Walnut Polyphenol Extract Protects against Malathion- and Chlorpyrifos-Induced Immunotoxicity by Modulating TLRx-NOX-ROS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Animals
2.3. Preparation of Splenocytes
2.4. Preparation of the WPE
2.5. Cell Viability Assay
2.6. Flow Cytometry
2.7. ELISA
2.8. Antioxidant Enzyme Activities and Biomarkers of Oxidative Stress
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
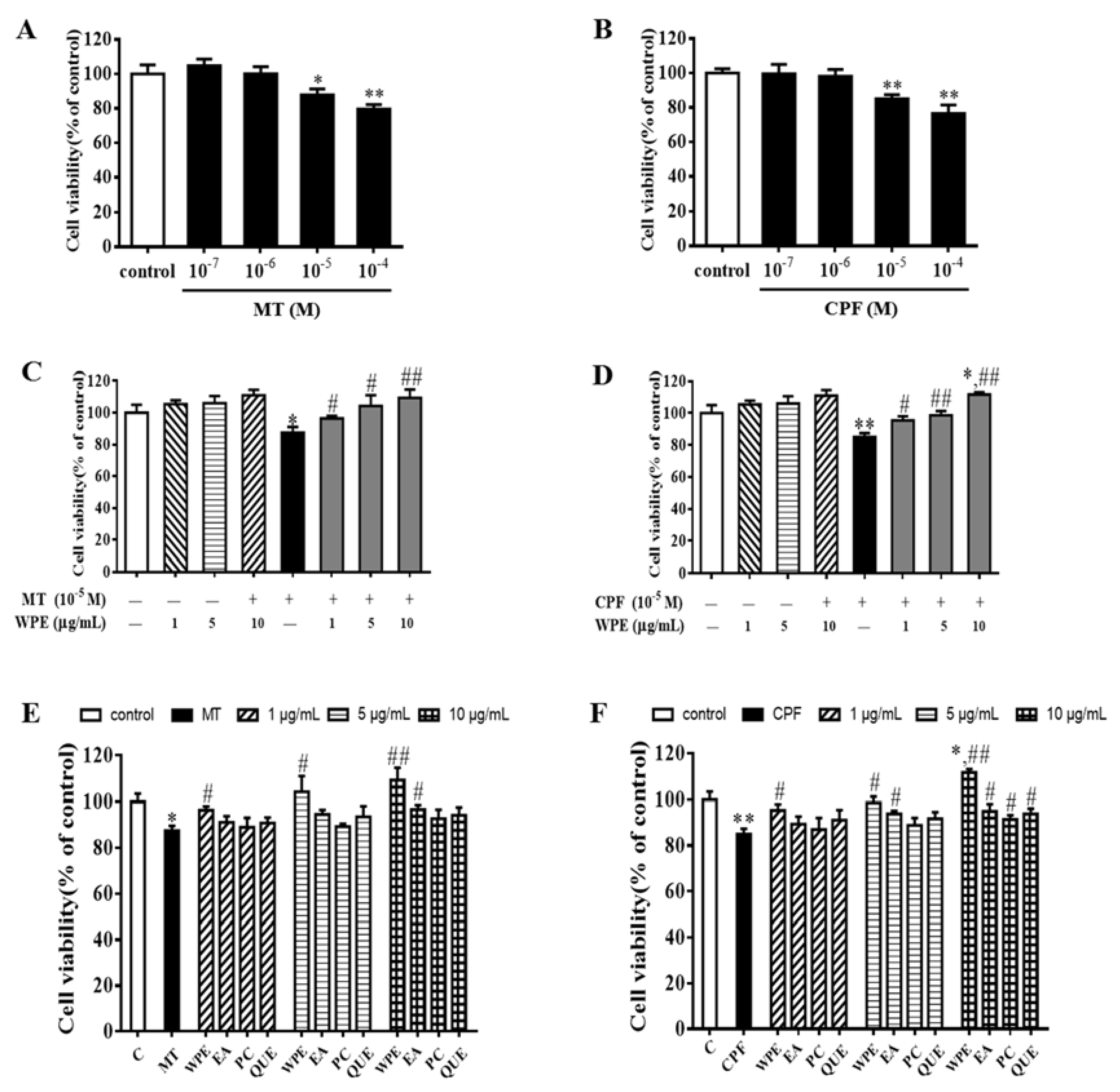
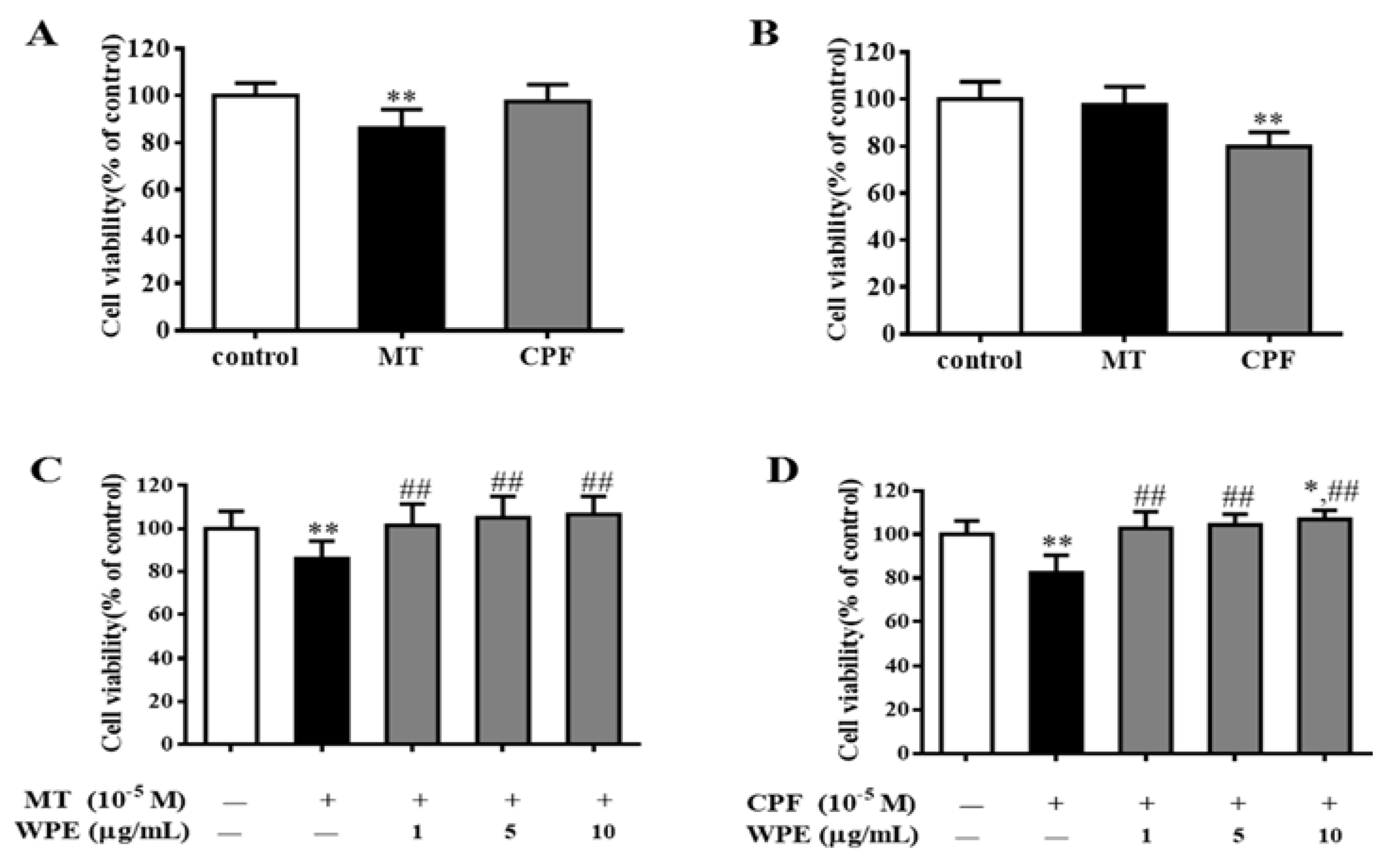
3.1. Effects of WPE on MT- and CPF-Induced Toxicity in Splenocytes
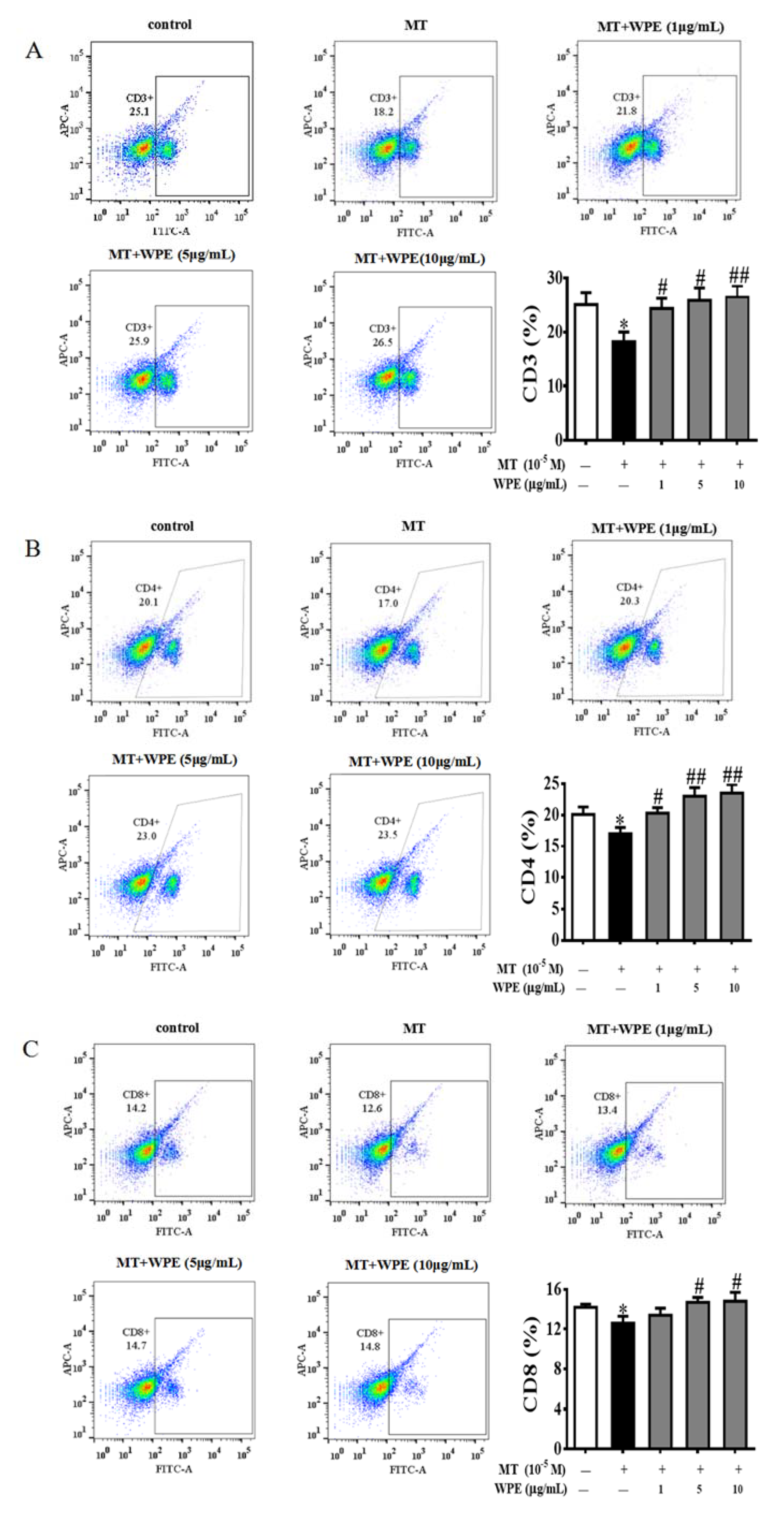
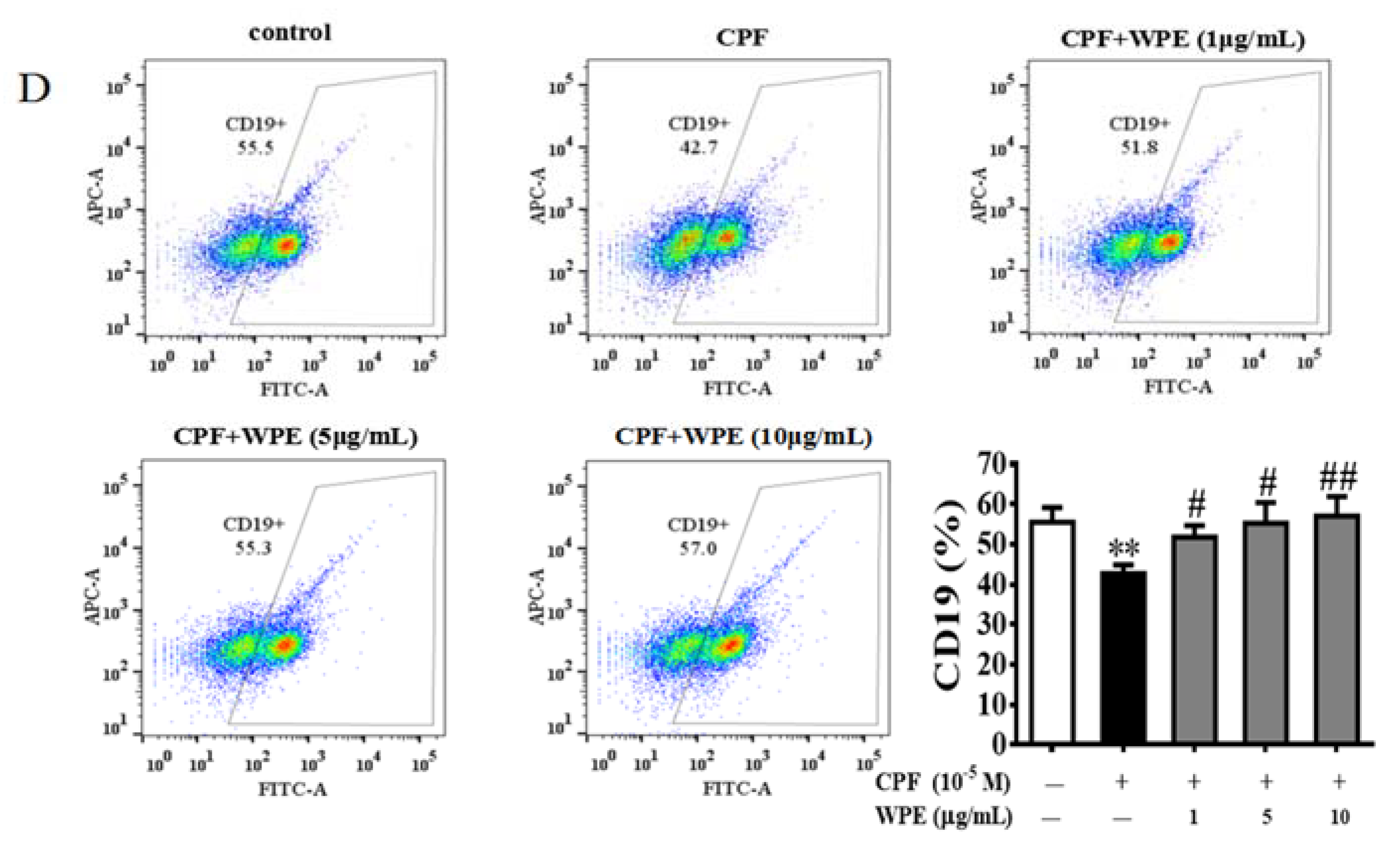
3.2. Effects of WPE on the Cytotoxicity of MT and CPF on Splenic Lymphocyte Subpopulations
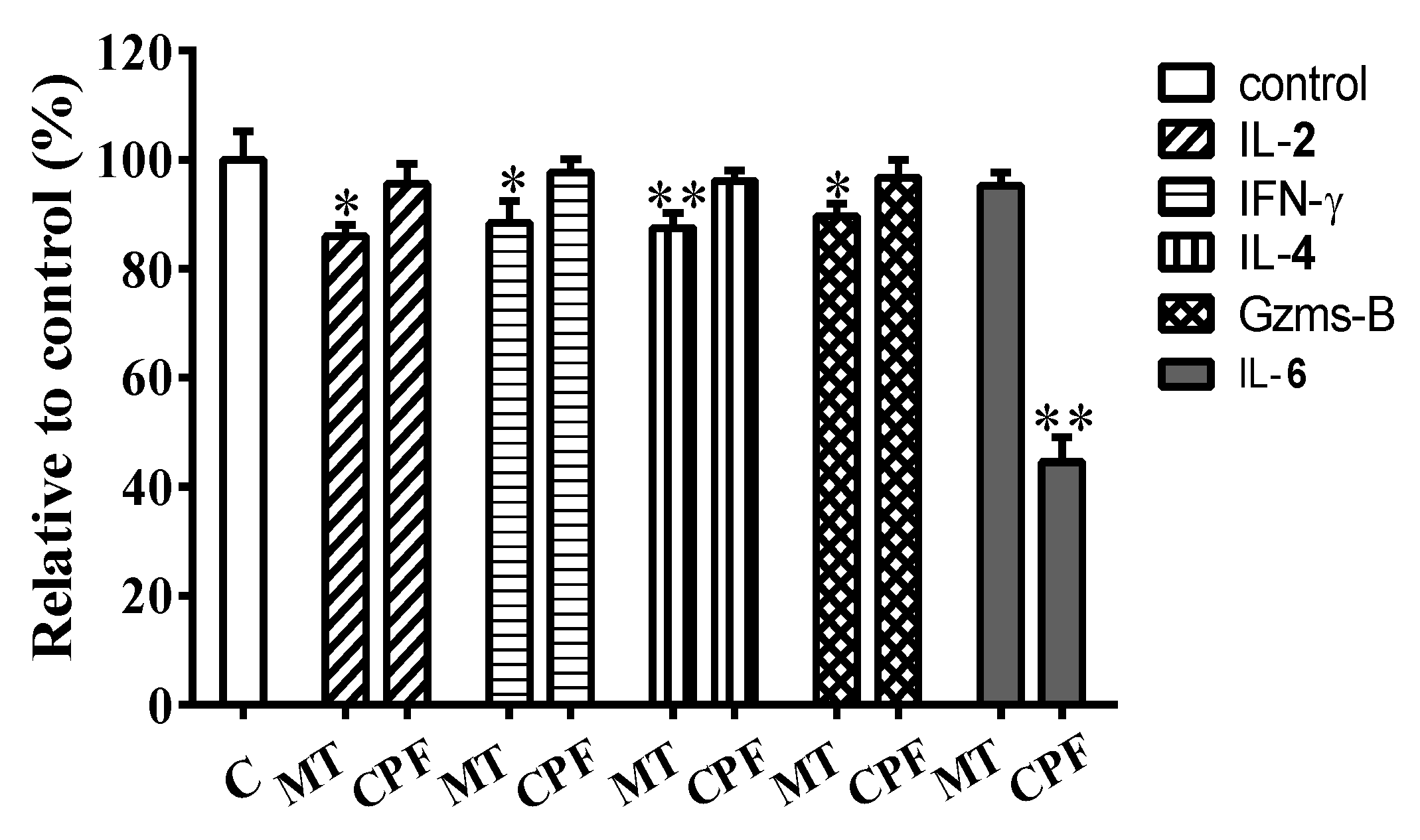
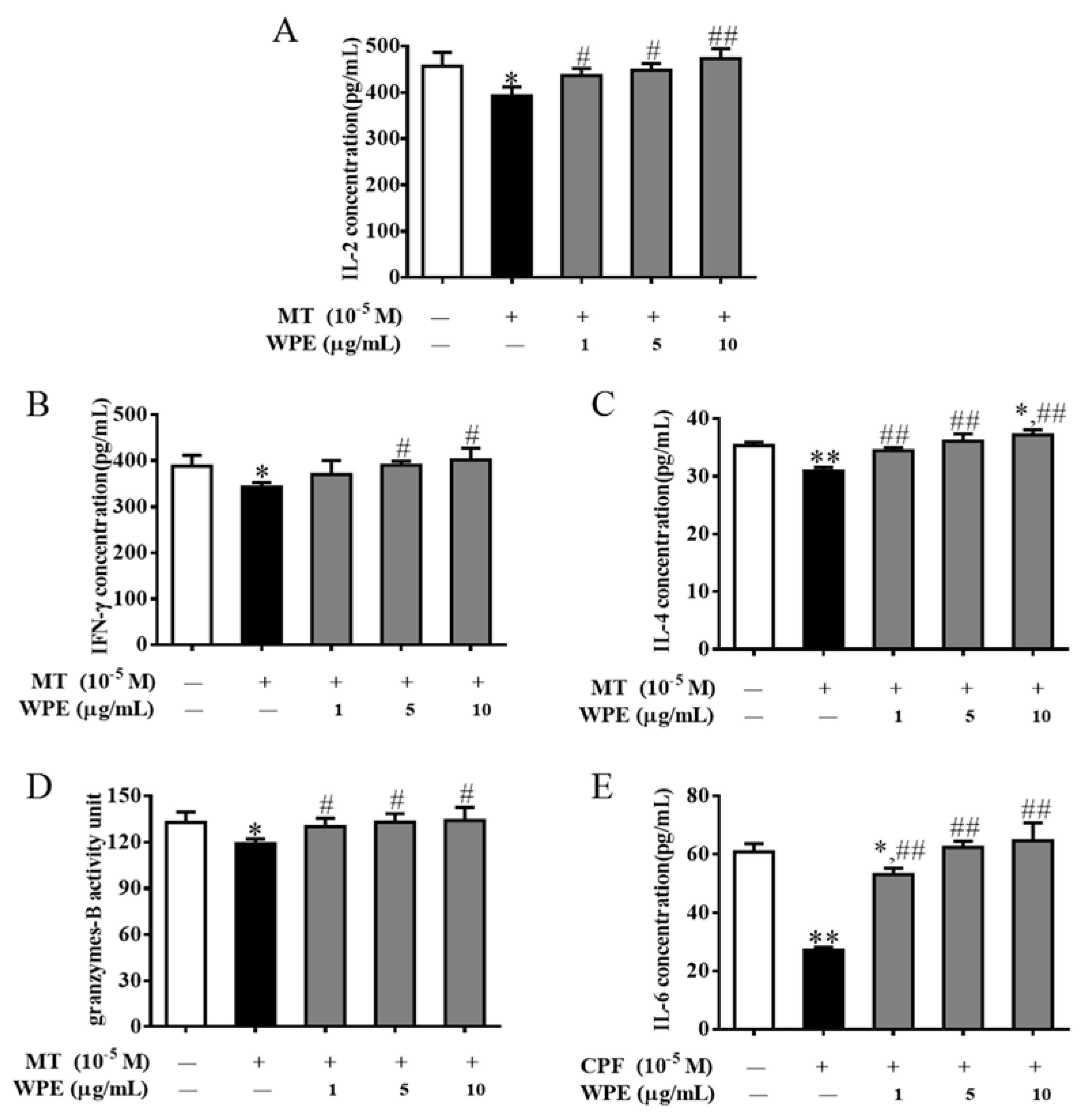
3.3. Effects of WPE on Cytokine Production by MT- or CPF-Stimulated Cells
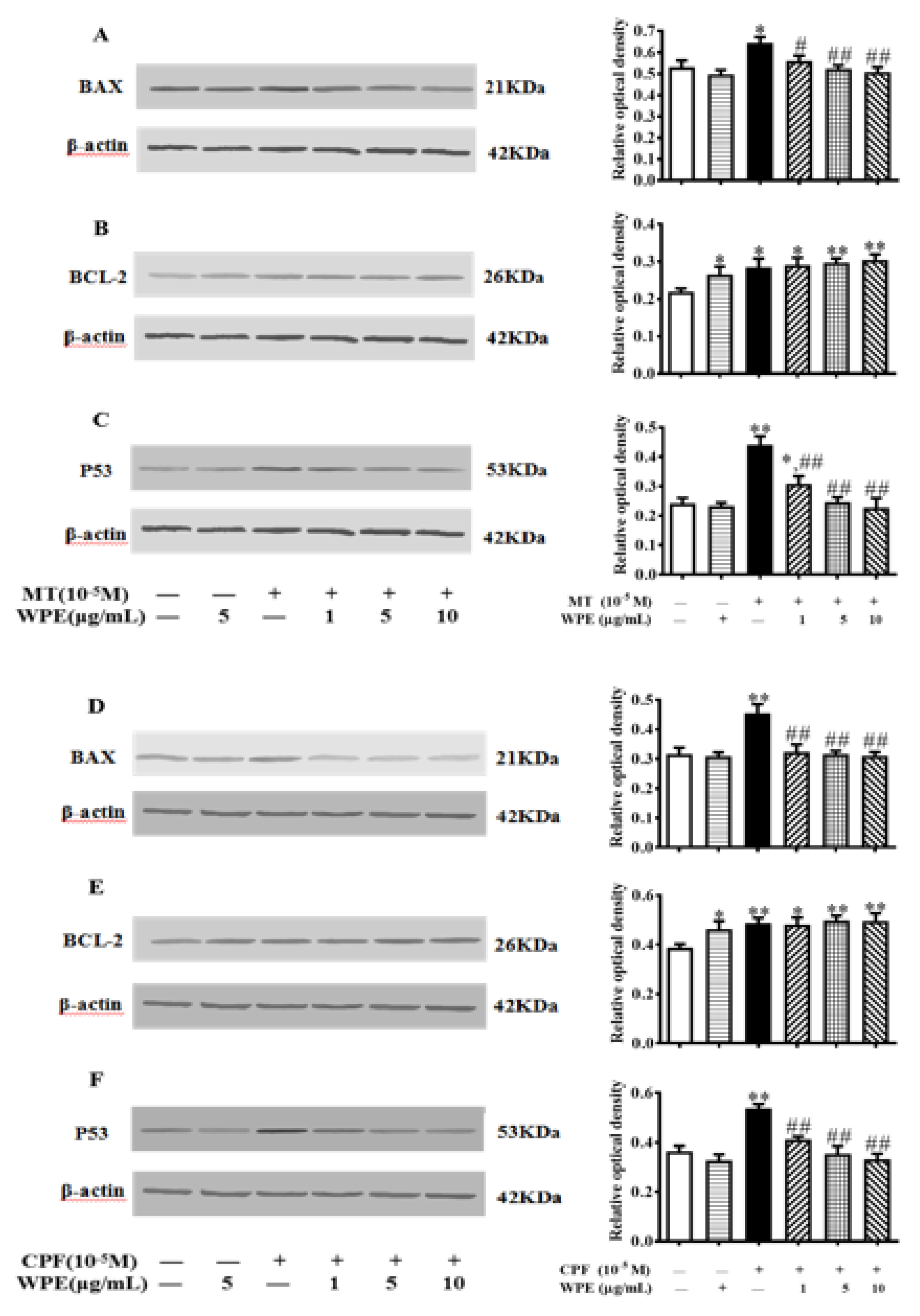
3.4. Effects of WPE on Levels of Apoptosis-Associated Proteins
3.5. Effects of WPE on the Production of ROS in MT- and CPF-Stimulated Cells
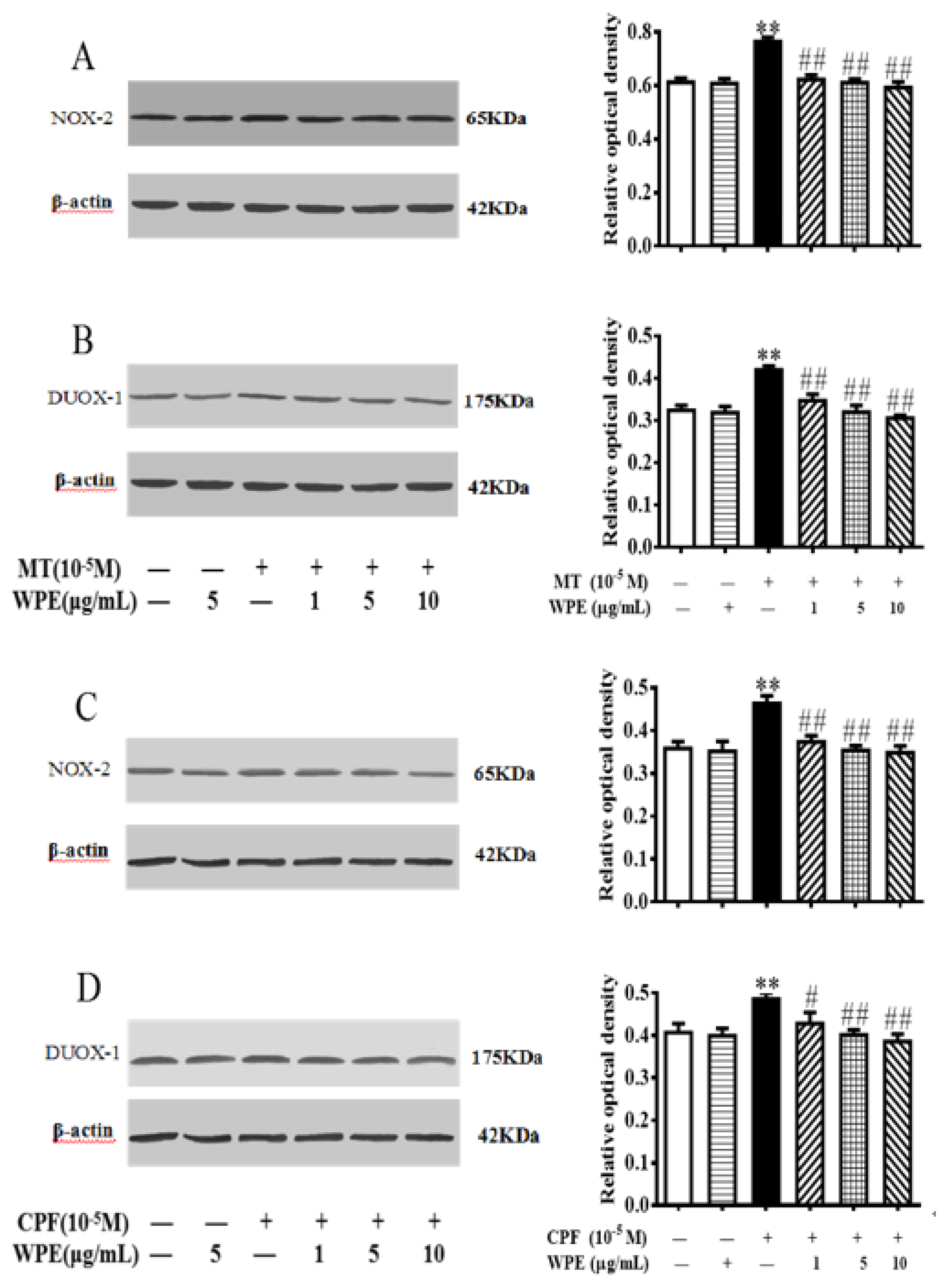
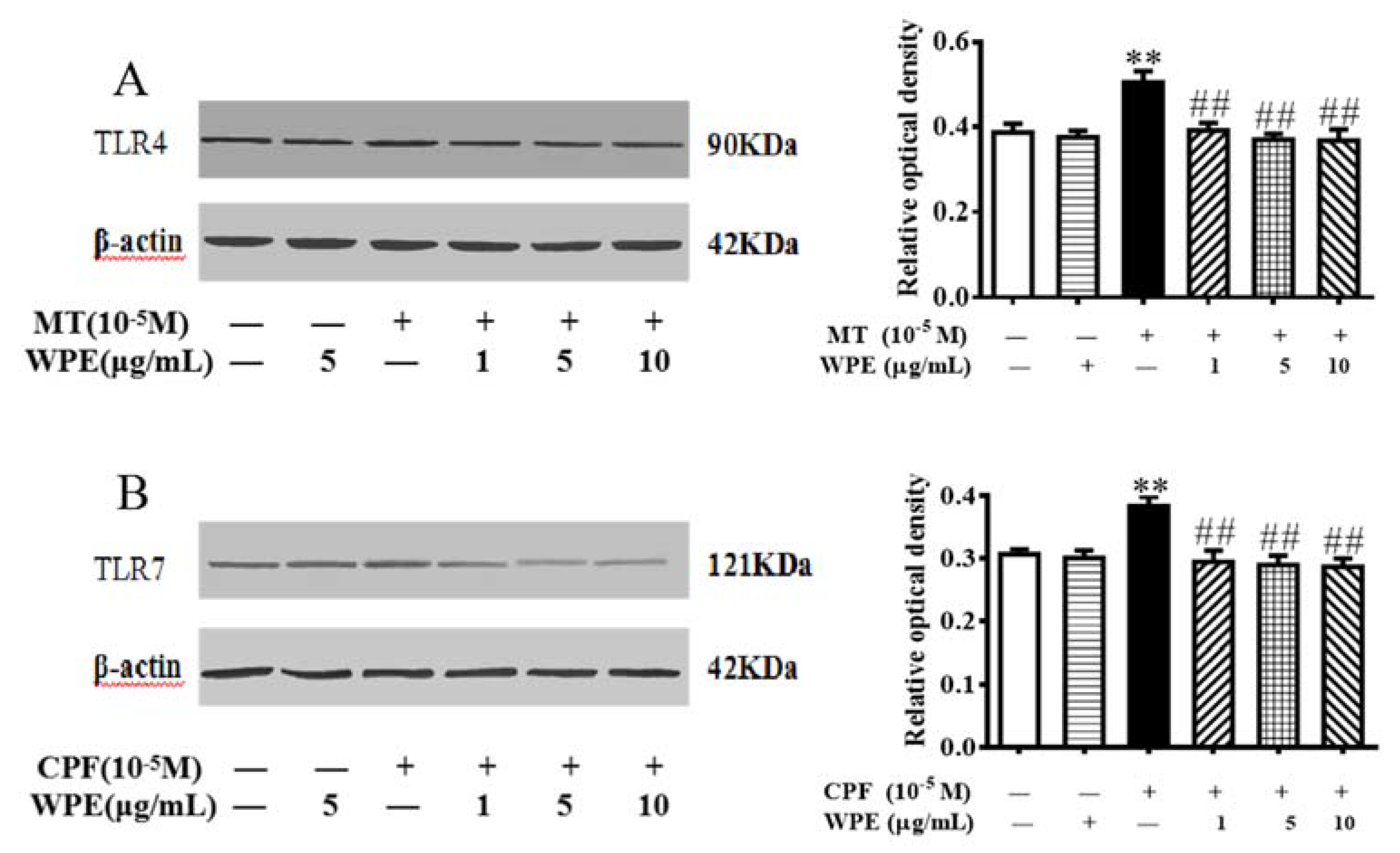
3.6. Effects of WPE on MT- or CPF-Induced NOX and TLR Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shapiro, H.; Micucci, S. Pesticide use for west Nile virus. Can. Med. Assoc. J. 2003, 289, 2719–2721. [Google Scholar]
- Saeed, G.; Maliheh, S.; Amir, A. Quercetin ameliorates chlorpyrifos-induced oxidative stress in the rat brain: Possible involvment of PON2 pathway. J. Food. Biochem. 2018, 42, e12530–e12537. [Google Scholar]
- Hernández, A.F.; Gómez, A.M.; Pérez, V.; García-Lario, J.V.; Pena, G.; Gil, F.; López, O.; Rodrigo, L.; Pino, G.; Pla, A. Influence of exposure to pesticides on serum components and enzyme activities of cytotoxicity among intensive agriculture farmers. Environ. Res. 2006, 102, 70–76. [Google Scholar] [CrossRef]
- Sodhi, S.; Sharma, A.; Brar, R.S. A protective effect of vitamin E and selenium in ameliorating the immunotoxicity of malathion in chicks. Vet. Res. Commun. 2006, 30, 935–942. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Sun, Y.J.; Yang, L.; Wu, Y.J. Cadmium and chlorpyrifos inhibit cellular immune response in spleen of rats. Environ. Toxicol. 2017, 32, 1927–1936. [Google Scholar] [CrossRef]
