Association of Vegetable and Animal Flesh Intake with Inflammation in Pregnant Women from India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Ethics Statement
2.3. Data Collection
2.4. Laboratory Assessments
2.5. Statistical Analyses
3. Results
3.1. Study Population and Characteristics
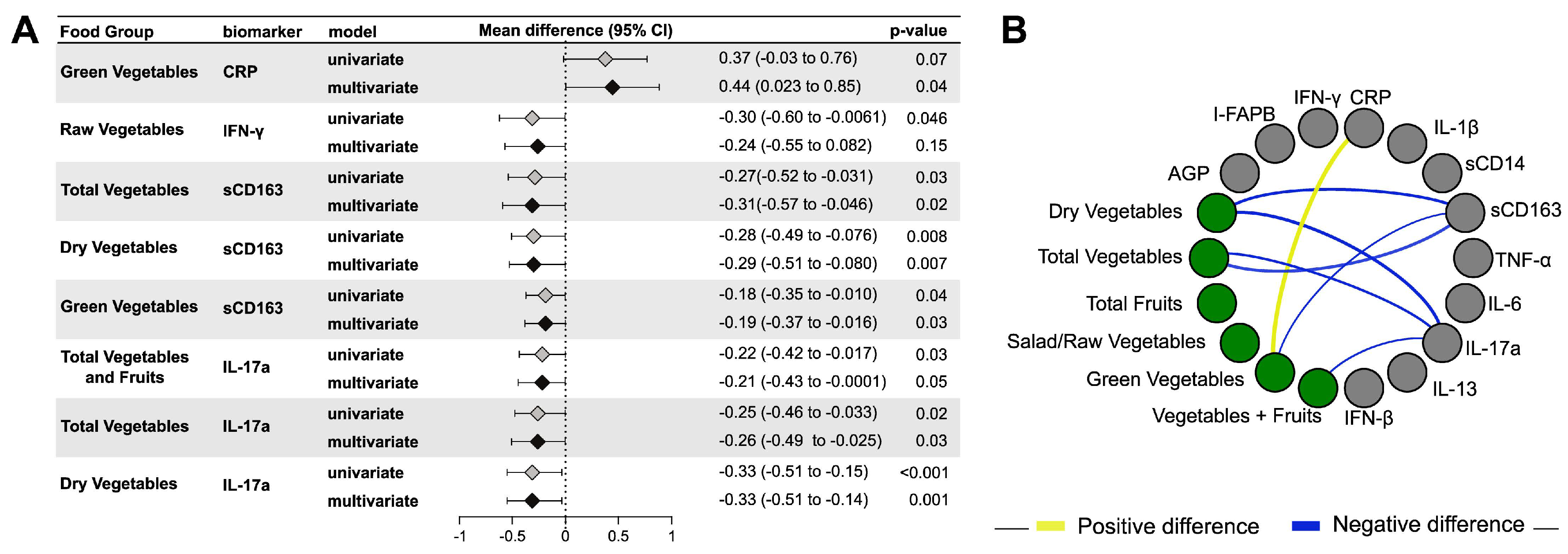
3.2. Vegetable/Fruit Intake and Maternal Inflammation
3.3. Animal Flesh Intake and Maternal Inflammation
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ershler, W.B.; Keller, E.T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000, 51, 245–270. [Google Scholar] [CrossRef]
- Kieolt-Glaser, J.K.; McGuire, L.; Robles, T.F.; Glaser, R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annu Rev. Psychol. 2002, 53, 83–107. [Google Scholar] [CrossRef]
- Catov, J.M.; Bodnar, L.M.; Ness, R.B.; Barron, S.J.; Roberts, J.M. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am. J. Epidemiol. 2007, 166, 1312–1319. [Google Scholar] [CrossRef]
- Bartha, J.L.; Fernandez-Deudero, A.; Bugatto, F.; Fajardo-Exposito, M.A.; Gonzalez-Gonzalez, N.; Hervias-Vivancos, B. Inflammation and cardiovascular risk in women with preterm labor. J. Womens Health 2012, 21, 643–648. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef]
- Calleja-Agius, J.; Jauniaux, E.; Pizzey, A.R.; Muttukrishna, S. Investigation of systemic inflammatory response in first trimester pregnancy failure. Hum. Reprod. 2012, 27, 349–357. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Vikki, A.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns and markers of systemic inflammation among Iranian women. J. Nutr. 2007, 137, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Vegetarian-Based Dietary Patterns and their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 433–451. [Google Scholar] [CrossRef]
- Montonen, J.; Boeing, H.; Fritsche, A.; Schleicher, E.; Joost, H.-G.; Schulze, M.; Steffen, A.; Pischon, T. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur. J. Nutr. 2013, 52, 337–345. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Liu, K.; Daviglus, M.L.; Jenny, N.S.; Mayer-Davis, E.; Jiang, R.; Steffen, L.; Siscovick, D.; Tsai, M.; Herrington, D. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2009, 103, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.A.; Miller, M.D.; Cobiac, L. Investigation of the effects of a high fish diet on inflammatory cytokines, blood pressure, and lipids in healthy older Australians. Food Nutr. Res. 2014, 58. [Google Scholar] [CrossRef]
