POCU1b, the n-Butanol Soluble Fraction of Polygoni Cuspidati Rhizoma et Radix, Attenuates Obesity, Non-Alcoholic Fatty Liver, and Insulin Resistance via Inhibitions of Pancreatic Lipase, cAMP-Dependent PDE Activity, AMPK Activation, and SOCS-3 Suppression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and High Performance Liquid Chromatography (HPLC) Analysis of POCU1b
2.2. Short-Term Animal Study for Serum Triglyceride (TG) Levels After Oral Administration of a Lipid Emulsion
2.3. Preparation of Fat Pads and Measurements of Glycerol Release and Cyclic Adenosine Monophosphate (cAMP)
2.4. Phosphodiesterase (PDE) Activity Assay
2.5. Long-Term Animal Study of Lipolytic Effect
2.6. Serum Analysis
2.7. Liver TG Measurement
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Carnitine Palmitoyl Transferase (CPT)-1 Activity Assay
2.10. Western Blot Analysis
2.11. RNA Isolation and Reverse Transcription (RT)-PCR
2.12. Histological Analysis
2.13. Immunohistochemistry
2.14. Measurment of NF-κB DNA Binding Using an ELISA-Based Method
2.15. Statistical Analysis
3. Results
3.1. Preparation and HPLC Analysis of POCU1b
3.2. Effects of POCU1b on Levels of Serum TG and cAMP, Glycerol Release, and PDE Activity
3.3. Effects of POCU1b on Body Weight and Food Intake in HFD-Fed Rats
3.4. Effect of POCU1b on Fat Weight and Adipocyte Size in HFD-Fed Rats
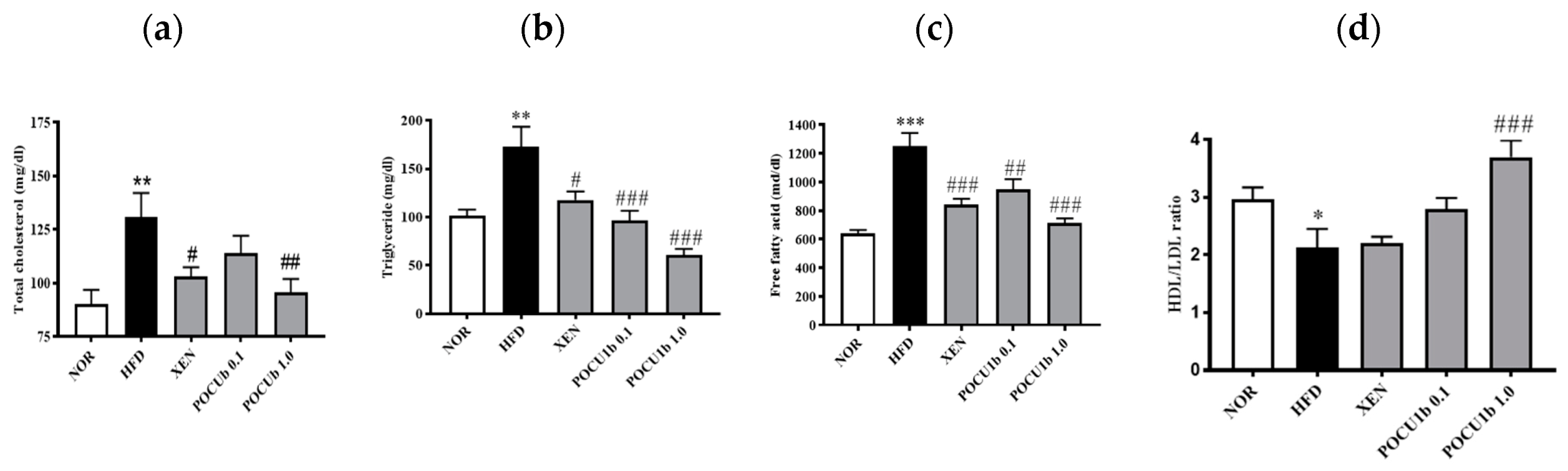
3.5. Effects of POCU1b on Serum Lipid Levels in HFD-Fed Rats
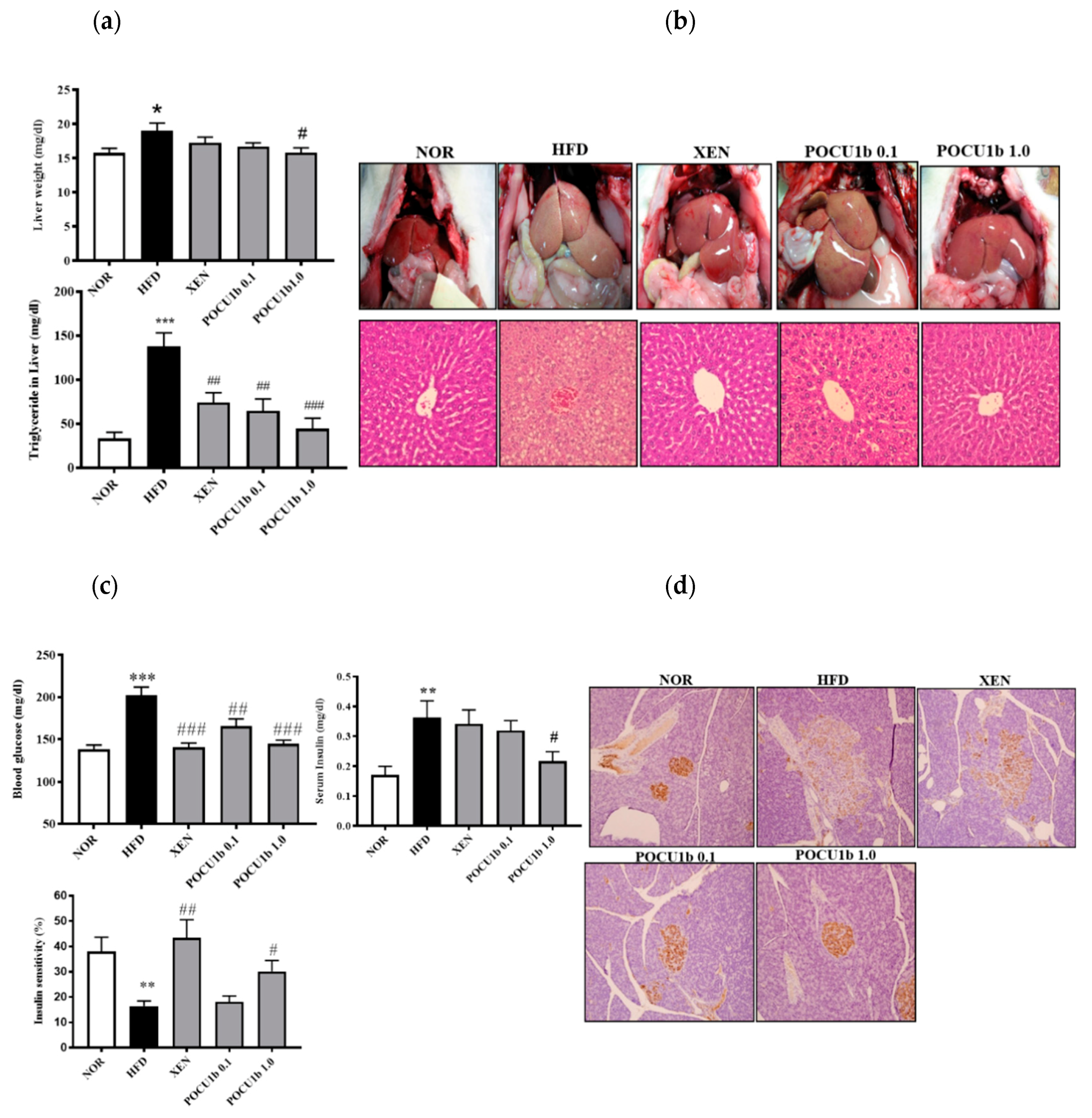
3.6. Effects of POCU1b on NAFL and IR in HFD-Fed Rats
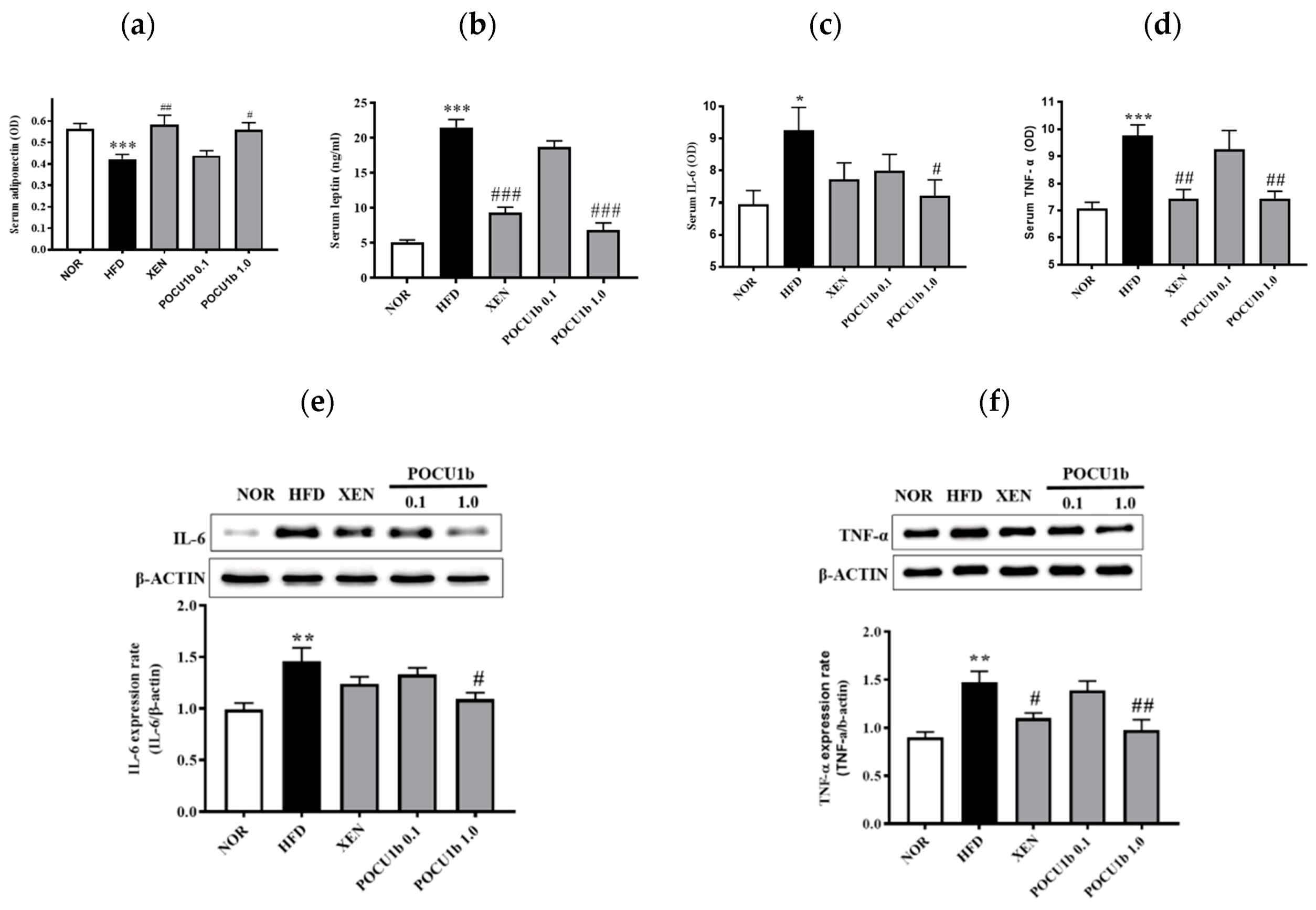
3.7. Effects of POCU1b on Adipokines in the Serum and/or Liver of HFD-Fed Rats
