Effects of 8 Weeks of 2S-Hesperidin Supplementation on Performance in Amateur Cyclists
Abstract
1. Introduction
2. Methodology
2.1. Participants
2.2. Study Design
2.3. Procedures
2.4. Testing
2.4.1. Medical Exam
2.4.2. Incremetal Test
2.4.3. Step Test
2.4.4. Wingate Test
2.4.5. Blood Samples
2.4.6. Urine Samples
2.5. Statistical Analysis
3. Results
3.1. Hesperidin Metabolites Urine
3.2. Incremental Test
3.3. Step Test
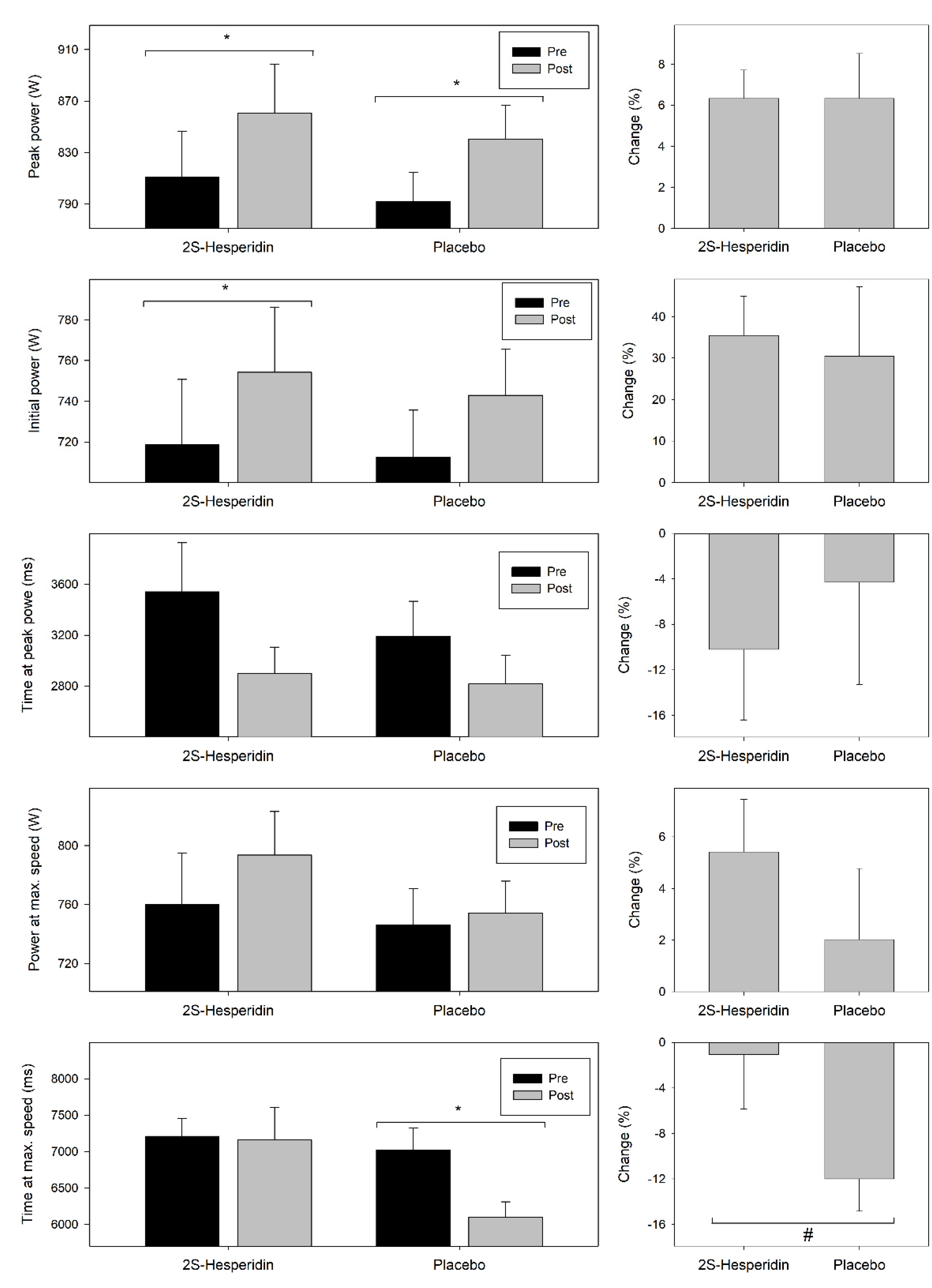
3.4. Wingate Test
4. Discussion
4.1. Incremental Test
4.2. Step Test
4.3. Wingate Test
4.4. Practical Applications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tomás-Barberán, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones–nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Yanez, J.A.; Andrews, P.K.; Davies, N.M. Methods of analysis and separation of chiral flavonoids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 848, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Aturki, Z.; Brandi, V.; Sinibaldi, M. Separation of Flavanone-7-O-glycoside Diastereomers and Analysis in Citrus Juices by Multidimensional Liquid Chromatography Coupled with Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 5303–5308. [Google Scholar] [CrossRef] [PubMed]
- Brand, W.; Shao, J.; Hoek-van den Hil, E.F.; van Elk, K.N.; Spenkelink, B.; de Haan, L.H.J.; Rein, M.J.; Dionisi, F.; Williamson, G.; van Bladeren, P.J.; et al. Stereoselective Conjugation, Transport and Bioactivity of S- and R-Hesperetin Enantiomers in Vitro. J. Agric. Food Chem. 2010, 58, 6119–6125. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Cai, L.; Hu, C.M.; Zhang, L. Suppression of adjuvant arthritis by hesperidin in rats and its mechanisms. J. Pharm. Pharmacol. 2008, 60, 221–228. [Google Scholar] [CrossRef]
- Jain, M.; Parmar, H.S. Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm. Res. 2011, 60, 483–491. [Google Scholar] [CrossRef]
- Ramelet, A.A. Clinical benefits of Daflon 500 mg in the most severe stages of chronic venous insufficiency. Angiology 2001, 52, S49–S56. [Google Scholar] [CrossRef]
- Li, D.; Mitsuhashi, S.; Ubukata, M. Protective effects of hesperidin derivatives and their stereoisomers against advanced glycation end-products formation. Pharm. Biol. 2012, 50, 1531–1535. [Google Scholar] [CrossRef]