- El-Bini, D.I.; Lasram, M.M.; Annabi, A.; Gharbi, N.; El-Fazaa, S. A comparative study on toxicity induced by carbosulfan and malathion in Wistar rat liver and spleen. Pestic. Biochem. Physiol. 2015, 124, 21–28. [Google Scholar] [CrossRef]
- Ojha, A.; Yaduvanshi, S.K.; Srivastava, N. Effect of combined exposure of commonly used organophosphate pesticides on lipid peroxidation and antioxidant enzymes in rat tissues. Pestic. Biochem. Physiol. 2011, 99, 148–156. [Google Scholar] [CrossRef]
- Campbell, A.M.; Kashgarian, M.; Shlomchik, M.J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 2012, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mangum, L.C.; Borazjani, A.; Stokes, J.V.; Matthews, A.T.; Lee, J.H.; Chambers, J.E.; Ross, M.K. Organochlorine insecticides induce NADPH oxidase-dependent reactive oxygen species in human monocytic cells via phospholipase A2/arachidonic acid. Chem. Res. Toxicol. 2015, 28, 570–584. [Google Scholar] [CrossRef] [Green Version]
- Kalyanaraman, B.; Hardy, M.; Zielonka, J. A critical review of methodologies to detect reactive oxygen and nitrogen species stimulated by NADPH oxidase enzymes: Implications in pesticide toxicity. Curr. Pharmacol. Rep. 2016, 2, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [Green Version]
- Holmberg, J.; Tuncel, J.; Yamada, H.; Lu, S.; Olofsson, P.; Holmdahl, R. Pristane, a non-antigenic adjuvant, induces MHC class II-restricted, arthritogenic T cells in the rat. J. Immunol. 2006, 176, 1172–1179. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Singh, V.; Tiwari, R.L.; Chandra, T.; Kumar, A.; Dikshit, M.; Barthwal, M.K. The IRAK-ERK-p67phox-Nox-2 axis mediates TLR4, 2-induced ROS production for IL-1β transcription and processing in monocytes. Cell. Mol. Immunol. 2016, 13, 745–763. [Google Scholar] [CrossRef]
- Hayashi, F.; Means, T.K.; Luster, A.D. Toll-like receptors stimulate human neutrophil function. Blood 2003, 102, 2660–2669. [Google Scholar] [CrossRef]
- Laurent, C.; Chabi, B.; Fouret, G.; Py, G.; Sairafi, B.; Elong, C.; Gaillet, S.; Cristol, J.P.; Coudray, C.; Feillet-Coudray, C. Polyphenols decreased liver NADPH oxidase activity, increased muscle mitochondrial biogenesis and decreased gastrocnemius age-dependent autophagy in aged rats. Free Radic. Res. 2012, 46, 1140–1149. [Google Scholar] [CrossRef]
- Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Nowak, M.; de Graft-Johnson, J.; Padula, G.; Bialasiewicz, P.; Markow-ski, J.; et al. Consumption of strawberries on a daily basis increases the non-urate 2,2-diphenyl-1-picryl-hydrazyl(DPPH) radical scavenging activity of fasting plasma in healthy subjects. J. Clin. Biochem. Nutr. 2014, 55, 48–55. [Google Scholar] [CrossRef]
- Takahashi, M.; Satake, N.; Yamashita, H.; Tamura, A.; Sasaki, M.; Matsui-Yuasa, I.; Tabuchi, M.; Akahishi, Y.; Terada, M.; Kojima-Yuasa, A. Ecklonia cava polyphenol protects the liver against ethanol-induced injury in rats. Biochim. Biophys. Acta. 2012, 1820, 978–988. [Google Scholar] [CrossRef]
- Wei, B.L.; Weng, J.R.; Chiu, P.H.; Hung, C.F.; Wang, J.P.; Lin, C.N. Antiinflammatory flavonoids from artocarpus heterophyllus and artocarpus communis. J. Agric. Food Chem. 2005, 53, 3867–3871. [Google Scholar] [CrossRef]
- Nani, A.; Belarbi, M.; Ksouri-Megdiche, W.; Abdoul-Azize, S.; Benammar, C.; Ghiringhelli, F.; Hichami, A.; Khan, N.A. Effects of polyphenols and lipids from Pennisetum glaucum grains on T-cell activation: Modulation of Ca2+ and ERK1/ERK2 signaling. BMC Complement. Altern. Med. 2015, 15, 426. [Google Scholar] [CrossRef] [Green Version]
- Maiti, M.; Chattopadhyay, K.; Verma, M.; Chattopadhyay, B. Curcumin protects against nicotine-induced stress during protein malnutrition in female rat through immunomodulation with cellular amelioration. Mol. Biol. Rep. 2015, 42, 1623–1637. [Google Scholar] [CrossRef]
- Chabalgoity, J.A.; Baz, A.; Rial, A.; Grille, S. The relevance of cytokines for development of protective immunity and rational design of vaccines. Cytokine Growth Factor Rev. 2007, 18, 195–207. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Zhang, M.; Yue, L.-T.; Wang, C.-C.; Zhang, P.; Liu, Y.; Duan, R.S. Curcumin ameliorates experimental autoimmune myasthenia gravis by diverse immune cells. Neurosci. Lett. 2016, 626, 25–34. [Google Scholar] [CrossRef]
- Lee, N.; Shin, M.S.; Kang, Y.; Park, K.; Maeda, T.; Nishioka, H.; Fujii, H.; Kang, I. Oligonol, a lychee fruit-derived low-molecular form of polyphenol mixture, suppresses inflammatory cytokine production from human monocytes. Hum. Immunol. 2016, 77, 512–515. [Google Scholar] [CrossRef] [Green Version]