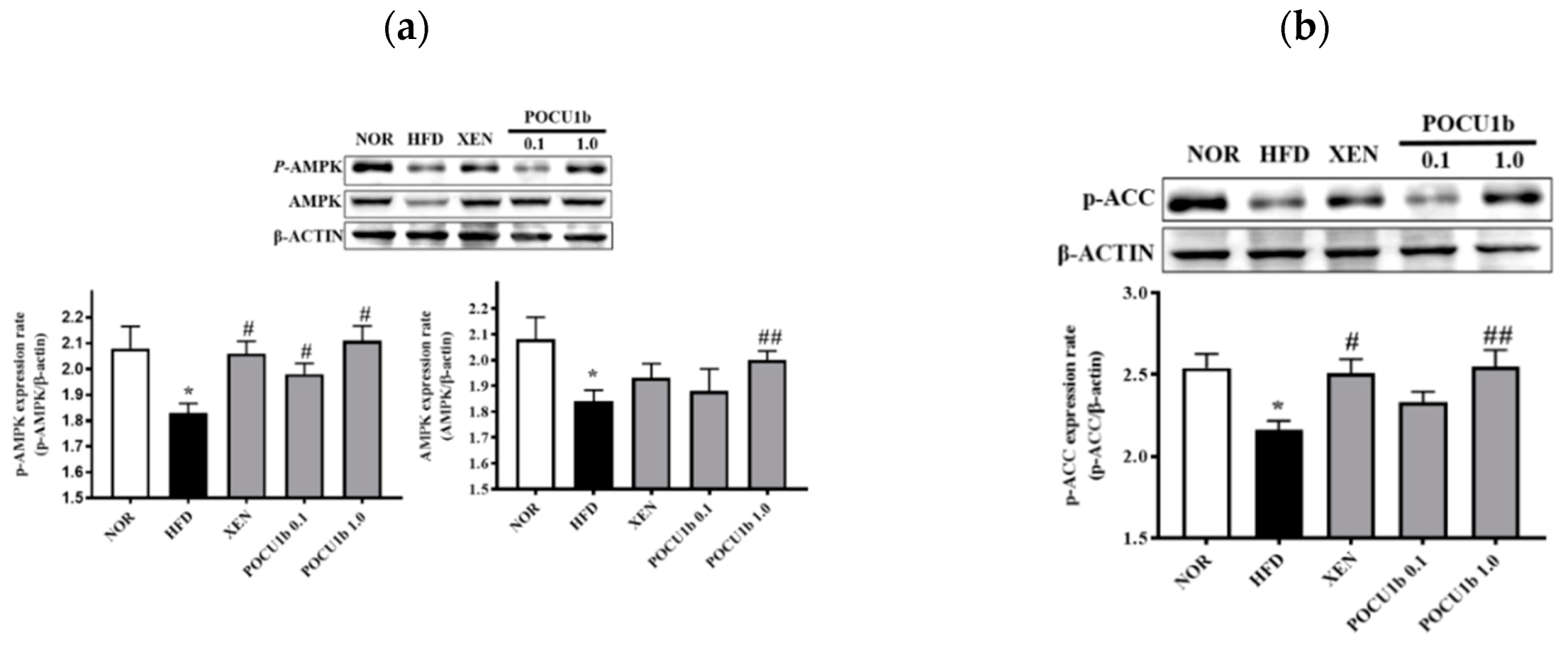
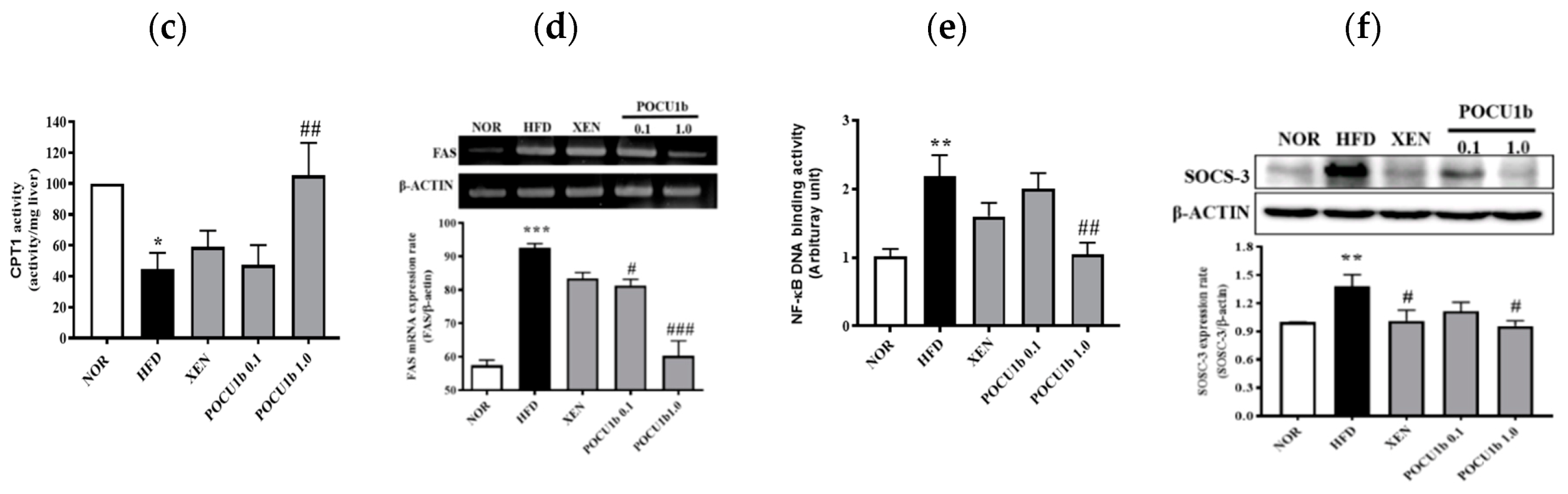
3.8. Effects of POCU1b on p-AMPK, AMPK, p-ACC, SOCS-3 Protein, and Fatty Acid Synthase (FAS) mRNA Expressions, and CPT-1 and NF-κB DNA-Binding Activities in HFD-Fed Rat Livers
4. Discussion
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Q.; Bengmark, S.; Qu, S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.K.; Choi, Y.J.; Huh, B.W.; Park, S.W.; Lee, E.J.; Cho, Y.W.; Huh, K.B. Nonalcoholic Fatty liver disease is associated with increased carotid intima-media thickness only in type 2 diabetic subjects with insulin resistance. J. Clin. Endocrinol. Metab. 2014, 99, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; McPherson, S.; Day, C.P. How big a problem is non-alcoholic fatty liver disease? BMJ 2011, 343, d3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Girotti, C.; Ginet, M.; Demarne, F.C.; Lagarde, M.; Geloen, A. Lipolytic activity of cirsimarin extracted from Microtea debilis. Planta Med. 2005, 71, 1170–1172. [Google Scholar] [CrossRef]
- Li, M.F.; Cheung, B.M. Rise and fall of anti-obesity drugs. World J. Diabetes 2011, 2, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjostrom, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Bray, G.A. Medications for obesity: Mechanisms and applications. Clin. Chest Med. 2009, 30, 525–538. [Google Scholar] [CrossRef]
- Ballinger, A.; Peikin, S.R. Orlistat: Its current status as an anti-obesity drug. Eur. J. Pharmacol. 2002, 440, 109–117. [Google Scholar] [CrossRef]
