang, L.C. Anti-inflammatory and quinone reductase inducing compounds from fermented noni (Morinda citrifolia) juice exudates. J. Nat. Prod. 2016, 79, 1508–1513. [Google Scholar] [CrossRef]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. New features on the fragmentation and differentiation of C-glycosidic flavone isomers by positive electrospray ionization and triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1834–1842. [Google Scholar] [CrossRef]
- Pandy, V.; Narasingam, M.; Kunasegaran, T.; Murugan, D.D.; Mohamed, Z. Effect of Noni (Morinda citrifolia Lin) fruit and its bioactive principles scopoletin and rutin on rat vas deferens contractility: An ex vivo study. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Pandi, P.; Selvam, P.; Mohan Gupta, V. LC-MS/MS studies on the fruit extracts of Morinda citrifolia L. (Noni) other sources. World J. Pharm. Res. 2015, 4, 2281–2298. [Google Scholar]
- Taechowisa, T.; Sarakoat, P.; Phutdhawon, W.S. Major chemical composition of fruit extracts of Morinda citrifolia L. and their antibacterial, antioxidant and cytotoxicity properties. J. Appl. Sci. 2019, 19, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Zhuhua, Z.; Zhiquan, W.; Zhen, Y.; Yixin, N.; Weiwei, Z.; Xiaoyong, L.; Yueming, L.; Hongmei, Z.; Li, Q.; Qing, S.; et al. A novel mice model of metabolic syndrome: The high-fat/high-fructose diet-fed ICR mice. Exp. Anim. 2015, 64, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Shokouh, P.; Jeppesen, P.; Hermansen, K.; Laustsen, C.; Stødkilde-Jørgensen, H.; Hamilton-Dutoit, S.; Søndergaard Schmedes, M.; Qi, H.; Stokholm Nørlinger, T.; Gregersen, S.; et al. Effects of unfiltered coffee and bioactive coffee compounds on the development of metabolic syndrome components in a high-fat-/high-fructose-fed rat model. Nutrients 2018, 10, 1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, B.S.; Marshall, J.R.; Isitor, G.; Adogwa, A. Hypoglycemic and hepatoprotective activity of fermented fruit juice of Morinda citrifolia (Noni) in diabetic rats. Evid. Based Complement. Alternat. Med. 2011, 2011, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Kikuzaki, H.; Csiszar, K.; Boyd, C.D.; Maunakea, A.; Fong, S.F.T.; Ghai, G.; Rosen, R.T.; Nakatani, N.; Ho, C.-T.; et al. Novel trisaccharide fatty acid ester identified from the fruits of Morinda citrifolia (Noni). J. Agric. Food Chem. 1999, 47, 4880–4882. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Indian Morinda species: A review. Phytother. Res. 2020, 34, 924–1007. [Google Scholar] [CrossRef]