- Balu, M. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem. Res. 2011, 36, 2096–2103. [Google Scholar]
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglans regia) chemical composition and research in human health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef]
- Shabani, M.; Nazeri, M.; Parsania, S.; Razavinasab, M.; Zangiabadi, N.; Esmaeilpour, K.; Abareghi, F. Walnut consumption protects rats against cisplatin-induced neurotoxicity. NeuroToxicology 2012, 33, 1314–1321. [Google Scholar] [CrossRef]
- Yang, L.; Ma, S.; Han, Y.; Wang, Y.; Guo, Y.; Weng, Q.; Xu, M. Walnut polyphenol extract attenuates immunotoxicity induced by 4-pentylphenol and 3-methyl-4-nitrophenol in murine splenic lymphocyte. Nutrients 2016, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wan, Y.F.; Wang, Y.X.; Zhao, Y.; Zhang, Y.; Zhang, A. Walnut polyphenol extract protects against fenitrothion-induced immunotoxicity in murine splenic lymphocytes. Nutrients 2018, 10, 1838. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, J.X.; Tian, J.L.; Wang, C.; Wang, Y.X.; Wan, Y.F.; Weng, Q.; Xu, M.Y. Selective effects of fenitrothion on murine splenic T-lymphocyte populations and cytokine/granzyme production. J. Environ. Sci. Health Part B 2018, 53, 319–326. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Inagaki, H.; Hirata, Y.; Sato, S.; Ishizaki, M.; Okamura, A.; Wang, D.; Nakajima, T.; Kamijima, M.; et al. Effect of oral exposure to fenitrothion and 3-methyl-4-nitrophenol on splenic cell populations and histopathological alterations in spleen in Wistar rats. Hum. Exp. Toxicol. 2011, 30, 665–674. [Google Scholar] [CrossRef]
- Dangroo, N.A.; Singh, J.; Gupta, N.; Singh, S.; Kaul, A.; Khuroo, M.A.; Sangwan, P.L. T- and B-cell immunosuppressive activity of novel α -santonin analogs with humoral and cellular immune response in Balb/c mice. Med. Chem. Commun. 