Hybrid Dietary-Blood Inflammatory Profiles and Postmenopausal Breast Cancer: A Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
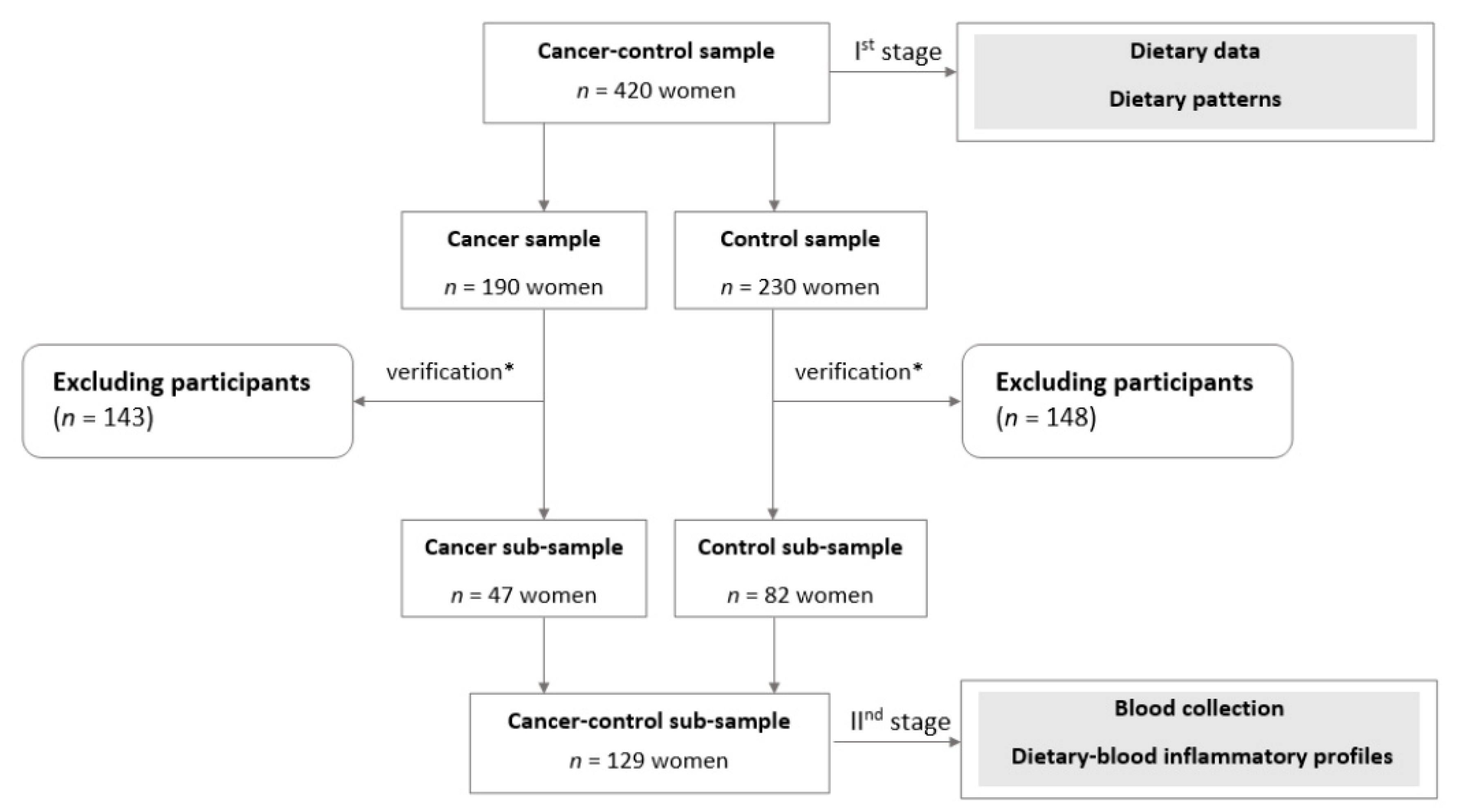
2.2. Study Design and Sample Collection
2.3. Dietary Data
2.4. Blood Markers
2.5. Hybrid Dietary-Blood Inflammatory Profiles
2.6. Statistical Analysis
3. Results
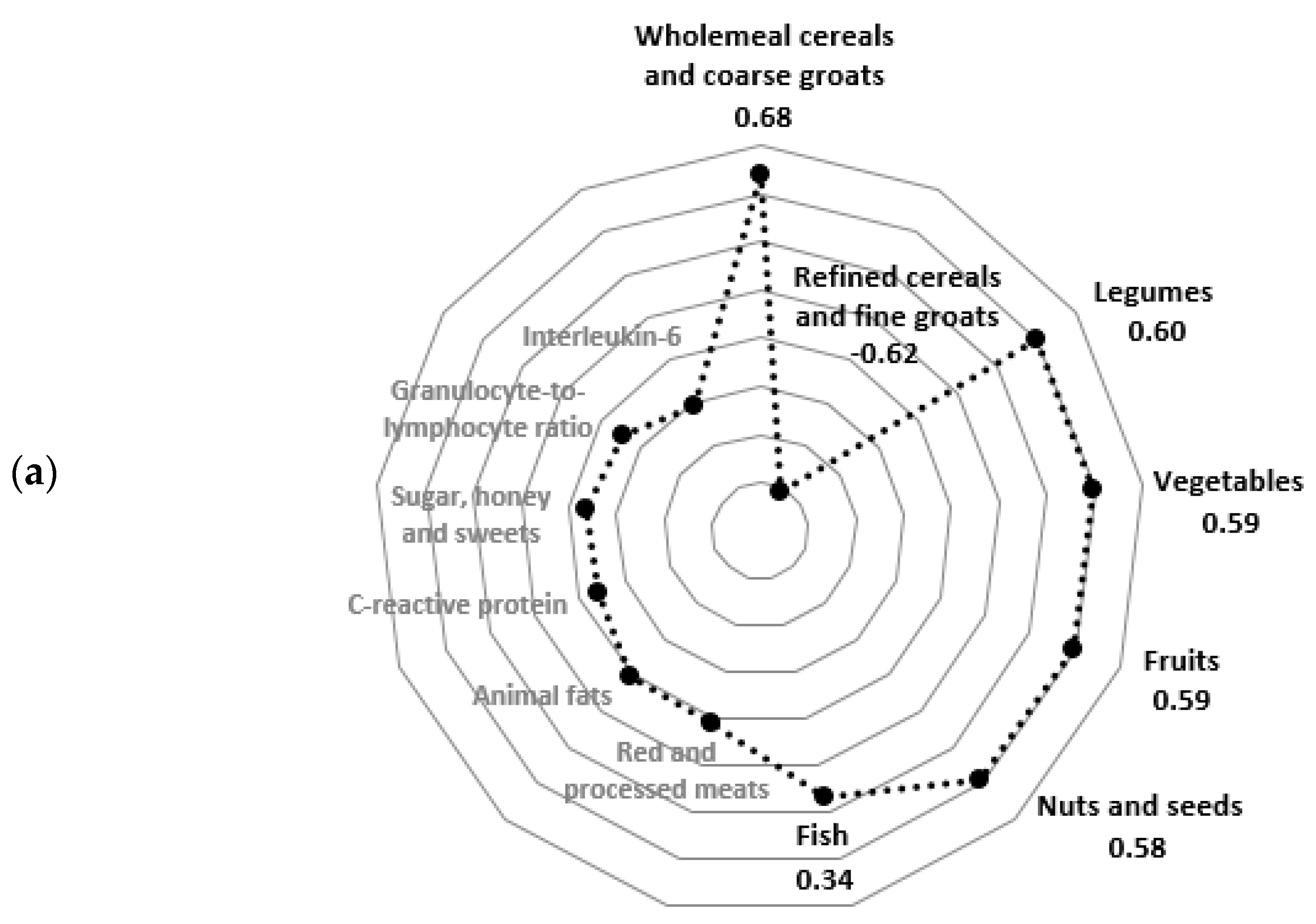
3.1. Hybrid Dietary-Blood Inflammatory Profiles
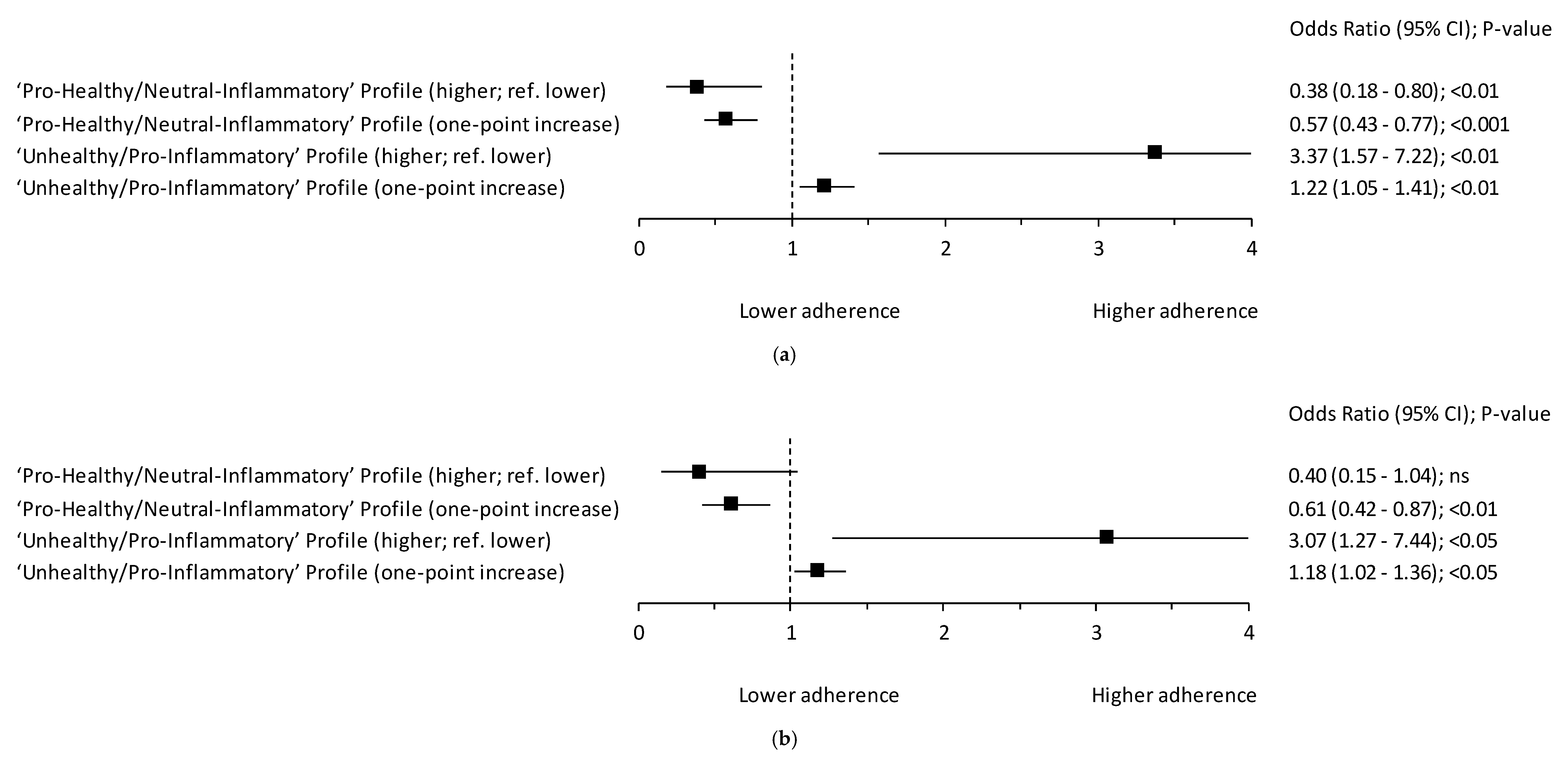
3.2. Hybrid Dietary-Blood Inflammatory Profiles and Breast Cancer
4. Discussion
4.1. ”Unhealthy/Pro-inflammatory” Profile and Breast Cancer
4.2. Inflammatory Markers and Breast Cancer
4.3. “Pro-Healthy/Neutral-Inflammatory” Profile and Breast Cancer
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 12 September 2018).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Krajowy Rejestr Nowotworów, Centrum Onkologii—Instytut im. Marii Skłodowskiej—Curie (Polish National Cancer Registry, Oncology Centre. Institute of M. Sklodowska-Curie). Available online: http://onkologia.org.pl/k/epidemiologia/ (accessed on 12 July 2020).
- World Cancer Research Fund; American Institute for Cancer Research. Diet, Nutrition, Physical Activity, and Breast Cancer 2017. Available online: https://www.wcrf.org/sites/default/files/Breast-Cancer-2017-Report.pdf (accessed on 12 July 2020).