- Fernandes, A.A.H.; Novelli, E.L.B.; Okoshi, K.; Okoshi, M.P.; Muzio, B.P.D.; Guimarães, J.F.C.; Junior, A.F. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed. Pharmacother. 2010, 64, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Emamjomeh, A.; Chenarani, N.; Bohlooli, M. Beneficial effects of rutin in diabetes-induced deficits in acquisition learning, retention memory and pain perception in rats. Nutr. Neurosci. 2020, 23, 563–574. [Google Scholar] [CrossRef]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef]
- Chiu, S.; Mulligan, K.; Schwarz, J.-M. Dietary carbohydrates and fatty liver disease: De novo lipogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 277–282. [Google Scholar] [CrossRef]
- Chawla, A.; Schwarz, E.J.; Dimaculangan, D.D.; Lazar, M.A. Peroxisome proliferator-activated receptor (PPAR) gamma: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 1994, 135, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Tanne, D.; Koren-Morag, N.; Graff, E.; Goldbourt, U. Blood lipids and first-ever ischemic stroke/transient ischemic attack in the bezafibrate infarction prevention (BIP) registry: High triglycerides constitute an independent risk factor. Circulation 2001, 104, 2892–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubins, H.B.; Robins, S.J.; Collins, D.; Fye, C.L.; Anderson, J.W.; Elam, M.B.; Faas, F.H.; Linares, E.; Schaefer, E.J.; Schectman, G.; et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N. Engl. J. Med. 1999, 341, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Guerre-Millo, M.; Rouault, C.; Poulain, P.; André, J.; Poitout, V.; Peters, J.M.; Gonzalez, F.J.; Fruchart, J.-C.; Reach, G.; Staels, B.; et al. PPAR-α–null mice are protected from high-fat diet–induced insulin resistance. Diabetes 2001, 50, 2809–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, C.-C.; Lin, C.-H.; Lin, W.-L.; Wu, J.-B. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J. Ethnopharmacol. 2009, 123, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Lau, C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine 1996, 2, 349–362. [Google Scholar] [CrossRef]
- Fukuchi, Y.; Hiramitsu, M.; Okada, M.; Hayashi, S.; Nabeno, Y.; Osawa, T.; Michitaka, N. Lemon polyphenols suppress diet-induced obesity by up-regulation of mRNA levels of the enzymes involved in β-oxidation in mouse white adipose tissue. J. Clin. Biochem. Nutr. 2008, 43, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Ferré, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12, 83–92. [Google Scholar] [CrossRef]
- Diraison, F.; Yankah, V.; Letexier, D.; Dusserre, E.; Jones, P.; Beylot, M. Differences in the regulation of adipose tissue and liver lipogenesis by carbohydrates in humans. J. Lipid Res. 2003, 44, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Softic, S.; Gupta, M.K.; Wang, G.-X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Invest. 2017, 127, 4059–4074. [Google Scholar] [CrossRef] [Green Version]