- Slanc, P.; Doljak, B.; Kreft, S.; Lunder, M.; Janes, D.; Strukelj, B. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytother. Res. 2009, 23, 874–877. [Google Scholar] [CrossRef]
- Allen, D.O.; Clark, J.F.; Ashmore, J. Study of phosphodiesterase inhibitors on lipolysis, phosphodiesterase activity and cyclic 3′,5′-adenosine monophosphate levels in isolated fat cells. J. Pharmacol. Exp. Ther. 1973, 185, 379–385. [Google Scholar] [PubMed]
- Park, C.S.; Lee, Y.C.; Kim, J.D.; Kim, H.M.; Kim, C.H. Inhibitory effects of Polygonum cuspidatum water extract (PCWE) and its component resveratrol [correction of rasveratrol] on acyl-coenzyme A-cholesterol acyltransferase activity for cholesteryl ester synthesis in HepG2 cells. Vascul. Pharmacol. 2004, 40, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, S.K.; Chang, K.W.; Han, S.K.; Yi, H.K.; Jeon, J.G. In vitro inhibitory effects of Polygonum cuspidatum on bacterial viability and virulence factors of Streptococcus mutans and Streptococcus sobrinus. Arch. Oral Biol. 2006, 51, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Yang, T.C.; Chang, K.W.; Han, S.K.; Yi, H.K.; Jeon, J.G. In vitro effects of a fraction separated from Polygonum cuspidatum root on the viability, in suspension and biofilms, and biofilm formation of mutans streptococci. J. Ethnopharmacol. 2007, 112, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.H.; Kwon, Y.R.; Pandit, S.; Lee, Y.S.; Yi, H.K.; Jeon, J.G. Effects of a bio-assay guided fraction from Polygonum cuspidatum root on the viability, acid production and glucosyltranferase of mutans streptococci. Fitoterapia 2010, 81, 30–34. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.X.; Kongstad, K.T.; Jager, A.K.; Staerk, D. Potential of Polygonum cuspidatum Root as an Antidiabetic Food: Dual High-Resolution alpha-Glucosidase and PTP1B Inhibition Profiling Combined with HPLC-HRMS and NMR for Identification of Antidiabetic Constituents. J. Agric. Food Chem. 2017, 65, 4421–4427. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, Y.M.; Kim, J.H.; Kim, J.S. Polygonum cuspidatum inhibits pancreatic lipase activity and adipogenesis via attenuation of lipid accumulation. BMC Complement. Altern. Med. 2013, 13, 282. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.H.; Han, J.H.; Yu, K.H.; Hong, M.; Lee, S.Y.; Park, K.H.; Lee, S.U.; Kwon, T.H. Antioxidant and Anti-Obesity Activities of Polygonum cuspidatum Extract through Alleviation of Lipid Accumulation on 3T3-L1 Adipocytes. J. Microbiol. Biotechnol. 2020, 30, 21–30. [Google Scholar] [CrossRef]
- Jayasuriya, H.; Koonchanok, N.M.; Geahlen, R.L.; McLaughlin, J.L.; Chang, C.J. Emodin, a protein tyrosine kinase inhibitor from Polygonum cuspidatum. J. Nat. Prod. 1992, 55, 696–698. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Jayasuriya, H.; Lee, E.S.; Koonchanok, N.M.; Geahlen, R.L.; Ashendel, C.L.; McLaughlin, J.L.; Chang, C.J. Kinase inhibitors from Polygonum cuspidatum. J. Nat. Prod. 1993, 56, 1805–1810. [Google Scholar] [CrossRef]
- Carmen, G.Y.; Victor, S.M. Signalling mechanisms regulating lipolysis. Cell. Signal. 