- Tao, Z.; Shi, A.; Lu, C.; Song, T.; Zhang, Z.; Zhao, J. Breast Cancer: Epidemiology and Etiology. Cell Biophys. 2015, 72, 333–338. [Google Scholar] [CrossRef]
- Baniyash, M.; Sade-Feldman, M.; Kanterman, J. Chronic inflammation and cancer: Suppressing the suppressors. Cancer Immunol. Immunother. 2013, 63, 11–20. [Google Scholar] [CrossRef]
- Gunter, M.J.; Wang, T.; Cushman, M.; Xue, X.; Wassertheil-Smoller, S.; Strickler, H.D.; Rohan, T.E.; Manson, J.E.; McTiernan, A.; Kaplan, R.C.; et al. Circulating Adipokines and Inflammatory Markers and Postmenopausal Breast Cancer Risk. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Agnoli, C.; Grioni, S.; Pala, V.; Allione, A.; Matullo, G.; Di Gaetano, C.; Tagliabue, G.; Sieri, S.; Krogh, V. Biomarkers of inflammation and breast cancer risk: A case-control study nested in the EPIC-Varese cohort. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Larouche, D.; Hanna, M.; Chang, S.-L.; Jacob, S.; Têtu, B.; Diorio, C. Evaluation of Antioxidant Intakes in Relation to Inflammatory Markers Expression Within the Normal Breast Tissue of Breast Cancer Patients. Integr. Cancer Ther. 2016, 16, 485–495. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Chen, I.-C.; Zhou, X.K.; Giri, D.D.; Falcone, D.J.; Winston, L.A.; Wang, H.; Williams, S.; Lu, Y.-S.; Hsueh, T.-H.; et al. Adiposity, Inflammation, and Breast Cancer Pathogenesis in Asian Women. Cancer Prev. Res. 2017, 11, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Liu, S.; Zhang, S.; Chen, Q.; Zhang, M.; Quan, P.; Lu, J.; Sun, X. C-reactive protein and risk of breast cancer: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 10508. [Google Scholar] [CrossRef]
- Wang, J.; Lee, I.-M.; Tworoger, S.S.; Buring, J.E.; Ridker, P.M.; Rosner, B.; Hankinson, S.E. Plasma C-Reactive Protein and Risk of Breast Cancer in Two Prospective Studies and a Meta-analysis. Cancer Epidemiology Biomarkers Prev. 2015, 24, 1199–1206. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.S.M.; Bandera, E.V.; Greenwood, D.C.; Norat, T. Circulating C-Reactive Protein and Breast Cancer Risk--Systematic Literature Review and Meta-analysis of Prospective Cohort Studies. Cancer Epidemiology Biomarkers Prev. 2015, 24, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Nelson, S.H.; Brasky, T.M.; Patterson, R.E.; Laughlin, G.A.; Kritz-Silverstein, N.; Edwards, B.J.; Lane, R.; Rohan, T.E.; Ho, G.Y.; Manson, J.E.; et al. The Association of the C-Reactive Protein Inflammatory Biomarker with Breast Cancer Incidence and Mortality in the Women’s Health Initiative. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1100–1106. [Google Scholar] [CrossRef] [Green Version]
- Tobias, D.K.; Akinkuolie, A.O.; Chandler, P.D.; Lawler, P.R.; Manson, J.E.; Buring, J.E.; Ridker, P.M.; Wang, L.; Lee, I.-M.; Mora, S. Markers of Inflammation and Incident Breast Cancer Risk in the Women’s Health Study. Am. J. Epidemiol. 2017, 187, 705–716. [Google Scholar] [CrossRef] [Green Version]
- Allin, K.H.; Nordestgaard, B.G.; Flyger, H.; Bojesen, S.E. Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: A cohort study. Breast Cancer Res. 2011, 13, R55. [Google Scholar] [CrossRef] [Green Version]
- Proctor, M.; Talwar, D.; Balmar, S.M.; O’Reilly, D.S.J.; Foulis, A.K.; Horgan, P.G.; Morrison, D.S.; McMillan, D.C. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br. J. Cancer 2010, 103, 870–876. [Google Scholar] [CrossRef]
- Allin, K.H.; Bojesen, S.E.; Nordestgaard, B.G. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. Int. J. Cancer 2016, 139, 1493–1500. [Google Scholar] [CrossRef]
- Busch, E.L.; Whitsel, E.A.; Kroenke, C.H.; Yang, Y.C. Social relationships, inflammation markers, and breast cancer incidence in the Women’s Health Initiative. Breast 2018, 39, 63–69. [Google Scholar] [CrossRef]
- Wulaningsih, W.; Holmberg, L.; Abeler-Doner, L.; Ng, T.; Rohrmann, S.; Van Hemelrijck, M. Associations of C-Reactive Protein, Granulocytes and Granulocyte-to-Lymphocyte Ratio with Mortality from Breast Cancer in Non-Institutionalized American Women. PLOS ONE 2016, 11, e0157482. [Google Scholar] [CrossRef] [Green Version]
