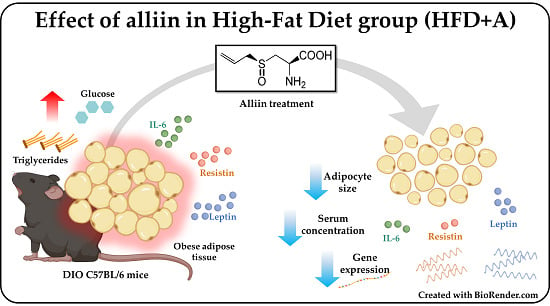
Alliin, An Allium sativum Nutraceutical, Reduces Metaflammation Markers in DIO Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alliin
2.2. Diets
2.3. Animals
2.4. Metabolic Tests
2.4.1. Oral Glucose Tolerance Test (OGTT) and Intraperitoneal Insulin Tolerance Test (IITT)
2.4.2. Cholesterol and Triglycerides Test
2.5. Serum Cytokines Measurement
2.6. Histological Examination and Hematoxylin–Eosin Staining
2.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Alliin Does Not Affect Body Weight but Significatively Improves Glucose Tolerance
3.2. The Obese Group Treated with Alliin Had Smaller Adipocyte Size Than the Obese Control Group
3.3. Alliin Significantly Decreased Gene Expression from Epididymal Adipose Tissue and Protein Serum Levels of Adipocytokines Leptin and Resistin.
3.4. Obesity-Related Serum Inflammatory Cytokine Concentrations Were Decreased or Homogenized by Alliin
3.5. The mRNA Expression of Antioxidant Enzymes in the Liver Was Not Modified by Alliin
4. Discussion
4.1. Alliin Reduces the Adipocyte Size and Improves Metabolism Leading to Homeostasis of Adipose Tissue
4.2. Alliin Showed a Relevant Anti-Inflammatory Potential in the Low-Grade Inflammatory State in Obesity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| cDNA | complementary DNA |
| DIO | Diet-Induced Obesity model |
| ERK | Extracellular Signal-Regulated Kinases |
| FG | Fasting Glucose |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GIP | Gastric Inhibitory Polypeptide |
| GLP-1 | Glucagon-Like Peptide-1 |
| GPx | Glutathione Peroxidase |
| H&E | Hematoxylin & Eosin |
| HFD | High-Fat Diet group |
| HFD+A | High-Fat Diet + Alliin treatment group |
| IITT | Intraperitoneal Insulin Tolerance Test |
| IKK | IκB kinase complex |
| IL-6 | Interleukine-6 |
| IRS | Insulin Receptor Substrate proteins |
| JNK | Jun kinase |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| mRNA | messenger RNA |
| OGTT | Oral Glucose Tolerance Test |
| PKC | protein kinase C |
| PP | Pancreatic Polypeptide |
| PPARγ | Peroxisome Proliferator-Activated Receptor gamma |
| PPY | Pancreatic Polypeptide Y |
| RNA | Ribonucleic Acid |
| RT-qPCR | Real-Time quantitative Polymerase Chain Reaction |
| SOD1 | Superoxide dismutase |
| STD | Standard Diet group |
| STD+A | Standard Diet + Alliin treatment group |
| TG | Triglycerides |
| TNFα | Tumor Necrosis Factor alpha |
| TLR4 | Toll-Like Receptor 4 |
References
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-x Direct Role in Obesity-Linked Insulin Resistance.pdf. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions—But do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef] [Green Version]
- Kalra, E.K. Nutraceutical-definition and introduction. AAPS Pharm. Sci. 2003, 5, 27–28. [Google Scholar] [CrossRef] [Green Version]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Rivlin, R.S. Historical Perspective on the Use of Garlic. J. Nutr. 2001, 131, 951S–954S. [Google Scholar] [CrossRef] [Green Version]
- Chung, L.Y. The Antioxidant Properties of Garlic Compounds: Allyl Cysteine, Alliin, Allicin, and Allyl Disulfide. J. Med. Food 2006, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Leontowicz, M.; Leontowicz, H.; Najman, K.; Namiesnik, J.; Park, Y.-S.; Jung, S.-T.; Kang, S.-G.; Trakhtenberg, S. Supplementation of garlic lowers lipids and increases antioxidant capacity in plasma of rats. Nutr. Res. 2006, 26, 362–368. [Google Scholar] [CrossRef]
- Colín-González, A.L.; Ali, S.F.; Túnez, I.; Santamaría, A. On the antioxidant, neuroprotective and anti-inflammatory properties of S-allyl cysteine: An update. Neurochem. Int. 2015, 89, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Ortuño-Sahagún, D.; Vázquez-Carrera, M.; López-Roa, R.I. Alliin, a Garlic (Allium sativum) Compound, Prevents LPS-Induced Inflammation in 3T3-L1 Adipocytes. Mediat. Inflamm. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Lin, Q.; Li, X.; Nie, Y.; Sun, S.; Deng, X.; Wang, L.; Lu, J.; Tang, Y.; Luo, F. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK-NF-κB/AP-1/STAT-1 inactivation and PPAR-γ activation. Mol. Nutr. Food Res. 2017, 61, 1601013. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [Green Version]