- Martinez-Noguera, F.J.; Marin-Pagan, C.; Carlos-Vivas, J.; Rubio-Arias, J.A.; Alcaraz, P.E. Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists. Nutrients 2019, 11, 1898. [Google Scholar] [CrossRef] [PubMed]
- Pittaluga, M.; Sgadari, A.; Tavazzi, B.; Fantini, C.; Sabatini, S.; Ceci, R.; Amorini, A.M.; Parisi, P.; Caporossi, D. Exercise-induced oxidative stress in elderly subjects: The effect of red orange supplementation on the biochemical and cellular response to a single bout of intense physical activity. Free Radic. Res. 2013, 47, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Overdevest, E.; Wouters, J.A.; Wolfs, K.H.M.; van Leeuwen, J.J.M.; Possemiers, S. Citrus Flavonoid Supplementation Improves Exercise Performance in Trained Athletes. J. Sports Sci. Med. 2018, 17, 24–30. [Google Scholar] [PubMed]
- Best, R.; McDonald, K.; Hurst, P.; Pickering, C. Can taste be ergogenic? Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Biesemann, N.; Ried, J.S.; Ding-Pfennigdorff, D.; Dietrich, A.; Rudolph, C.; Hahn, S.; Hennerici, W.; Asbrand, C.; Leeuw, T.; Strübing, C. High throughput screening of mitochondrial bioenergetics in human differentiated myotubes identifies novel enhancers of muscle performance in aged mice. Sci. Rep. 2018, 8, 9408. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Dourado, G.K.; Cesar, T.B. Hesperidin associated with continuous and interval swimming improved biochemical and oxidative biomarkers in rats. J. Int. Soc. Sports Nutr. 2013, 10, 27. [Google Scholar] [CrossRef]
- Ruiz-Iglesias, P.; Estruel-Amades, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Franch, A.; Perez-Cano, F.J.; Castell, M. Influence of Hesperidin on Systemic Immunity of Rats Following an Intensive Training and Exhausting Exercise. Nutrients 2020, 12, 1291. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerdà, P.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients 2019, 11, 783. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Urbaniak, G.; Plous, S. Research Randomizer. Version 4.0. 2013. Available online: https://www.randomizer.org (accessed on 10 October 2020).