2017, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ruwali, P.; Ambwani, T.K.; Gautam, P. In vitro immunomodulatory potential of Artemisia indica Willd. In chicken lymphocytes. Vet. World 2018, 11, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 2011, 108, 716–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, A.; Ahmad, S.F.; Bakheet, S.A.; Al-Harbi, N.O.; AL-Ayadhi, L.Y.; Attia, S.M.; Zoheir, K.M.A. Toll-like receptor 4 signaling is associated with upregulated NADPH oxidase expression in peripheral T cells of children with autism. Brain Behav. Immun. 2017, 61, 146–154. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.; Niu, X. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R.; Janeway, C.J. The Toll receptor family and microbial recognition. Trends Microbiol. 2000, 8, 452–456. [Google Scholar] [CrossRef]
- Charoenying, T.; Suriyo, T.; Thiantanawat, A.; Chaiyaroj, S.C.; Parkpian, P.; Satayavivad, J. Effects of paraoxon on neuronal and lymphocytic cholinergic systems. Environ. Toxicol. Pharmacol. 2011, 31, 119–128. [Google Scholar] [CrossRef]
- Handy, R.D.; Abd-El Samei, H.A.; Bayomy, M.F.; Mahran, A.M.; Abdeen, A.M.; El-Elaimy, E.A. Chronic diazinon exposure: Pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicology 2002, 172, 13–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Waqas, Y.; Yang, P.; Sun, X.J.; Liu, Y.; Ahmed, N.; Chen, B.; Li, Q.F.; Hu, L.S.; Huang, Y.F.; et al. Cytological study on the regulation of lymphocyte homing in the chicken spleen during LPS stimulation. Oncotarget 2017, 8, 7405–7419. [Google Scholar] [CrossRef]
- Sanbongi, C.; Suzuki, N.; Sakane, T. Polyphenols in chocolate, which have antioxidant activity, modulate immune functions in humansin vitro. Cell. Immunol. 1997, 177, 129–136. [Google Scholar] [CrossRef]
- Neeraj, K.; Anita, Y.; Sachin, G.; Kanupriya; Neeraj, A.; Ranjan, G. Antigenotoxic effect of curcumin and carvacrol against parathion induced DNA damage in cultured human peripheral blood lymphocytes and its relation to GSTM1 and GSTT1 polymorphism. J. Toxicol. 2014. [Google Scholar] [CrossRef]
- Magrone, T.; Fontana, S.; Laforgia, F.; Dragone, T.; Jirillo, E.; Passantino, L. Administration of a polyphenol-enriched feed to farmed sea bass (Dicentrarchus labrax L.) modulates intestinal and spleen immune responses. Oxid. Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Hirata, Y.; Piao, S.; Minami, M. The by-products generated during sarin synthesis in the Tokyo sarin disaster induced inhibition of natural killer and cytotoxic T lymphocyte activity. Toxicology 2000, 146, 209–220. [Google Scholar] [CrossRef]