2006, 18, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Lizaso, A.; Tan, K.T.; Lee, Y.H. β-adrenergic receptor-stimulated lipolysis requires the RAB7-mediated autolysosomal lipid degradation. Autophagy 2013, 9, 1228–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, P.B.; Esselstyn, J.M.; Loughney, K.; Wolda, S.L.; Florio, V.A. The role of cyclic nucleotide phosphodiesterases in the regulation of adipocyte lipolysis. J. Lipid Res. 2005, 46, 494–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Hui, J.M.; Hodge, A.; Farrell, G.C.; Kench, J.G.; Kriketos, A.; George, J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004, 40, 46–54. [Google Scholar] [CrossRef]
- Diez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Kern, P.A.; Di Gregorio, G.B.; Lu, T.; Rassouli, N.; Ranganathan, G. Adiponectin expression from human adipose tissue: Relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 2003, 52, 1779–1785. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, M.; Maruoka, S.; Katayose, S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J. Clin. Endocrinol. Metab. 2002, 87, 2764–2769. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Aygun, A.D.; Gungor, S.; Ustundag, B.; Gurgoze, M.K.; Sen, Y. Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediat. Inflamm. 2005, 2005, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappo, F.; Esposito, K.; Cioffi, M.; Giugliano, G.; Molinari, A.M.; Paolisso, G.; Marfella, R.; Giugliano, D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: Role of fat and carbohydrate meals. J. Am. Coll. Cardiol. 2002, 39, 1145–1150. [Google Scholar] [CrossRef] [Green Version]
- Mohrschladt, M.F.; Weverling-Rijnsburger, A.W.; de Man, F.H.; Stoeken, D.J.; Sturk, A.; Smelt, A.H.; Westendorp, R.G. Hyperlipoproteinemia affects cytokine production in whole blood samples ex vivo. The influence of lipid-lowering therapy. Atherosclerosis 2000, 148, 413–419. [Google Scholar] [CrossRef]