- Chatzi, L.; Garcia, R.; Roumeliotaki, T.; Basterrechea, M.; Begiristain, H.; Iniguez, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. INMA Study group; RHEA study group. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br. J. Nutr. 2013, 110, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Srugo, S.A.; Bloise, E.; Nguyen, T.; Connor, K.L. Impact of Maternal Malnutrition on Gut Barrier Defense: Implications for Pregnancy Health and Fetal Development. Nutrients 2019, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, R.; Gupte, N.; Kumar, N.P.; Kulkarni, V.; Balasubramanian, U.; Bhosale, R.; Sambrey, P.; Kinikar, A.; Bharadwaj, R.; Patil, S.; et al. Intestinal Barrier Dysfunction and Microbial Translocation in Human Immunodeficiency Virus-Infected Pregnant Women Are Associated With Preterm Birth. Clin. Infect. Dis. 2018, 67, 1103–1109. [Google Scholar] [CrossRef]
- López, M.; Figueras, F.; Coll, O.; Goncé, A.; Hernández, S.; Loncá, M.; Vila, J.; Gratacós, E.; Palacio, M. Inflammatory Markers Related to Microbial Translocation Among HIV-Infected Pregnant Women: A Risk Factor of Preterm Delivery. J. Infect. Dis. 2016, 213, 343–350. [Google Scholar] [CrossRef]
- Ito, M.; Nakashima, A.; Hidaka, T.; Okabe, M.; Bac, N.D.; Ina, S.; Yoneda, S.; Shiozaki, A.; Sumi, S.; Tsuneyama, K.; et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J. Reprod. Immunol. 2010, 84, 75–85. [Google Scholar] [CrossRef]
- Muller, O.; Krawinkel, M. Malnutrition and health in developing countries. CMAJ 2005, 173, 279–286. [Google Scholar] [CrossRef]
- Mehta, S.; Murrill, M.; Suryavanshi, N.; Bhosale, R.; Naik, S.; Patil, N.; Gupta, A.; Mathad, J.; Shivakoti, R.; Alexander, M. Assessing TB-related knowledge and stigma among pregnant women in low resource settings of Pune, India. In Proceedings of the 50th Union World Conference on Lung Health, Hyderabad, India, 30 October–2 November 2019. [Google Scholar]
- Fakier, A.; Petro, G.; Fawcus, S. Mid-upper arm circumference: A surrogate for body mass index in pregnant women. S. Afr. Med. J. 2017, 107, 606–610. [Google Scholar] [CrossRef]
- Daniel, C.R.; Kapur, K.; McAdams, M.J.; Dixit-Joshi, S.; Devasenapathy, N.; Shetty, H.; Hariharan, S.; George, P.S.; Mathew, A.; Sinha, R. Development of a field-friendly automated dietary assessment tool and nutrient database for India. Br. J. Nutr. 2014, 111, 160–171. [Google Scholar] [CrossRef]
- Rajagopalan, K.; Alexander, M.; Naik, S.; Patil, N.; Mehta, S.; Leu, C.S.; Bhosale, R.; Mathad, J.; Caulfield, L.E.; Gupta, A.; et al. Validation of NINA-DISH food frequency questionnaire with multiple 24-hour dietary recalls among pregnant women in Pune, India. Br. J. Nutr. 2020. in review. [Google Scholar]
- Energy and Nutrient Composition of Food. 2011. Available online: https://focos.hpb.gov.sg/eservices/ENCF/ (accessed on 8 August 2020).