- Galland, L. Diet and Inflammation. Nutr. Clin. Pr. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Hermsdorff, H.H.M.; Zulet, M.; Abete, I.; Martínez, J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur. J. Nutr. 2010, 50, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.; Zulet, M.A.; Puchau, B.; Martínez, J.A. Fruit and vegetable consumption and proinflammatory gene expression from peripheral blood mononuclear cells in young adults: A translational study. Nutr. Metab. 2010, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas-Agustench, P.; López-Uriarte, P.; Bulló, M.; Ros, E.; Cabré-Vila, J.; Salas-Salvadó, J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Esmaillzadeh, A. Red Meat Intake Is Associated with Metabolic Syndrome and the Plasma C-Reactive Protein Concentration in Women. J. Nutr. 2008, 139, 335–339. [Google Scholar] [CrossRef]
- Shivappa, N.; Sandin, S.; Löf, M.; Hébert, J.R.; Adami, H.-O.; Weiderpass, E. Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br. J. Cancer 2015, 113, 1099–1103. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Hébert, J.R.; Rosato, V.; Montella, M.; Serraino, D.; La Vecchia, C. Association between the dietary inflammatory index and breast cancer in a large Italian case-control study. Mol. Nutr. Food Res. 2016, 61, 1600500. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Blair, C.K.; Prizment, A.E.; Jacobs, D.R.; Hébert, J.R. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-Q.; Mo, X.-F.; Ye, Y.-B.; Shivappa, N.; Lin, F.-Y.; Huang, J.; Hébert, J.R.; Yan, B.; Zhang, C.-X. A higher Dietary Inflammatory Index score is associated with a higher risk of breast cancer among Chinese women: A case–control study. Br. J. Nutr. 2017, 117, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Quiambao, A.L.; Lee, J.; Ro, J.; Lee, E.-S.; Jung, S.-Y.; Sung, M.-K.; Kim, J. Dietary Inflammatory Index and Risk of Breast Cancer Based on Hormone Receptor Status: A Case-Control Study in Korea. Nutrients 2019, 11, 1949. [Google Scholar] [CrossRef] [Green Version]
- Tabung, F.K.; Steck, S.E.; Liese, A.D.; Zhang, J.; Ma, Y.; Caan, B.; Chlebowski, R.T.; Freudenheim, J.L.; Hou, L.; Mossavar-Rahmani, Y.; et al. Association between dietary inflammatory potential and breast cancer incidence and death: Results from the Women’s Health Initiative. Br. J. Cancer 2016, 114, 1277–1285. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Liese, A.D.; Zhang, J.; Ma, Y.; Johnson, K.C.; Lane, D.S.; Qi, L.; Snetselaar, L.; Vitolins, M.Z.; et al. Patterns of change over time and history of the inflammatory potential of diet and risk of breast cancer among postmenopausal women. Breast Cancer Res. Treat. 2016, 159, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.-Y.; Gao, X.-P.; Zhu, S.; Liu, Y.-H.; Wang, L.; Jing, C.-X.; Zeng, F.-F. Dietary inflammatory index and risk of gynecological cancers: A systematic review and meta-analysis of observational studies. J. Gynecol. Oncol. 2019, 30, e23. [Google Scholar] [CrossRef] [PubMed]
- Norat, T.; Scoccianti, C.; Boutron-Ruault, M.-C.; Anderson, A.; Berrino, F.; Cecchini, M.; Espina, C.; Key, T.; Leitzmann, M.; Powers, H.; et al. European Code against Cancer 4th Edition: Diet and cancer. Cancer Epidemiol. 2015, 39, S56–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Wei, W.; Zhan, L. Red and processed meat intake and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2015, 151, 191–198. [Google Scholar] [CrossRef]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113, S102–S110. [Google Scholar] [CrossRef]
- Castello, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baenacanada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Munoz, M.T.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case–control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Krusinska, B.; Wadolowska, L.; Slowinska, M.A.; Biernacki, M.; Drozdowski, M.; Chadzynski, T. Associations of Dietary Patterns and Metabolic-Hormone Profiles with Breast Cancer Risk: A Case-Control Study. Nutrients 2018, 10, 2013. [Google Scholar] [CrossRef] [Green Version]