- Jonkers, I.J.; Mohrschladt, M.F.; Westendorp, R.G.; van der Laarse, A.; Smelt, A.H. Severe hypertriglyceridemia with insulin resistance is associated with systemic inflammation: Reversal with bezafibrate therapy in a randomized controlled trial. Am. J. Med. 2002, 112, 275–280. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; Ricart, W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr. Rev. 2003, 24, 278–301. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Q.; Guan, Q.; Gao, L.; Zhao, J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats’ skeletal muscle. Biochem. Biophys. Res. Commun. 2006, 339, 701–707. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Capo, X.; Mascaro, C.M.; Monserrat-Mesquida, M.; Quetglas-Llabres, M.M.; Pons, A.; Tur, J.A.; Sureda, A. Hepatoprotective effects of resveratrol in non-alcoholic fatty live disease. Curr. Pharm. Des. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gong, L.; Wang, C.; Hu, N.; Tang, Y.; Zheng, L.; Dai, X.; Li, Y. Radix Polygoni Multiflori and Its Main Component Emodin Attenuate Non-Alcoholic Fatty Liver Disease in Zebrafish by Regulation of AMPK Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 1493–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Mao, X.; Wang, L.; Liu, M.; Wetzel, M.D.; Guan, K.L.; Dong, L.Q.; Liu, F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J. Biol. Chem. 2007, 282, 7991–7996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boden, G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997, 46, 3–10. [Google Scholar] [CrossRef]