- Shiota, M.; Galassetti, P.; Igawa, K.; Neal, D.W.; Cherrington, A.D. Inclusion of low amounts of fructose with an intraportal glucose load increases net hepatic glucose uptake in the presence of relative insulin deficiency in dog. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E1160–E1167. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T. Fructose induced lipogenesis: From sugar to fat to insulin resistance. Trends Endocrinol. Metab. 2011, 22, 60–65. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.L.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? BBA-Mol. Cell Biol. L. 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.-C.; Lin, J.-K. Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet. Food Funct. 2012, 3, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Youn, B.-S.; Choi, H.Y.; Lee, S.Y.; Hong, H.C.; Yang, S.J.; Yoo, H.J.; Kim, B.-H.; Baik, S.H.; Choi, K.M.; et al. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem. Pharmacol. 2013, 86, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Nangami, G.; Sakwe, A.; Moye, C.; Alvarez, J.; Whalen, D.; Thomas, P.; Lammers, P. Impact of fetuin-A (AHSG) on tumor progression and type 2 diabetes. Int. J. Mol. Sci. 2018, 19, 2211. [Google Scholar] [CrossRef] [Green Version]
- Bakan, I.; Laplante, M. Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 2012, 23, 226–234. [Google Scholar] [CrossRef]




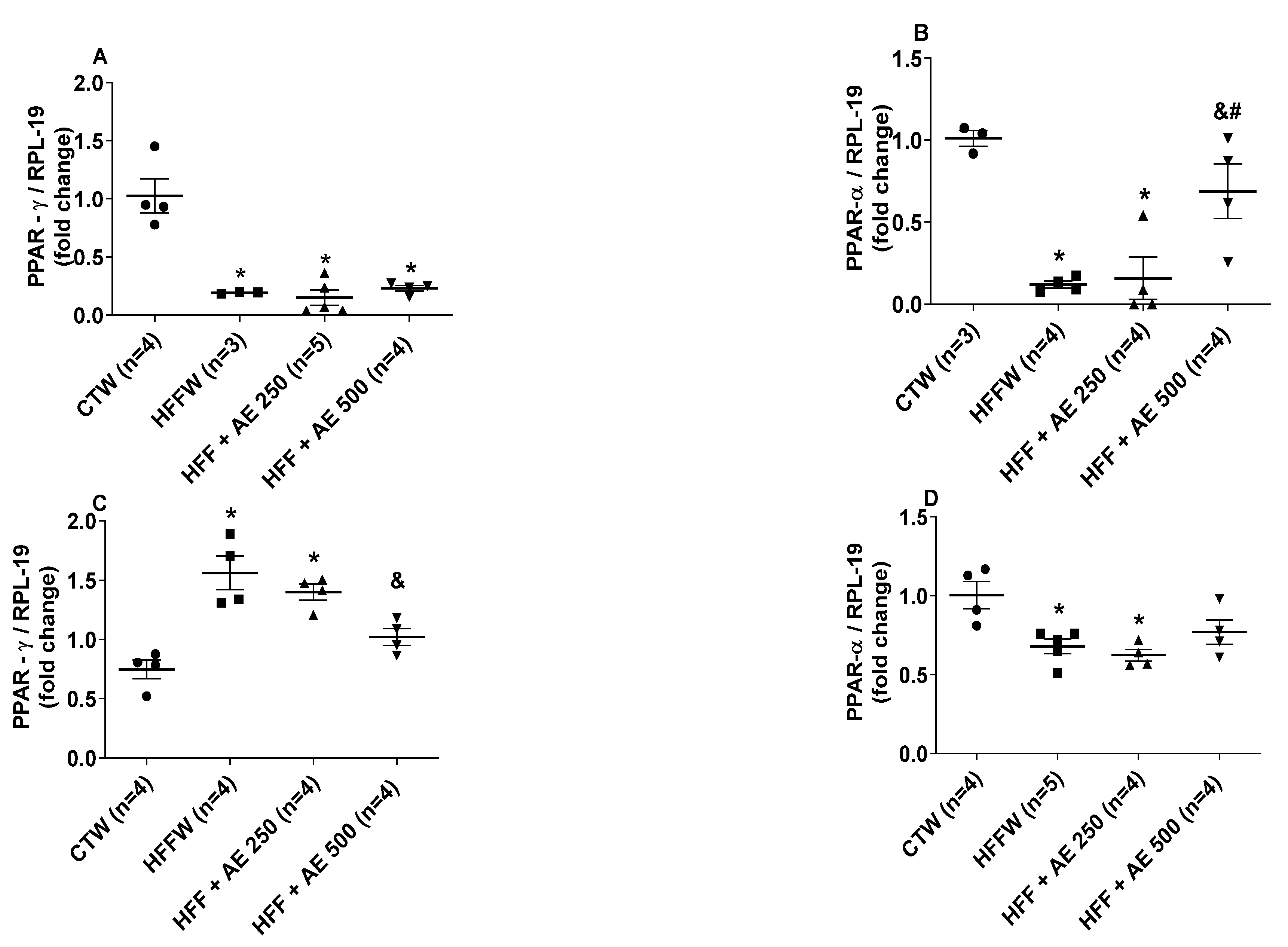
| Gene | Sequence | Amplicon (bp) | Accession Number |
|---|---|---|---|
| Peroxisome Proliferator Activated Receptor Gamma (PPAR-γ) | Fwd: ATCTTAACTGCCGGATCCAC Rev: CAAACCTGATGGCATTGTGAG | 102 | NM_001127330.2 |
| Peroxisome Proliferator Activated Receptor Alpha (PPAR-α) | Fwd: TGCAATTCGCTTTGGAAGAA Rev: CTTGCCCAGAGATTTGAGGT | 118 | NM_011144.6 |
| Fat Acid Synthase (FAS) | Fwd: GATTCGGTGTATCCTGCTGTC Rev: CATGCTTTAGCACCTGCTGT | 95 | NM_007988.3 |
| Glucose-6-Phosphatase (G6P) | Fwd: CCGGATCTACCTTGCTGCTC Rev: GCATTGTAGATGCCCCGGAT | 105 | NM_008061.4 |
| Fetuin-A | Fwd: GGAGATTTCCCGGGCTCAAA Rev: TGCAGTACAGTCAGTGGCAG | 82 | NM_001276450.1 |
| Carbohydrate Response Element Binding Protein (ChREBP) | Fwd: CAGCATCGATCCGACACTCA Rev: GGCCTTTGAAGTTCTTCCACT | 96 | NM_021455.5 |
| Sterol Regulatory Element Binding Transcription Factor 1-c (SREBP1-c) | Fwd: TGACGGAGACAGGGAGTTCT Rev: CAGAGAAACTGCAAGCAGGA | 95 | NM_001313979.1 |
| Ribosomal Protein L19 (RPL19) | Fwd: CAATGCCAACTCCCGTCA Rev: GTGTTTTTCCGGCAACGAG | 102 | NM_009078.2 |
| N° | Rt (min) | Compound | Molecular Formula * | MS (−) | MS 2 | UV λmax (nm) |
|---|---|---|---|---|---|---|
| 1 | 1.2 | di-O-hexoside | C12H22O11 | 341.1091 (M − H)− | - | - |
| 2 | 1.2 | tri-O-hexoside | C18H32O16 | 503,1636 (M − H)− 549.1679 (M + HCOO)− | 549.1679: 221, 179, 161 | - |