- Niedzwiedzka, E.; Wadolowska, L.; Kowalkowska, J. Reproducibility of A Non-Quantitative Food Frequency Questionnaire (62-Item FFQ-6) and PCA-Driven Dietary Pattern Identification in 13-21-Year-Old Females. Nutrients 2019, 11, 2183. [Google Scholar] [CrossRef] [Green Version]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. (Eds.) Clinical Methods: History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- International Committee for Standardization in Haematology; Expert Panel on Cytometry. The assignment of values to fresh blood used for calibrating automated blood cell counters. Clin. Lab. Haemat. 1988, 10, 203–212. [Google Scholar] [CrossRef]
- Armitage, P.; Berry, G.; Matthews, J.N.S. Statistical Methods in Medical Research, 4th ed.; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Zahedi, H.; Djalalinia, S.; Sadeghi, O.; Asayesh, H.; Noroozi, M.; Gorabi, A.M.; Mohammadi, R.; Qorbani, M. Dietary Inflammatory Potential Score and Risk of Breast Cancer: Systematic Review and Meta-analysis. Clin. Breast Cancer 2018, 18, e561–e570. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Azadbakht, L. Association between the dietary inflammatory index and the incidence of cancer: A systematic review and meta-analysis of prospective studies. Public Health 2018, 164, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ge, I.; Rudolph, A.; Shivappa, N.; Flesch-Janys, D.; Hébert, J.R.; Chang-Claude, J. Dietary inflammation potential and postmenopausal breast cancer risk in a German case-control study. Breast 2015, 24, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Emadi, A.; Shab-Bidar, S. Dietary Inflammatory Index and Site-Specific Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis. Adv. Nutr. 2018, 9, 388–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, K.W.; Weissfeld, J.L.; Modugno, F.; Diergaarde, B. Circulating levels of inflammatory markers and mammographic density among postmenopausal women. Breast Cancer Res. Treat. 2010, 127, 555–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.A.; Iyengar, N.M.; Zhou, X.K.; Gucalp, A.; Subbaramaiah, K.; Wang, H.; Giri, D.D.; Morrow, M.; Falcone, D.J.; Wendel, N.K.; et al. Menopause Is a Determinant of Breast Aromatase Expression and Its Associations With BMI, Inflammation, and Systemic Markers. J. Clin. Endocrinol. Metab. 2017, 102, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Link, L.B.; Canchola, A.J.; Bernstein, L.; A Clarke, C.; O Stram, D.; Ursin, G.; Horn-Ross, P.L. Dietary patterns and breast cancer risk in the California Teachers Study cohort. Am. J. Clin. Nutr. 2013, 98, 1524–1532. [Google Scholar] [CrossRef] [Green Version]
- Sieri, S.; Agnoli, C.; Pala, V.; Mattiello, A.; Panico, S.; Masala, G.; Assedi, M.; Tumino, R.; Frasca, G.; Sacerdote, C.; et al. Dietary habits and cancer: The experience of EPIC-Italy. Epidemiol. Prev. 2015, 39, 333–338. [Google Scholar]
- Baglietto, L.; Krishnan, K.; Severi, G.; Hodge, A.; Brinkman, M.; English, D.R.; McLean, C.; Hopper, J.L.; Giles, G.G. Dietary patterns and risk of breast cancer. Br. J. Cancer 2010, 104, 524–531. [Google Scholar] [CrossRef]
- Penniecook-Sawyers, J.A.; Jaceldo-Siegl, K.; Fan, J.; Beeson, L.; Knutsen, S.; Herring, P.; E Fraser, G. Vegetarian dietary patterns and the risk of breast cancer in a low-risk population. Br. J. Nutr. 2016, 115, 1790–1797. [Google Scholar] [CrossRef] [Green Version]
- Christodoulou, C.C.; Hadjisavvas, A.; Loizidou, M.A.; Loucaides, G.; Neophytou, I.; Sieri, S.; Kakouri, E.; Middleton, N.; Vineis, P.; Kyriacou, K. The mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: A case-control study. BMC Cancer 2012, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014, 12, 3597–3665. [CrossRef]
- Marciniak, W.; Derkacz, R.; Muszyńska, M.; Baszuk, P.; Gronwald, J.; Huzarski, T.; Cybulski, C.; Jakubowska, A.; Falco, M.; Dębniak, T.; et al. Blood arsenic levels and the risk of familial breast cancer in Poland. Int. J. Cancer 2020, 146, 2721–2727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Chatterji, U. Arsenic abrogates the estrogen-signaling pathway in the rat uterus. Reprod. Biol. Endocrinol. 2010, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.W.; Chung, K.C. Observational Studies: Cohort and Case-Control Studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. STEPS Sample Size Calculator and Sampling Spreadsheet. Available online: https://www.who.int/ncds/surveillance/steps/resources/sampling/en/ (accessed on 20 July 2019).