- Binder, R.K.; Wonisch, M.; Corra, U.; Cohen-Solal, A.; Vanhees, L.; Saner, H.; Schmid, J.P. Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 726–734. [Google Scholar] [CrossRef]
- Davis, J.A. Anaerobic threshold: Review of the concept and directions for future research. Med. Sci. Sports Exerc. 1985, 17, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Beaver, W.L.; Whipp, B.J. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 1990, 81, Ii14–Ii30. [Google Scholar] [PubMed]
- Gavin, T.P.; Van Meter, J.B.; Brophy, P.M.; Dubis, G.S.; Potts, K.N.; Hickner, R.C. Comparison of a Field-Based Test to Estimate Functional Threshold Power and Power Output at Lactate Threshold. J. Strength Cond. Res. 2012, 26, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.; Scott-Hamilton, J.; Hagstrom, A.D.; Gray, A.J. Cycling Power Outputs Predict Functional Threshold Power And Maximum Oxygen Uptake. J. Strength Cond. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A.; Tucker, W.J.; Sawyer, B.J.; Bhammar, D.M.; Angadi, S.S. Cycling efficiency and energy cost of walking in young and older adults. J. Appl. Physiol. 2018, 124, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, O. The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med. 1987, 4, 381–394. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Tomas-Navarro, M.; Vallejo, F.; Sentandreu, E.; Navarro, J.L.; Tomas-Barberan, F.A. Volunteer stratification is more relevant than technological treatment in orange juice flavanone bioavailability. J. Agric. Food Chem. 2014, 62, 24–27. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Loscalzo, J. Nitrite and Nitrate in Human Health and Disease; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Adefegha, S.A.; Rosa Leal, D.B.; Olabiyi, A.A.; Oboh, G.; Castilhos, L.G. Hesperidin attenuates inflammation and oxidative damage in pleural exudates and liver of rat model of pleurisy. Redox Rep. 2017, 22, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Murakami, Y.; Shoji, M.; Kadoma, Y.; Fujisawa, S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005, 25, 3367–3374. [Google Scholar] [PubMed]
- Liu, L.; Xu, D.M.; Cheng, Y.Y. Distinct effects of naringenin and hesperetin on nitric oxide production from endothelial cells. J. Agric. Food Chem. 2008, 56, 824–829. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.-A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus Polyphenol Hesperidin Stimulates Production of Nitric Oxide in Endothelial Cells while Improving Endothelial Function and Reducing Inflammatory Markers in Patients with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Hickner, R.C.; Fisher, J.S.; Ehsani, A.A.; Kohrt, W.M. Role of nitric oxide in skeletal muscle blood flow at rest and during dynamic exercise in humans. Am. J. Physiol. 1997, 273, H405–H410. [Google Scholar] [CrossRef]
- Elavarasan, J.; Velusamy, P.; Ganesan, T.; Ramakrishnan, S.K.; Rajasekaran, D.; Periandavan, K. Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart. J. Pharm. Pharmacol. 2012, 64, 1472–1482. [Google Scholar] [CrossRef]
- Clements, W.T.; Lee, S.R.; Bloomer, R.J. Nitrate ingestion: A review of the health and physical performance effects. Nutrients 2014, 6, 5224–5264. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef]