| 3 | 2.4 | Deacetylasperulosidic acid | C16H22O11 | 389.1090 (M − H)− | 389.1090: 209, 165 | - |
| 4 | 2.7 | Unknown | C17H26O12 | 421.1323 (M − H)− | - | - |
| 5 | 5.1 | Unknown | C16H28O12 | 411.1500 (M − H)− | - | - |
| 6 | 7.1 | Unknown | C15H26O11 | 381.1405 (M − H)− | - | - |
| 7 | 7.5 | Unknown | C15H26O11 | 381.1407 (M − H)− | - | - |
| 8 | 9.2 | Nonioside (hemiterpene disaccharide) | C17H30O11 | 409.1735 (M − H)− | - | - |
| 9 | 9.6 | Unknown | C16H24O10 | 375.1285 (M − H)− | - | - |
| 10 | 9.9 | Asperulosidic acid | C18H24O12 | 431.1217 (M − H)− | 431.1217: 165, 147 | - |
| 11 | 11.2 | Nonioside | C16H28O11 | 395.1593 (M − H)−, 441.1623 (M + HCOO)− | - | - |
| 12 | 11.6 | Nonioside (hemiterpene disaccharide) | C16H28O10 | 379.1636 (M − H)−, 425.1692 (M + HCOO) | - | - |
| 13 | 12.1 | Nonioside (hemiterpene disaccharide) | C16H28O10 | 379.1647 (M − H)−, 425.1679 (M + HCOO) | - | - |
| 14 | 15.3 | Nonioside (fatty acid ester disaccharide) | C18H32O12 | 439.1831 (M − H)−, | - | - |
| 15 | 18.5 | Rutin | C27H30O16 | 609.1480 (M − H)− | - | 291, 347 |
| 16 | 24.3 | Nonioside (fatty acid ester disaccharide) | C20H36O12 | 467.2161 (M − H)− | - | - |
| 17 | 24.5 | Nonioside (fatty acid ester disaccharide) | C22H34O12 | 489.2001 (M − H)− | - | 279 |
| Parameter | Groups | |||
|---|---|---|---|---|
| CTW (n = 11) | HFFW (n = 10) | HFF + AE 250 (n = 11) | HFF + AE 500 (n = 10) | |
| Epididymal fat (mg/g) | 28.68 ± 2.69 | 42.10 ± 3.77 | 39.71 ± 3.95 | 48.67 ± 3.66 * |
| Retroperitoneal fat (mg/g) | 8.13 ± 0.45 | 16.81 ± 1.96 * | 16.09 ± 1.28 * | 16.56 ± 1.00 * |
| Perirenal fat (mg/g) | 6.79 ± 0.71 | 10.75 ± 1.14 * | 15.90 ± 0.94 *& | 14.27 ± 1.17 * |
| Omental fat (mg/g) | 0.42 ± 0.07 | 1.04 ± 0.25 | 0.71 ± 0.17 | 0.62 ± 0.21 |
| Mesenteric fat (mg/g) | 18.47 ± 1.04 | 29.20 ± 2.86 * | 34.14 ± 1.55 * | 34.29 ± 1.3 4 * |
| Liver (mg/g) | 38.11 ± 0.60 | 42.62 ± 3.87 | 42.83 ± 2.55 | 40.33 ± 2.92 |
| Adiposity index (%) | 6.25 ± 0.46 | 9.99 ± 0.62 * | 10.65 ± 0.49 * | 11.44 ± 0.61 * |
| Variable | Groups | |||
|---|---|---|---|---|
| Liver Changes | CTW (n = 10) | HFFW (n = 10) | HFF + AE 250 (n = 10) | HFF + AE 500 (n = 11) |
| Steatosis *(p < 0.0001) | ||||
| Score: 0 = none; 1 = light, 2 = moderate; 3 = severe | 0.0 ± 0.0 | 2.0 ± 0.15 * | 1.9 ± 0.18 * | 2.273 ± 0.14 * |
| Steatosis Localization | None | Zone 3 | Zone 3 | Zone 3 |
| Microvesicular Steatosis (p = 0.09) | ||||
| 0 = absent; 1 = present (number of mice) | 0 | 0 | 1 (8 out of 10) | 0 |
| Lobular Inflammation*(p < 0.0001) | ||||
| Score: 0 = Absent; 1 = < 2 focus/field; 2 = 2-4 focus/field | 0.1 ± 0.1 | 0.9 ± 0.18 * | 1.0 ± 0 * | 1.182 ± 0.18 * |
| Ballooning *(p = 0.05) | ||||
| Score: 0 = Absent; 1 = Few cells, 2 = Many cells | 1.0 ± 0.15 | 1.7 ± 0.15 * | 1.7 ± 0.15 * | 1.64 ± 0.15 * |