- Catrysse, L.; van Loo, G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-κB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? Oxidative stress and diabetes mellitus. J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Spahis, S.; Delvin, E.; Borys, J.-M.; Levy, E. Oxidative Stress as a Critical Factor in Nonalcoholic Fatty Liver Disease Pathogenesis. Antioxid Redox Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. IJMS 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Ghyasi, R.; Mohaddes, G.; Naderi, R. Combination effect of voluntary exercise and garlic (Allium sativum) on oxidative stress, cholesterol level and histopathology of heart tissue in type 1 diabetic rats. J. Cardiovasc. Thorac. Res. 2019, 11, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Sangeetha, T.; Darlin Quine, S. Preventive effect of S-allyl cysteine sulphoxide (Alliin) on mitochondrial dysfunction in normal and isoproterenol induced cardiotoxicity in male Wistar rats: A histopathological study. Mol. Cell. Biochem. 2009, 328, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Dou, C.; Li, N.; Kang, F.; Wang, Y.; Cao, Z.; Yang, X.; Dong, S. Alliin Attenuated RANKL-Induced Osteoclastogenesis by Scavenging Reactive Oxygen Species through Inhibiting Nox1. IJMS 2016, 17, 1516. [Google Scholar] [CrossRef] [Green Version]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [Green Version]
- Rosini, T.C.; Ramos Da Silva, A.S.; De Moraes, C. Diet-induced obesity: Rodent model for the study of obesity-related disorders. Rev. Assoc. Med. Bras. 2012, 58, 383–387. [Google Scholar]
- Zhai, B.; Zhang, C.; Sheng, Y.; Zhao, C.; He, X.; Xu, W.; Huang, K.; Luo, Y. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabani, E.; Sayemiri, K.; Mohammadpour, M. The effect of garlic on lipid profile and glucose parameters in diabetic patients: A systematic review and meta-analysis. Prima Care Diabetes 2019, 13, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xie, K.; Liu, Z.; Nakasone, Y.; Sakao, K.; Hossain, M.A.; Hou, D.-X. Preventive Effects and Mechanisms of Garlic on Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2019, 11, 1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrouj, H.; Ziamajidi, N.; Abbasalipourkabir, R.; Goodarzi, M.T.; Saidijam, M. Hypoglycemic and antioxidant effects of oral administration of garlic extract in the livers of type 1 diabetic rats. JBCPP 2019, 30, 245–250. [Google Scholar] [CrossRef]
- Sun, Y.-E.; Wang, W.; Qin, J. Anti-hyperlipidemia of garlic by reducing the level of total cholesterol and low-density lipoprotein: A meta-analysis. Medicine 2018, 97, e0255. [Google Scholar] [CrossRef]
- Yu, J.; Li, P. The size matters: Regulation of lipid storage by lipid droplet dynamics. Sci. China Life Sci. 2017, 60, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yeh, Y.-Y. Water-soluble organosulfur compounds of garlic inhibit fatty acid and triglyceride syntheses in cultured rat hepatocytes. Lipids 2001, 36, 395–400. [Google Scholar] [CrossRef]
- Liu, L.; Yeh, Y.-Y. Inhibition of cholesterol biosynthesis by organosulfur compounds derived from garlic. Lipids 2000, 35, 197–203. [Google Scholar] [CrossRef]
- Choudhary, P.R.; Jani, R.D.; Sharma, M.S. Effect of Raw Crushed Garlic (Allium sativum L.) on Components of Metabolic Syndrome. J. Diet. Suppl. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Jernås, M.; Palming, J.; Sjöholm, K.; Jennische, E.; Svensson, P.-A.; Gabrielsson, B.G.; Levin, M.; Sjögren, A.; Rudemo, M.; Lystig, T.C.; et al. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006, 20, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cao, F.; Tang, Q.-Z.; Yan, L.; Dong, Y.-G.; Zhu, L.-H.; Wang, L.; Bian, Z.-Y.; Li, H. Allicin protects against cardiac hypertrophy and fibrosis via attenuating reactive oxygen species-dependent signaling pathways. J. Nutr. Biochem. 2010, 21, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Louis, X.L.; Murphy, R.; Thandapilly, S.J.; Yu, L.; Netticadan, T. Garlic extracts prevent oxidative stress, hypertrophy and apoptosis in cardiomyocytes: A role for nitric oxide and hydrogen sulfide. BMC Complement. Altern. Med. 2012, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Colitti, M.; Grasso, S. Nutraceuticals and regulation of adipocyte life: Premises or promises: Nutraceuticals and Regulation of Adipocyte Life. BioFactors 2014, 40, 398–418. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; He, W.; Chen, R.; Zhuang, R. Effects of alliin on LPS-induced acute lung injury by activating PPARγ. Microb. Pathog. 2017, 110, 375–379. [Google Scholar] [CrossRef]