- McCance and Widdowson’s “Composition of Foods Integrated Dataset” on the Nutrient Content of the UK Food Supply. 2015. Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 8 August 2020).
- Food Surveys Research Group. 2015–2016 Food and Nutrient Database for Dietary Studies. 2018. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds-download-databases/ (accessed on 12 August 2020).
- USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov (accessed on 12 August 2020).
- AUSNUT 2011–2013 Food Nutrient Database. 2011–2013. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/ausnutdatafiles/Pages/foodnutrient.aspx (accessed on 12 August 2020).
- Institute of Medical Research. Malaysian Food Composition Database. 1997–2015. Available online: https://myfcd.moh.gov.my/ (accessed on 14 August 2020).
- National Cancer Institute. Learn More about Outliers. Available online: https://dietassessmentprimer.cancer.gov/learn/outliers.html (accessed on 5 June 2020).
- Frøslie, K.F.; Røislien, J.; Laake, P.; Henriksen, T.; Qvigstad, E.; Veierød, M.B. Categorisation of continuous exposure variables revisited. A response to the Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study. BMC Med. Res. Methodol. 2010, 10, 103. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Colditz, G.A.; Rosner, B.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Frequent nut consumption and risk of coronary heart disease in women: Prospective cohort study. Br. Med. J. 1998, 317, 1341–1345. [Google Scholar] [CrossRef]
- Daniel, C.R.; Cross, A.J.; Koebnick, C.; Sinha, R. Trends in meat consumption in the USA. Public Health Nutr. 2010, 14, 575–583. [Google Scholar] [CrossRef]
- Robertson, A.; Tirado, C.; Lobstein, T.; Jermini, M.; Knai, C.; Jensen, J.H.; Ferro-Luzzai, A.; James, W.P.T. Food and Health in Europe: A New Basic for Action; WHO Regional Publications European Series No. 96; WHO Regional Office for Europe: Copenhagen, Denmark, 2004. [Google Scholar]
- Martin, C.L.; Sotres-Alvarez, D.; Siega-Riz, A.M. Maternal Dietary Patterns during the Second Trimester Are Associated with Preterm Birth. J. Nutr. 2015, 145, 1857–1864. [Google Scholar] [CrossRef]
- Saunders, L.; Guldner, L.; Costet, N.; Kadhel, P.; Rouget, F.; Monfort, C.; Thome, J.P.; Multigner, L.; Cordier, S. Effect of a Mediterranean Diet during Pregnancy on Fetal Growth and Preterm Delivery: Results From a French Caribbean Mother-Child Cohort Study (TIMOUN). Paediatr. Perinat. Epidemiol. 2014, 28, 235–244. [Google Scholar] [CrossRef]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012, 93, 75–81. [Google Scholar] [CrossRef]
- Nakashima, A.; Ito, M.; Shima, T.; Bac, N.D.; Hidaka, T.; Saito, S. Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am. J. Reprod. Immunol. 2010, 64, 4–11. [Google Scholar] [CrossRef]
- Wang, W.J.; Hao, C.F.; Yi-Lin Yin, G.J.; Bao, S.H.; Qiu, L.H.; Lin, Q.D. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol. 2010, 84, 164–170. [Google Scholar] [CrossRef]