| Variable | Hybrid Dietary-Blood Inflammatory Profiles (Levels) | |||||
|---|---|---|---|---|---|---|
| “Pro-healthy/Neutral-inflammatory” | “Unhealthy/Pro-inflammatory” | |||||
| lower (<Me) | higher (≥Me) | p-Value | lower (<Me) | higher (≥Me) | p-Value | |
| Sample Size (n) | 64 | 65 | 64 | 65 | ||
| Frequency of food consumption (times/day) | ||||||
| Sugar, honey and sweets # | 1.82 (1.51; 2.14) | 1.61 (1.30; 1.91) | ns | 1.14 (0.92; 1.35) | 2.29 (1.96; 2.61) | <0.0001 |
| at least once a day | 50.0 | 53.8 | ns | 34.4 | 69.2 | <0.0001 |
| Wholemeal cereals, coarse groats # | 0.65 (0.50; 0.81) | 1.41 (1.24; 1.58) | <0.0001 | 1.08 (0.91; 1.25) | 0.99 (0.79; 1.19) | ns |
| at least once a day | 31.3 | 75.4 | <0.0001 | 56.3 | 50.8 | ns |
| Refined cereals and fine groats # | 1.16 (0.97; 1.34) | 0.46 (0.34; 0.57) | <0.0001 | 0.64 (0.47; 0.80) | 0.97 (0.80; 1.14) | 0.0015 |
| at least several times/week | 78.1 | 38.5 | <0.0001 | 48.4 | 67.7 | 0.0267 |
| Animal fats # | 1.11 (0.93; 1.29) | 1.26 (1.05; 1.48) | ns | 0.73 (0.61; 0.84) | 1.64 (1.44; 1.84) | <0.0001 |
| at least once a day | 46.9 | 53.8 | ns | 28.1 | 72.3 | <0.0001 |
| Fruits # | 0.71 (0.63; 0.78) | 1.14 (1.03; 1.25) | <0.0001 | 0.95 (0.85; 1.06) | 0.89 (0.79; 1.00) | ns |
| at least once a day | 32.8 | 89.2 | <0.0001 | 65.6 | 56.9 | ns |
| Vegetables # | 0.93 (0.83; 1.03) | 1.38 (1.25; 1.50) | <0.0001 | 1.17 (1.05; 1.29) | 1.15 (1.02; 1.28) | ns |
| at least once a day | 62.5 | 98.5 | <0.0001 | 84.4 | 76.9 | ns |
| Nuts and seeds # | 0.18 (0.11; 0.25) | 0.69 (0.52; 0.86) | <0.0001 | 0.54 (0.37; 0.71) | 0.34 (0.21; 0.46) | 0.0254 |
| at least several times/month | 43.8 | 78.5 | <0.0001 | 68.8 | 53.8 | ns |
| Legumes # | 0.09 (0.07; 0.11) | 0.26 (0.19; 0.33) | <0.0001 | 0.16 (0.10; 0.22) | 0.19 (0.14; 0.24) | ns |
| at least several times/month | 28.1 | 72.3 | <0.0001 | 42.2 | 58.5 | ns |
| Red and processed meats # | 1.00 (0.83; 1.16) | 1.17 (0.98; 1.36) | ns | 0.64 (0.51; 0.77) | 1.52 (1.36; 1.67) | <0.0001 |
| at least once a day | 45.3 | 55.4 | ns | 25.0 | 75.4 | <0.0001 |
| Fish # | 0.18 (0.13; 0.24) | 0.32 (0.24; 0.40) | 0.0326 | 0.31 (0.23; 0.39) | 0.20 (0.14; 0.25) | ns |
| at least several times/month | 50.0 | 63.1 | ns | 57.8 | 55.4 | ns |
| Blood concentration of inflammatory biomarkers | ||||||
| C-reactive protein (mg/L) # | 2.26 (1.25; 3.26) | 2.87 (1.25; 4.49) | ns | 1.23 (0.91; 1.54) | 3.89 (2.07; 5.70) | 0.0013 |
| ≥1.00 | 51.6 | 53.8 | ns | 43.8 | 61.5 | 0.0430 |
| Interleukin-6 (pg/mL) # | 3.30 (2.33; 4.27) | 2.82 (2.34; 3.29) | ns | 2.59 (2.26; 2.92) | 3.52 (2.51; 4.53) | ns |
| ≥2.30 | 54.7 | 47.7 | ns | 46.9 | 55.4 | ns |
| Leukocyte count (103 cells/µL) # | 6.27 (5.86; 6.67) | 6.22 (5.74; 6.69) | ns | 5.53 (5.23; 5.83) | 6.94 (6.45; 7.43) | <0.0001 |
| ≥5.95 | 57.8 | 43.1 | ns | 32.8 | 67.7 | <0.0001 |
| Granulocyte count (103 cells/µL) # | 3.77 (3.44; 4.10) | 3.70 (3.33; 4.07) | ns | 3.15 (2.89; 3.41) | 4.31 (3.94; 4.67) | <0.0001 |
| ≥3.56 | 56.3 | 44.6 | ns | 26.6 | 73.8 | <0.0001 |
| Neutrophil count (103 cells/µL) # | 3.60 (3.27; 3.93) | 3.45 (3.09; 3.80) | ns | 2.98 (2.72; 3.25) | 4.05 (3.69; 4.41) | <0.0001 |
| ≥3.31 | 54.7 | 46.2 | ns | 29.7 | 70.8 | <0.0001 |