- Sakazaki, H.; Ueno, H.; Umetani, K.; Utsumi, H.; Nakamuro, K. Immunotoxicological evaluation of environmental chemicals utilizing mouse lymphocyte mitogenesis test. J. Health Sci. 2001, 47, 258–271. [Google Scholar] [CrossRef] [Green Version]
- Nishino, R.; Fukuyama, T.; Kosaka, T.; Hayashi, K.; Watanabe, Y.; Kurosawa, Y.; Ueda, H.; Harada, T. Effects of short-term oral combined exposure to environmental immunotoxic chemicals in mice. J. Immunotoxicol. 2014, 11, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Eom, J.H.; Cho, H.Y.; Cho, Y.J.; Kim, J.Y.; Lee, J.K.; Kim, S.H.; Park, K.L. Evaluation of immunotoxicity induced by pirimiphos-methyl in male Balb/c mice following exposure to for 28 days. J. Toxicol. Environ. Health A 2007, 70, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Shawna, M.; Bryan, D.V.; Mary, C.D.; Janice, S. Hyper-responsive Toll-like receptor 7 and 9 activation in NADPH oxidase-deficient B lymphoblasts. Immunology 2015, 146, 595–606. [Google Scholar]
- Alluwaimi, A.M.; Hussein, Y. Diazinon immunotoxicity in mice: Modulation of cytokines level and their gene expression. Toxicology 2007, 236, 123–131. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Pang, Q.; Song, S.; Miao, R.; Chen, W.; Zhou, Y.; Liu, C. The protective role of curcumin in zymosan-induced multiple organ dysfunction syndrome in mice. SHOCK 2016, 45, 209–219. [Google Scholar] [CrossRef]
- Choudhury, S.; Ghosh, S.; Mukherjee, S.; Gupta, P.; Bhattacharya, S.; Adhikary, A.; Chattopadhyay, S. Pomegranate protects against arsenic-induced p53-dependent ROS-mediated inflammation and apoptosis in liver cells. J. Nutr. Biochem. 2016, 38, 25–40. [Google Scholar] [CrossRef]
- Mansour, S.; Fadia, S.; Youssef, A.E.; Ganna, P.; Ahmed, H.; El-Khatib, D.; Maria, M.; Mohamed, L.A.; Michael, W. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem. Toxicol. 2018, 113, 145–153. [Google Scholar]