- Han, Y.Y.; Forno, E.; Brehm, J.M.; Acosta-Perez, E.; Alvarez, M.; Colon-Semidey, A.; Rivera-Soto, W.; Campos, H.; Litonjua, A.A.; Alcorn, J.F.; et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann. Allergy Asthma Immunol. 2015, 115, 288–293. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef]
- Faas, M.M.; Vos, P. Maternal monocytes in pregnancy and preeclampsia in humans and in rats. J. Reprod. Immunol. 2017, 119, 91–97. [Google Scholar] [CrossRef][Green Version]
- Bari, M.F.; Weickert, M.O.; Sivakumar, K.; James, S.G.; Snead, D.R.; Tan, B.K.; Randeva, H.S.; Bastie, C.C.; Vatish, M. Elevated soluble CD163 in gestational diabetes mellitus: Secretion from human placenta and adipose tissue. PLoS ONE 2014, 9, e101327. [Google Scholar] [CrossRef]
- Hu, T.Y.; Lee, S.Y.; Shih, C.K.; Chou, M.J.; Wu, M.C.; Teng, I.C.; Bai, C.H.; Sabrina, N.; Tinkov, A.A.; Skalny, A.V.; et al. Soluble CD163-Associated Dietary Patterns and the Risk of Metabolic Syndrome. Nutrients 2019, 11, 940. [Google Scholar] [CrossRef]
- Unruh, D.; Srinivasan, R.; Benson, T.; Haigh, S.; Coyle, D.; Batra, N.; Keil, R.; Sturm, R.; Blanco, V.; Palascak, M.; et al. Red Blood Cell Dysfunction Induced by High-Fat Diet: Potential Implications for Obesity-Related Atherosclerosis. Circulation 2015, 132, 1898–1908. [Google Scholar] [CrossRef]
- Chang, T.Y.; Liu, K.L.; Chang, C.S.; Su, C.T.; Chen, S.H.; Lee, Y.C.; Chang, J.S. Ferric Citrate Supplementation Reduces Red-Blood-Cell Aggregation and Improves CD163+ Macrophage-Mediated Hemoglobin Metabolism in a Rat Model of High-Fat-Diet-Induced Obesity. Mol. Nutr. Food. Res. 2018, 62. [Google Scholar] [CrossRef]
- Okita, M.; Sasagawa, T.; Kotani, M.; Hayashi, M.; Yamashita, H.; Kimoto, M.; Suzuki, K.; Tsuji, H.; Tabei, T. Green vegetable juice increases polyunsaturated fatty acid of erythrocyte membrane phospholipid in hypercholesterolaemic patients. Asia Pac. J. Clin. Nutr. 2000, 9, 309–313. [Google Scholar] [CrossRef]
- World Health Organization. WHO Healthy Diet; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Chen, C.W.; Lin, Y.L.; Lin, T.K.; Lin, C.T.; Chen, B.C.; Lin, C.L. Total cardiovascular risk profile of Taiwanese vegetarians. Eur. J. Clin. Nutr. 2008, 62, 138–144. [Google Scholar] [CrossRef]
- Franco-de-Moraes, A.C.; de Almeida-Pititto, B.; da Rocha Fernandes, G.; Gomes, E.P.; da Costa Pereira, A.; Ferreira, S.R.G. Worse inflammatory profile in omnivores than in vegetarians associates with the gut microbiota composition. Diabetol. Metab. Syndr. 2017, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Chen, Y.A.; Tuohy, K.M. A comparative in vitro investigation into the effects of cooked meats on the human faecal microbiota. Anaerobe 2010, 16, 572–577. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Doherty, T.M. Interleukin-13: A review. J. Immunol. Immunopharmacol. 1995, 15, 81. [Google Scholar]
- Feijen, M.; Gerritsen, J.; Postma, D.S. Genetics of allergic diseases. Br. Med. Bull. 2000, 56, 894–907. [Google Scholar] [CrossRef]
- Kull, I.; Bergstrom, A.; Lilja, G.; Pershagen, G.; Wickman, M. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy 2006, 61, 1009–1015. [Google Scholar] [CrossRef]