- Askari, G.; Ghiasvand, R.; Paknahad, Z.; Karimian, J.; Rabiee, K.; Sharifirad, G.; Feizi, A. The effects of quercetin supplementation on body composition, exercise performance and muscle damage indices in athletes. Int. J. Prev. Med. 2013, 4, 21–26. [Google Scholar]
- Decroix, L.; Soares, D.D.; Meeusen, R.; Heyman, E.; Tonoli, C. Cocoa Flavanol Supplementation and Exercise: A Systematic Review. Sports Med. 2018, 48, 867–892. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.J.; Menaspà, P.; Villerius, V.; Quod, M.; Peiffer, J.J.; Govus, A.D.; Abbiss, C.R. Within-Season Distribution of External Training and Racing Workload in Professional Male Road Cyclists. Int. J. Sports Physiol. Perform. 2017, 12, S2142–S2146. [Google Scholar] [CrossRef] [PubMed]
- Hüttemann, M.; Lee, I.; Malek, M.H. (-)-Epicatechin maintains endurance training adaptation in mice after 14 days of detraining. FASEB J. 2012, 26, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Shokri Afra, H.; Zangooei, M.; Meshkani, R.; Ghahremani, M.H.; Ilbeigi, D.; Khedri, A.; Shahmohamadnejad, S.; Khaghani, S.; Nourbakhsh, M. Hesperetin is a potent bioactivator that activates SIRT1-AMPK signaling pathway in HepG2 cells. J. Physiol. Biochem. 2019, 75, 125–133. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Chen, X.; Zhu, D.; Ma, J.; Yan, Y.; Si, M.; Li, X.; Sun, C.; Yang, B.; et al. Neohesperidin Exerts Lipid-Regulating Effects in vitro and in vivo via Fibroblast Growth Factor 21 and AMP-Activated Protein Kinase/Sirtuin Type 1/Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1α Signaling Axis. Pharmacology 2017, 100, 115–126. [Google Scholar] [CrossRef]
- Lantier, L.; Fentz, J.; Mounier, R.; Leclerc, J.; Treebak, J.T.; Pehmøller, C.; Sanz, N.; Sakakibara, I.; Saint-Amand, E.; Rimbaud, S.; et al. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J. 2014, 28, 3211–3224. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Eynon, N.; Alves, A.J.; Sagiv, M.; Yamin, C.; Sagiv, M.; Meckel, Y. Interaction between SNPs in the NRF2 gene and elite endurance performance. Physiol. Genom. 2010, 41, 78–81. [Google Scholar] [CrossRef]
- Bentley, D.J.; Ackerman, J.; Clifford, T.; Slattery, K.S. Acute and chronic effects of antioxidant supplementation on exercise performance. In Antioxidants in Sport Nutrition; CRC: Boca Raton, FL, USA, 2014; Volume 141. [Google Scholar]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef]
- Coyle, E.F.; Martin, W.H., 3rd; Sinacore, D.R.; Joyner, M.J.; Hagberg, J.M.; Holloszy, J.O. Time course of loss of adaptations after stopping prolonged intense endurance training. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 1984, 57, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Marroyo, J.A.; Villa, J.G.; Pernia, R.; Foster, C. Decrement in Professional Cyclists’ Performance After a Grand Tour. Int. J. Sports Physiol. Perform. 2017, 12, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.T.; Lazaro, C.M.; De Mateo, F.G.; Campos, M.C.; Mezencio, J.G.; Claudino, M.A.; de O Carvalho, P.; Webb, R.C.; Priviero, F.B. Effects of glucosyl-hesperidin and physical training on body weight, plasma lipids, oxidative status and vascular reactivity of rats fed with high-fat diet. Diabetes Metab. Syndr. Obes. 2018, 11, 321–332. [Google Scholar] [CrossRef]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Habib, J.J.; Pérez-López, A.; Vega, T.; Morales-Alamo, D.; et