| Mallory’s Hyaline *(p = 0.07) | ||||
| Score: 0 = Absent; 1 = Rare, 2 = Several | 0.2 ± 0.13 | 0.3 ± 0.2 | 1.1 ± 0.28 * | 1.1 ± 0.25 * |
| Apoptosis (p = 0.45) | ||||
| Score: 0 = Absent; 1 = Present | 0.1 ± 0.1 | 0.6 ± 0.16 | 0.5 ± 0.17 | 0.36 ± 0.15 |
| Glycogenate Nucleus (p = 0.12) | ||||
| Score: 0 = Absent/Rare; 1 = Some | 0.6 ± 0.16 | 0.1 ± 0.1 | 0.5 ± 0.16 | 0.36 ± 0.15 |
| Variable | Groups | |||
|---|---|---|---|---|
| Pancreas Changes | CTW (n = 11) | HFFW (n = 10) | HFF + AE 250 (n = 11) | HFF + AE 500 (n = 10) |
| Islet of Langerhans (p = 0.11) | ||||
| Score: 0 = No change; 1 = Discrete Atrophy; 2 =Atrophy | 0.3 ± 0.15 | 1.25 ± 0.3 | 0.62 ± 0.3 | 0.7 ± 0.29 |
| Pancreatic acini (p = 0.09) | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Score: 0 = No change; 1 = Necrosis/Atrophy | ||||
| Inflammatory cells (p = 0.33) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| Score: 0 = No change; 1 = Perinsulitis | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inada, A.C.; Silva, G.T.; Silva, L.P.R.d.; Alves, F.M.; Filiú, W.F.d.O.; Asato, M.A.; Junior, W.H.K.; Corsino, J.; Figueiredo, P.d.O.; Garcez, F.R.; et al. Therapeutic Effects of Morinda citrifolia Linn. (Noni) Aqueous Fruit Extract on the Glucose and Lipid Metabolism in High-Fat/High-Fructose-Fed Swiss Mice. Nutrients 2020, 12, 3439. https://doi.org/10.3390/nu12113439
Inada AC, Silva GT, Silva LPRd, Alves FM, Filiú WFdO, Asato MA, Junior WHK, Corsino J, Figueiredo PdO, Garcez FR, et al. Therapeutic Effects of Morinda citrifolia Linn. (Noni) Aqueous Fruit Extract on the Glucose and Lipid Metabolism in High-Fat/High-Fructose-Fed Swiss Mice. Nutrients. 2020; 12(11):3439. https://doi.org/10.3390/nu12113439
Chicago/Turabian StyleInada, Aline Carla, Gabriela Torres Silva, Laleska Pâmela Rodrigues da Silva, Flávio Macedo Alves, Wander Fernando de Oliveira Filiú, Marcel Arakaki Asato, Wilson Hino Kato Junior, Joaquim Corsino, Patrícia de Oliveira Figueiredo, Fernanda Rodrigues Garcez, and et al. 2020. "Therapeutic Effects of Morinda citrifolia Linn. (Noni) Aqueous Fruit Extract on the Glucose and Lipid Metabolism in High-Fat/High-Fructose-Fed Swiss Mice" Nutrients 12, no. 11: 3439. https://doi.org/10.3390/nu12113439
APA StyleInada, A. C., Silva, G. T., Silva, L. P. R. d., Alves, F. M., Filiú, W. F. d. O., Asato, M. A., Junior, W. H. K., Corsino, J., Figueiredo, P. d. O., Garcez, F. R., Garcez, W. S., Silva, R. d. N. O. d., Santos-Eichler, R. A. d., Guimarães, R. d. C. A., Freitas, K. d. C., & Hiane, P. A. (2020). Therapeutic Effects of Morinda citrifolia Linn. (Noni) Aqueous Fruit Extract on the Glucose and Lipid Metabolism in High-Fat/High-Fructose-Fed Swiss Mice. Nutrients, 12(11), 3439. https://doi.org/10.3390/nu12113439