- Alm, B.; Aberg, N.; Erdes, L.; Mollborg, P.; Pettersson, R.; Norvenius, S.G.; Goksor, E.; Wennergren, G. Early introduction of fish decreases the risk of eczema in infants. Arch. Dis. Child. 2009, 94, 11–15. [Google Scholar] [CrossRef]

| Overall | |
|---|---|
| N (%) | |
| Age, median (range) | 24 (18–40) |
| Income | |
| ≤Rs. 10,255 | 64 (34.9) |
| >Rs. 10,255 | 120 (65.1) |
| Education | |
| None to primary | 43 (23.1) |
| Middle school to high school | 119 (63.9) |
| Post high school | 24 (12.9) |
| Mid-upper arm circumference | |
| <23 cm | 54 (29.0) |
| 23–30.5 cm | 117 (62.9) |
| >30.5 cm | 15 (8.1) |
| Current Smoker | |
| Yes | 21 (11.3) |
| No | 165 (88.7) |
| Gestational age at sampling, median (IQR) | 29.3 (28.5–30.6) |
| Preeclampsia | |
| Yes | 21 (11.3) |
| No | 165 (88.7) |
| Gestational Diabetes status | |
| Gestational diabetes | 18 (10.1) |
| No gestational diabetes | 165 (89.9) |
| LTBI | |
| Yes | 131 (70.4) |
| No | 55 (29.6) |
| HIV | |
| Yes | 58 (31. 2) |
| No | 128 (68.8) |
| Vegetarian | |
| Yes | 23 (12.4) |
| No | 163 (87.6) |
| (grams/day) | N | Median | IQR |
|---|---|---|---|
| Total vegetables and fruits | 186 | 432.0 | (322.5, 602.5) |
| Total vegetables | 186 | 324.0 | (244.4, 450.5) |
| Dry vegetables | 186 | 185.7 | (135.0, 260.3) |
| Green vegetables | 186 | 76.7 | (50.0, 105.5) |
| Raw vegetables/Salad | 186 | 57.6 | (36.0, 85.7) |
| Fruits | 186 | 79.8 | (27.4, 180.4) |
| Animal flesh (red meat + poultry + seafood) 1 | 157 | 74.4 | (45.4, 135.8) |
| Red Meat | 99 | 37.7 | (17.6, 50.3) |
| Poultry | 150 | 47.0 | (23.5, 50.4) |
| Seafood | 113 | 31.5 | (13.7, 54.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadana, S.; Talegawkar, S.A.; Mathad, J.S.; Alexander, M.; Rajagopalan, K.; Kumar, P.; Naik, S.; Leu, C.-S.; Kulkarni, V.; Deshpande, P.; et al. Association of Vegetable and Animal Flesh Intake with Inflammation in Pregnant Women from India. Nutrients 2020, 12, 3767. https://doi.org/10.3390/nu12123767
Yadana S, Talegawkar SA, Mathad JS, Alexander M, Rajagopalan K, Kumar P, Naik S, Leu C-S, Kulkarni V, Deshpande P, et al. Association of Vegetable and Animal Flesh Intake with Inflammation in Pregnant Women from India. Nutrients. 2020; 12(12):3767. https://doi.org/10.3390/nu12123767
Chicago/Turabian StyleYadana, Su, Sameera A. Talegawkar, Jyoti S. Mathad, Mallika Alexander, Kripa Rajagopalan, Pavan Kumar, Shilpa Naik, Cheng-Shiun Leu, Vandana Kulkarni, Prasad Deshpande, and et al. 2020. "Association of Vegetable and Animal Flesh Intake with Inflammation in Pregnant Women from India" Nutrients 12, no. 12: 3767. https://doi.org/10.3390/nu12123767
APA StyleYadana, S., Talegawkar, S. A., Mathad, J. S., Alexander, M., Rajagopalan, K., Kumar, P., Naik, S., Leu, C.-S., Kulkarni, V., Deshpande, P., Araujo-Pereira, M., Bhosale, R., Babu, S., Andrade, B. B., Caulfield, L. E., Gupta, A., & Shivakoti, R. (2020). Association of Vegetable and Animal Flesh Intake with Inflammation in Pregnant Women from India. Nutrients, 12(12), 3767. https://doi.org/10.3390/nu12123767