- Tapas, A.R.; Sakarkar, D.; Kakde, R. Flavonoids as nutraceuticals: A review. Trop. J. Pharma. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Binienda, Z. A fetal rat model of acute perinatal ischemia-hypoxia. Ann. N. Y. Acad. Sci. 1995, 72, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Perfettini, J.L.; Roumier, T.; Castedo, M.; Larochette, N.; Boya, P.; Raynal, B.; Lazar, V.; Ciccosanti, F.; Nardacci, R.; Penninger, J. NF-κB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J. Exp. Med. 2004, 199, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Ji, G.W.; Wen, P.X.; Ju, F.G.; Hai, J.C.; Ming, J.Y.; Bo, W.; You, W.H.; Li, M.T. Cytotoxic effects of Avermectin on human HepG2 cells in vitro bioassays. Environ. Pollut. 2017, 220, 1127–1137. [Google Scholar]
- Angelini, D.J.; Moyer, R.A.; Cole, S.; Willis, K.L.; Oyler, J.; Dorsey, R.M.; Salem, H. The pesticide metabolites paraoxon and malaoxon induce cellular death by different mechanisms in cultured human pulmonary cells. Int. J. Toxicol. 2015, 34, 433–441. [Google Scholar] [CrossRef]
- Ling, Z.; Yang, L.; Jian, Y.L.; Ling, Z.L.; Yong, L.Z.; Hai, Y.G.; Ying, C. Protective effect of rosamultin against H2O2-induced oxidative stress and apoptosis in H9c2 cardiomyocytes. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef] [Green Version]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit. Rev. Food Sci. Nutr. 2016, 56, 46–59. [Google Scholar]
- Luo, H.; Rankin, G.O.; Juliano, N.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFkB-cMyc-p21 pathway. Food Chem. 2012, 130, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Mishra, M.; Sharma, A.; Balaji, T.D.; Kumar, R.; Mishra, R.K.; Chowdhuri, D.K. Chlorpyrifos induces apoptosis and DNA damage in Drosophila through generation of reactive oxygen species. Ecotoxicol. Environ. Saf. 2010, 73, 1415–1423. [Google Scholar] [CrossRef]
- Dal-Ros, S.; Zoll, J.; Lang, A.-L.; Auger, C.; Keller, N.; Bronner, C.; Geny, B.; Schini-Kerth, V.B. Chronic intake of red wine polyphenols by young rats prevents aging-induced endothelial dysfunction and decline in physical performance: Role of NADPH oxidase. Biochem. Biophys. Res. Commun. 2011, 404, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Gupta, Y.K. Study of commonly used organophosphate pesticides that induced oxidative stress and apoptosis in peripheral blood lymphocytes of rats. Hum. Exp. Toxicol. 2017, 36. [Google Scholar] [CrossRef] [PubMed]