- Malhi, H.; Gores, G.J. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 2008, 28, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Qatanani, M.; Lazar, M.A. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev. 2007, 21, 1443–1455. [Google Scholar] [CrossRef] [Green Version]
- Senn, J.J.; Klover, P.J.; Nowak, I.A.; Zimmers, T.A.; Koniaris, L.G.; Furlanetto, R.W.; Mooney, R.A. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J. Biol. Chem. 2003, 278, 13740–13746. [Google Scholar] [CrossRef] [Green Version]
- Karimi, G.; Sabran, M.R.; Jamaluddin, R.; Parvaneh, K.; Mohtarrudin, N.; Ahmad, Z.; Khazaai, H.; Khodavandi, A. The anti-obesity effects of Lactobacillus casei strain Shirota versus Orlistat on high fat diet-induced obese rats. Food Nutr. Res. 2015, 59, 29273. [Google Scholar] [CrossRef] [Green Version]
- Kujawska-Luczak, M.; Musialik, K.; Szulinska, M.; Swora-Cwynar, E.; Kargulewicz, A.; Grzymislawska, M.; Pupek-Musialik, D.; Bogdanski, P. The effect of orlistat versus metformin on body composition and insulin resistance in obese premenopausal women: 3-month randomized prospective open-label study. Arch. Med. Sci. 2017, 13, 725–731. [Google Scholar] [CrossRef] [Green Version]
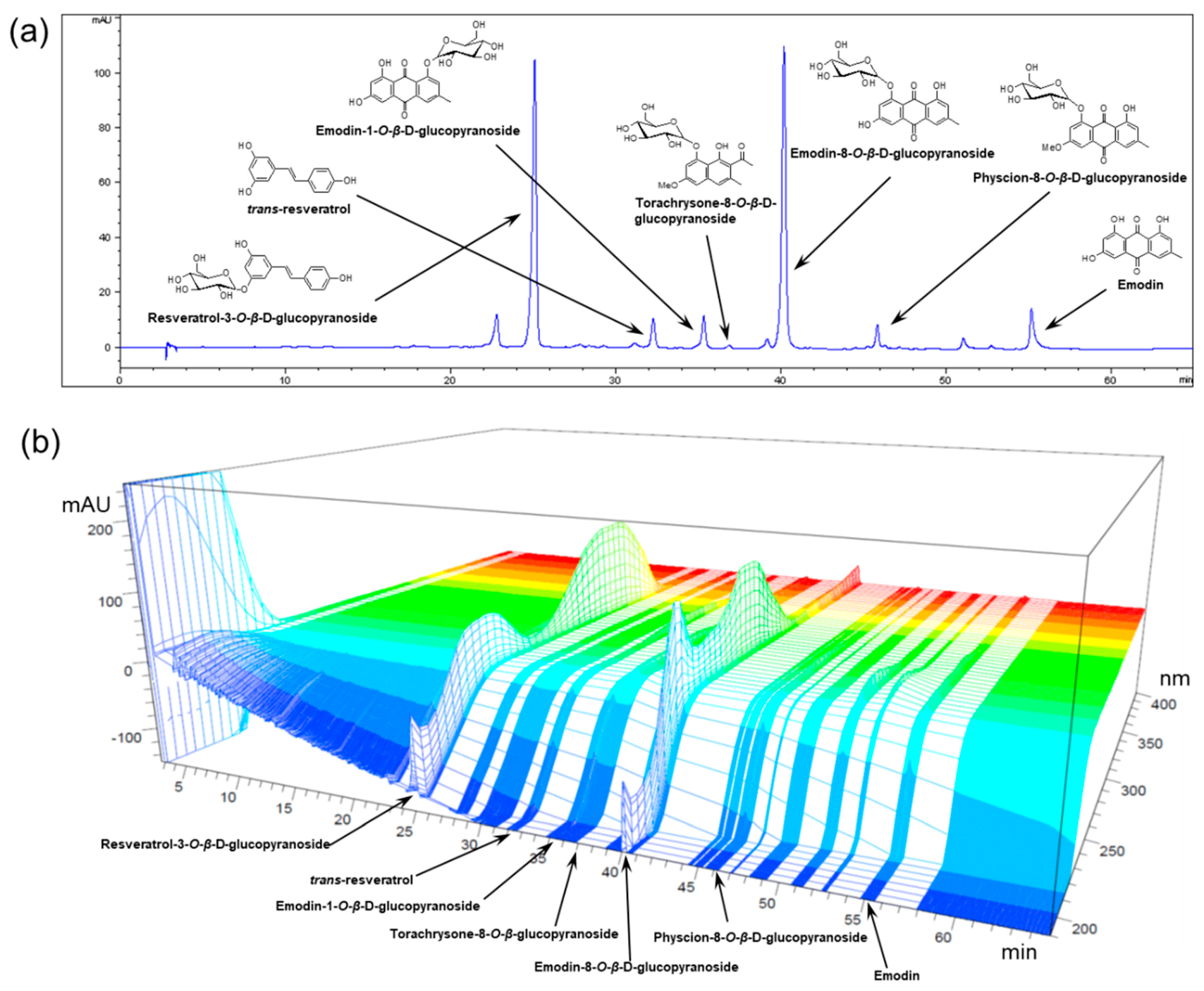



| Compound | Content (Mean ± SD, n = 3) |
|---|---|
| mg/g | |
| Resveratrol-3-O-β-d-glucopyranoside | 43.68 ± 2.21 |
| Resveratrol | 1.37 ± 0.07 |
| Emodin-1-O-β-d-glucopyranoside | 4.71 ± 0.28 |
| Torachrysone-8-O-β-d-glucopyranoside | 5.99 ± 0.28 |
| Emodin-8-O-β-d-glucopyranoside | 62.91 ± 3.22 |
| Physcion-8-O-β-d-glucopyranoside | 18.82 ± 0.96 |