| Agranulocyte count (103 cells/µL) # | 2.49 (2.31; 2.67) | 2.58 (2.40; 2.75) | ns | 2.38 (2.22; 2.53) | 2.69 (2.49; 2.88) | 0.0393 |
| ≥2.40 | 56.3 | 55.4 | ns | 51.6 | 60.0 | ns |
| Lymphocyte count (103 cells/µL) # | 2.02 (1.85; 2.19) | 2.13 (1.98; 2.28) | ns | 1.96 (1.81; 2.12) | 2.19 (2.02; 2.36) | ns |
| ≥2.00 | 50.0 | 50.8 | ns | 42.2 | 58.5 | ns |
| Granulocyte-to-Lymphocyte ratio # | 2.06 (1.83; 2.29) | 1.84 (1.63; 2.05) | ns | 1.79 (1.56; 2.03) | 2.10 (1.90; 2.31) | 0.0094 |
| ≥1.75 | 59.4 | 40.0 | 0.0278 | 35.9 | 63.1 | 0.0021 |
| Variable | Cancer-Control Sub-Sample | Cancer | Control | p-Value |
|---|---|---|---|---|
| Sub-Sample | Sub-Sample | |||
| Sample Size | 129 | 47 | 82 | |
| “Pro-healthy/Neutral-inflammatory” profile | ||||
| Score (points) # | 0.92 (0.62; 1.23) | 0.09 (−0.52; 0.70) | 1.42 (1.14; 1.70) | 0.0001 |
| levels | ||||
| lower (<Me) | 49.6 | 64.6 | 40.7 | 0.0089 |
| higher (≥Me) | 50.4 | 35.4 | 59.3 | |
| “Unhealthy/Pro-inflammatory” profile | ||||
| Score (points) # | 5.14 (4.34; 5.95) | 6.73 (4.83; 8.63) | 4.19 (3.65; 4.73) | 0.003 |
| levels | ||||
| lower (<Me) | 49.6 | 31.2 | 60.5 | 0.0013 |
| higher (≥Me) | 50.4 | 68.8 | 39.5 | |
| Blood concentration of inflammatory biomarkers | ||||
| C-reactive protein (mg/L) # | 2.58 (1.65; 3.52) | 3.85 (1.57; 6.13) | 1.80 (1.24; 2.36) | ns |
| ≥1.00 | 52.7 | 54 | 51.9 | ns |
| Interleukin-6 (pg/mL) # | 3.06 (2.53; 3.59) | 4.06 (2.75; 5.36) | 2.46 (2.18; 2.74) | 0.006 |
| ≥2.30 | 51.5 | 61.2 | 45.7 | ns |
| Leukocyte count (103 cells/µL) # | 6.26 (5.95; 6.57) | 7.10 (6.48; 7.73) | 5.74 (5.46; 6.02) | 0.0003 |
| ≥5.95 | 50.4 | 66 | 40.7 | 0.005 |
| Absolute granulocyte count (103 cells/µL) # | 3.73 (3.49; 3.97) | 4.51 (4.06; 4.95) | 3.26 (3.02; 3.49) | <0.0001 |
| ≥3.56 | 50 | 77.6 | 33.3 | <0.0001 |
| Neutrophil count (103 cells/µL) # | 3.54 (3.30; 3.78) | 4.39 (3.95; 4.82) | 3.02 (2.80; 3.23) | <0.0001 |
| ≥3.31 | 50.4 | 78 | 33.3 | <0.0001 |
| Absolute agranulocyte count (103 cells/µL) # | 2.53 (2.41; 2.66) | 2.55 (2.32; 2.79) | 2.52 (2.38; 2.67) | ns |
| ≥2.40 | 55.7 | 56 | 55.6 | ns |
| Lymphocyte count (103 cells/µL) # | 2.08 (1.96; 2.19) | 2.04 (1.84; 2.25) | 2.10 (1.96; 2.24) | ns |
| ≥2.00 | 50.4 | 48 | 51.9 | ns |
| Granulocyte-to-Lymphocyte (G/L) ratio # | 1.95 (1.80; 2.10) | 2.36 (2.12; 2.61) | 1.70 (1.52; 1.88) | <0.0001 |
| ≥1.75 | 50 | 73.5 | 35.8 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiewicz, B.; Wadolowska, L.; Biernacki, M.; Slowinska, M.A.; Drozdowski, M. Hybrid Dietary-Blood Inflammatory Profiles and Postmenopausal Breast Cancer: A Case-Control Study. Nutrients 2020, 12, 3503. https://doi.org/10.3390/nu12113503
Stasiewicz B, Wadolowska L, Biernacki M, Slowinska MA, Drozdowski M. Hybrid Dietary-Blood Inflammatory Profiles and Postmenopausal Breast Cancer: A Case-Control Study. Nutrients. 2020; 12(11):3503. https://doi.org/10.3390/nu12113503
Chicago/Turabian StyleStasiewicz, Beata, Lidia Wadolowska, Maciej Biernacki, Malgorzata Anna Slowinska, and Marek Drozdowski. 2020. "Hybrid Dietary-Blood Inflammatory Profiles and Postmenopausal Breast Cancer: A Case-Control Study" Nutrients 12, no. 11: 3503. https://doi.org/10.3390/nu12113503