al. Enhancement of Exercise Performance by 48 Hours, and 15-Day Supplementation with Mangiferin and Luteolin in Men. Nutrients 2019, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Gelal, A.; Jacob, P., III; Yu, L.; Benowitz, N.L. Disposition kinetics and effects of menthol. Clin. Pharmacol. Ther. 1999, 66, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F. Placebo effects: From the neurobiological paradigm to translational implications. Neuron 2014, 84, 623–637. [Google Scholar] [CrossRef]
- Beedie, C.; Foad, A.; Hurst, P. Capitalizing on the placebo component of treatments. Curr. Sports Med. Rep. 2015, 14, 284–287. [Google Scholar] [CrossRef]
- Lok, R.; Zerbini, G.; Gordijn, M.C.M.; Beersma, D.G.M.; Hut, R.A. Gold, silver or bronze: Circadian variation strongly affects performance in Olympic athletes. Sci. Rep. 2020, 10, 16088. [Google Scholar] [CrossRef]
- Best, R.; Temm, D.; Hucker, H.; McDonald, K. Repeated Menthol Mouth Swilling Affects Neither Strength nor Power Performance. Sports 2020, 8, 90. [Google Scholar] [CrossRef]



| 2S-Hesperidin | Placebo | p-Value | |
|---|---|---|---|
| Age (years) | 35.0 (9.20) | 32.6 (8.90) | 0.407 |
| Body mass (kg) | 71.0 (6.98) | 70.4 (6.06) | 0.773 |
| Height (cm) | 175.3 (6.20) | 176.5 (6.10) | 0.541 |
| BMI (kg·m−2) | 23.1 (1.53) | 22.6 (1.43) | 0.292 |
| BF (%) | 8.9 (1.63) | 9.0 (1.64) | 0.803 |
| VO2MAX (L·min−1) | 3.99 (0.36) | 3.98 (0.63) | 0.971 |
| VO2MAX (mL·kg−1·min−1) | 57.5 (6.97) | 57.9 (9.53) | 0.880 |
| HRMAX (bpm) | 184.9 (11.11) | 183.2 (8.68) | 0.593 |
| VT1 (%) | 50.9 (5.63) | 50.0 (4.78) | 0.610 |
| VT2 (%) | 84.9 (5.85) | 84.1 (5.70) | 0.644 |
| Training variables | 2S-Hesperidin | Placebo | p-value |
| Total distance (km) | 1121.12 (534.99) | 1082.43 (810.46) | 0.868 |
| HRAVG (bpm) | 144.76 (8.88) | 137.48 (13.11) | 0.067 |
| WAVG (W) | 174.9 (15.79) | 163.5 (32.49) | 0.435 |
| RPE | 6.34 (0.82) | 6.33 (1.16) | 0.975 |
| Pre-Intervention | Post-Intervention | |||||
|---|---|---|---|---|---|---|
| 2S-Hesperidin | Placebo | p-Value | 2S-Hesperidin | Placebo | p-Value | |
| Kcal | 2163.6 (519.02) | 2100.2 (515.77) | 0.708 | 1974.1 (377.97) | 2133.5 (437.98) | 0.237 |
| Kcal/BM | 31.1 (9.34) | 30.2 (8.71) | 0.768 | 27.9 (6.53) | 30.3 (6.46) | 0.249 |
| CHO (g) | 245.7 (73.46) | 222.0 (69.68) | 0.312 | 216.6 (63.47) | 248.3 (58.15) | 0.117 |
| CHO/BM | 3.5 (1.31) | 3.2 (1.14) | 0.416 | 3.1 (1.08) | 3.5 (0.94) | 0.173 |
| PRO (g) | 113.5 (25.21) | 115.2 (25.37) | 0.837 | 109.0 (23.05) | 101.5 (23.67) | 0.332 |
| PRO/BM | 1.6 (0.41) | 1.7 (0.48) | 0.778 | 1.5 (0.35) | 1.5 (0.42) | 0.596 |
| LP (g) | 80.8 (27.24) | 83.5 (23.65) | 0.739 | 71.5 (17.61) | 71.6 (18.89) | 0.985 |
| LP/BM | 1.2 (0.45) | 1.2 (0.37) | 0.758 | 1.0 (0.27) | 1.0 (0.29) | 0.823 |
| 2S-Hesperidin | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | p-Value | Pre-Intervention | Post-Intervention | p-Value | ηp2 | ES | |
| FatMax | ||||||||
| VO2 (L·min−1) | 2.23 (0.50) | 2.02 (0.37) | 0.063 | 2.27 (0.48) | 2.10 (0.57) | 0.151 | 0.005 | 0.08 |
| VO2R (ml·kg−1·min−1) | 31.45 (6.17) | 28.54 (5.43) | 0.060 | 32.40 (6.82) | 29.51 (6.99) | 0.100 | 0.003 | 0.00 |
| CHO (g·min−1) | 2.20 (0.58) | 2.01 (0.37) | 0.169 | 2.20 (0.50) | 2.27 (0.56) | 0.521 | 0.090 | 0.47 |
| FAT (g·min−1) | 0.29 (0.90) | 0.26 (0.14) | 0.247 | 0.32 (0.14) | 0.21 (0.14) | 0.007 | 0.064 | 0.59 |