- El-Benna, J.; Dang, P.M.; Gougerot-Pocidalo, M.A. Priming of the neutrophil NADPH oxidase activation: Role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008, 30, 279–289. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; D’Amore, S.; Gnoni, A.; Pellegrino, M.; Storelli, C.; De Caterina, R.; Palasciano, G.; Carluccio, M.A. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J. Nutr. Biochem. 2016, 28, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Bielinski, D.F.; Carrihill-Knoll, K.L.; Rabin, B.M.; Shukitt-Hale, B. Protective effects of blueberry- and strawberry diets on neuronal stress following exposure to (56)Fe particles. Brain Res. 2014, 1593, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zanin-Zhorov, A.; Tal-Lapidot, G.; Cahalon, L.; Cohen-Sfady, M.; Pevsner-Fischer, M.; Lider, O.; Cohen, I.R. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J. Immunol. 2007, 179, 41–44. [Google Scholar] [CrossRef]
- To, E.E.; Broughton, B.R.S.; Hendricks, K.S.; Vlahos, R.; Selemidis, S. Influenza A virus and TLR7 activation potentiate NOX2 oxidase-dependent ROS production in macrophages. Free Radic. Res. 2014, 48, 940–947. [Google Scholar] [CrossRef]
- Le Sage, F.; Meilhac, O.; Gonthier, M.-P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacol. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Fan, C.; Zhang, A.; Zhang, Y.; Wang, F.; Weng, Q.; Xu, M. Walnut Polyphenol Extract Protects against Malathion- and Chlorpyrifos-Induced Immunotoxicity by Modulating TLRx-NOX-ROS. Nutrients 2020, 12, 616. https://doi.org/10.3390/nu12030616
Zhao Y, Fan C, Zhang A, Zhang Y, Wang F, Weng Q, Xu M. Walnut Polyphenol Extract Protects against Malathion- and Chlorpyrifos-Induced Immunotoxicity by Modulating TLRx-NOX-ROS. Nutrients. 2020; 12(3):616. https://doi.org/10.3390/nu12030616
Chicago/Turabian StyleZhao, Yue, Chang Fan, Ao Zhang, Yue Zhang, Fengjun Wang, Qiang Weng, and Meiyu Xu. 2020. "Walnut Polyphenol Extract Protects against Malathion- and Chlorpyrifos-Induced Immunotoxicity by Modulating TLRx-NOX-ROS" Nutrients 12, no. 3: 616. https://doi.org/10.3390/nu12030616
APA StyleZhao, Y., Fan, C., Zhang, A., Zhang, Y., Wang, F., Weng, Q., & Xu, M. (2020). Walnut Polyphenol Extract Protects against Malathion- and Chlorpyrifos-Induced Immunotoxicity by Modulating TLRx-NOX-ROS. Nutrients, 12(3), 616. https://doi.org/10.3390/nu12030616