| Emodin | 7.20 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, C.-S.; Jo, K.; Lee, I.S.; Kim, J.-H.; Kim, J.S. POCU1b, the n-Butanol Soluble Fraction of Polygoni Cuspidati Rhizoma et Radix, Attenuates Obesity, Non-Alcoholic Fatty Liver, and Insulin Resistance via Inhibitions of Pancreatic Lipase, cAMP-Dependent PDE Activity, AMPK Activation, and SOCS-3 Suppression. Nutrients 2020, 12, 3612. https://doi.org/10.3390/nu12123612
Kim J, Kim C-S, Jo K, Lee IS, Kim J-H, Kim JS. POCU1b, the n-Butanol Soluble Fraction of Polygoni Cuspidati Rhizoma et Radix, Attenuates Obesity, Non-Alcoholic Fatty Liver, and Insulin Resistance via Inhibitions of Pancreatic Lipase, cAMP-Dependent PDE Activity, AMPK Activation, and SOCS-3 Suppression. Nutrients. 2020; 12(12):3612. https://doi.org/10.3390/nu12123612
Chicago/Turabian StyleKim, Junghyun, Chan-Sik Kim, Kyuhyung Jo, Ik Soo Lee, Joo-Hwan Kim, and Jin Sook Kim. 2020. "POCU1b, the n-Butanol Soluble Fraction of Polygoni Cuspidati Rhizoma et Radix, Attenuates Obesity, Non-Alcoholic Fatty Liver, and Insulin Resistance via Inhibitions of Pancreatic Lipase, cAMP-Dependent PDE Activity, AMPK Activation, and SOCS-3 Suppression" Nutrients 12, no. 12: 3612. https://doi.org/10.3390/nu12123612
APA StyleKim, J., Kim, C.-S., Jo, K., Lee, I. S., Kim, J.-H., & Kim, J. S. (2020). POCU1b, the n-Butanol Soluble Fraction of Polygoni Cuspidati Rhizoma et Radix, Attenuates Obesity, Non-Alcoholic Fatty Liver, and Insulin Resistance via Inhibitions of Pancreatic Lipase, cAMP-Dependent PDE Activity, AMPK Activation, and SOCS-3 Suppression. Nutrients, 12(12), 3612. https://doi.org/10.3390/nu12123612