| Efficiency (%) | 26.68 (2.95) | 26.05 (3.90) | 0.411 | 26.94 (2.79) | 24.62 (2.27) | 0.010 | 0.064 | 0.49 |
| VT1 | ||||||||
| VO2 (L·min−1) | 2.19 (0.39) | 2.10 (0.35) | 0.396 | 2.10 (0.41) | 2.09 (0.47) | 0.961 | 0.001 | 0.17 |
| VO2R (ml·kg−1·min−1) | 31.05 (5.34) | 29.62 (5.20) | 0.357 | 29.96 (5.84) | 29.64 (6.37) | 0.824 | 0.001 | 0.17 |
| CHO (g·min−1) | 2.08 (0.47) | 2.07 (0.30) | 0.974 | 1.86 (0.47) | 2.19 (0.49) | 0.020 | 0.028 | 0.57 |
| FAT (g·min−1) | 0.31 (0.10) | 0.27 (0.15) | 0.184 | 0.35 (0.12) | 0.23 (0.14) | 0.003 | 0.044 | 0.53 |
| Efficiency (%) | 26.55 (2.62) | 25.25 (5.38) | 0.250 | 27.49 (3.25) | 25.86 (5.85) | 0.282 | <0.001 | 0.77 |
| VT2 | ||||||||
| VO2 (L·min−1) | 3.49 (0.43) | 3.36 (0.41) | 0.135 | 3.63 (0.52) | 3.33 (0.54) | 0.002 | 0.039 | 0.49 |
| VO2R (ml·kg−1·min−1) | 49.48 (6.83) | 48.25 (6.84) | 0.211 | 51.90 (8.17) | 47.29 (7.76) | 0.002 † | 0.084 | 0.67 |
| CHO (g·min−1) | 5.11 (1.18) | 5.42 (1.37) | 0.349 | 5.53 (1.45) | 5.25 (1.13) | 0.369 | 0.022 | 0.43 |
| FAT (g·min−1) | 0.04 (0.08) | 0.04 (0.09) | 1.000 | 0.02 (0.06) | 0.01 (0.03) | 0.334 | 0.048 | 0.03 |
| Efficiency (%) | 20.58 (3.09) | 19.65 (3.37) | 0.272 | 20.15 (2.25) | 20.20 (4.30) | 0.965 | 0.009 | 0.24 |
| 2S-Hesperidin | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | p-Value | Pre-Intervention | Post-Intervention | p-Value | ηp2 | ES | |
| Initial power absolute (W) | 718.8 (143.05) | 754.3 (143.09) | 0.001 * | 712.5 (103.46) | 743.0 (101.78) | 0.084 | 0.003 | 0.08 |
| Initial power relative (W) | 10.2 (1.82) | 10.6 (1.78) | 0.004 * | 10.1 (1.38) | 10.6 (1.29) | 0.078 | <0.001 | 0.01 |
| Absolute peak power (W) | 810.8 (160.26) | 860.6 (170.37) | <0.001 * | 792.0 (100.96) | 840.2 (118.93) | 0.016 * | <0.001 | 0.02 |
| Relative peak power (W) | 11.5 (2.04) | 12.1 (2.27) | 0.001 * | 11.3 (1.37) | 11.9 (1.49) | 0.014 * | <0.001 | 0.02 |
| Power at maximum speed (W) | 760.0 (156.45) | 793.5 (132.23) | 0.051 † | 746.3 (110.30) | 754.3 (96.14) | 0.709 | 0.044 | 0.30 |
| Time at peak power (ms) | 3541.4 (1722.52) | 2900.2 (923.99) | 0.052 † | 3193.4 (1218.48) | 2816.9 (1013.54) | 0.138 | 0.001 | 0.82 |
| Time at maximum speed (ms) | 7208.7 (1098.24) | 7157.9 (2005.11) | 0.888 | 7024.4 (1347.65) | 6095.2 (957.33) | 0.001 * | 0.119 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Noguera, F.J.; Marín-Pagán, C.; Carlos-Vivas, J.; Alcaraz, P.E. Effects of 8 Weeks of 2S-Hesperidin Supplementation on Performance in Amateur Cyclists. Nutrients 2020, 12, 3911. https://doi.org/10.3390/nu12123911
Martínez-Noguera FJ, Marín-Pagán C, Carlos-Vivas J, Alcaraz PE. Effects of 8 Weeks of 2S-Hesperidin Supplementation on Performance in Amateur Cyclists. Nutrients. 2020; 12(12):3911. https://doi.org/10.3390/nu12123911
Chicago/Turabian StyleMartínez-Noguera, Francisco Javier, Cristian Marín-Pagán, Jorge Carlos-Vivas, and Pedro E. Alcaraz. 2020. "Effects of 8 Weeks of 2S-Hesperidin Supplementation on Performance in Amateur Cyclists" Nutrients 12, no. 12: 3911. https://doi.org/10.3390/nu12123911
APA StyleMartínez-Noguera, F. J., Marín-Pagán, C., Carlos-Vivas, J., & Alcaraz, P. E. (2020). Effects of 8 Weeks of 2S-Hesperidin Supplementation on Performance in Amateur Cyclists. Nutrients, 12(12), 3911. https://doi.org/10.3390/nu12123911