- Cariou, B.; Charbonnel, B.; Staels, B. Thiazolidinediones and PPARγ agonists: Time for a reassessment. Trends Endocrinol. Metab. 2012, 23, 205–215. [Google Scholar] [CrossRef]
- Chao, L.; Marcus-Samuels, B.; Mason, M.M.; Moitra, J.; Vinson, C.; Arioglu, E.; Gavrilova, O.; Reitman, M.L. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J. Clin. Invest. 2000, 106, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Nantz, M.P.; Rowe, C.A.; Muller, C.E.; Creasy, R.A.; Stanilka, J.M.; Percival, S.S. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind, placebo-controlled nutrition intervention. Clin. Nutr. 2012, 31, 337–344. [Google Scholar] [CrossRef]
- Park, S.Y.; Seetharaman, R.; Ko, M.J.; Kim, D.Y.; Kim, T.H.; Yoon, M.K.; Kwak, J.H.; Lee, S.J.; Bae, Y.S.; Choi, Y.W. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int. Immunopharmacol. 2014, 19, 253–261. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Linking resistin, inflammation, and cardiometabolic diseases. Korean J. Intern. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Alonso-Iglesias, E. Resistin: Insulin resistance to malignancy. Clin. Chim. Acta 2015, 438, 46–54. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Khetani, S.A.; Zahner, G.J.; Spaulding, K.A.; Schaller, M.S.; Gasper, W.J.; Hills, N.K.; Schafer, A.L.; Grenon, S.M. Serum resistin is associated with impaired endothelial function and a higher rate of adverse cardiac events in patients with peripheral artery disease. J. Vasc. Surg. 2019, 69, 497–506. [Google Scholar] [CrossRef]
- Miao, J.; Benomar, Y.; Al Rifai, S.; Poizat, G.; Riffault, L.; Crépin, D.; Taouis, M. Resistin inhibits neuronal autophagy through Toll-like receptor 4. J. Endocrinol. 2018, 238, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Azamar-Llamas, D.; Hernández-Molina, G.; Ramos-Ávalos, B.; Furuzawa-Carballeda, J. Adipokine Contribution to the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2017, 2017, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.C.; Li, J.; Gambini, L.; Batugedara, H.M.; Sati, S.; Lazar, M.A.; Fan, L.; Pellecchia, M.; Nair, M.G. Human resistin protects against endotoxic shock by blocking LPS–TLR4 interaction. Proc. Natl. Acad. Sci. USA 2017, 114, E10399–E10408. [Google Scholar] [CrossRef] [Green Version]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, Y.; Schultz, R.D.; Li, N.; He, Z.; Zhang, Z.; Caron, A.; Zhu, Q.; Sun, K.; Xiong, W.; et al. Partial Leptin Reduction as an Insulin Sensitization and Weight Loss Strategy. Cell Metab. 2019, 30, 706–719. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, C.A.; Leibel, R.L. Auto-Regulation of Leptin Neurobiology. Cell Metab. 2019, 30, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.G.; Albrecht, U.; Häussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins—Regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell Biol. 2012, 91, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Caputo, T.; Gilardi, F.; Desvergne, B. From chronic overnutrition to metaflammation and insulin resistance: Adipose tissue and liver contributions. FEBS Lett. 2017, 591, 3061–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braune, J.; Weyer, U.; Hobusch, C.; Mauer, J.; Brüning, J.C.; Bechmann, I.; Gericke, M. IL-6 Regulates M2 Polarization and Local Proliferation of Adipose Tissue Macrophages in Obesity. J. Immunol. 2017, 198, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- León-Pedroza, J.I.; González-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodríguez, D.; Escobedo, G.; González-Chávez, A. Inflamación sistémica de grado bajo y su relación con el desarrollo de enfermedades metabólicas: De la evidencia molecular a la aplicación clínica. Cirugía Cir. 2015, 83, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, X.; Zheng, T.; Xing, S.; Wu, Y.; Bian, F.; Wu, G.; Li, Y.; Li, J.; Bai, X.; et al. TNF-α stimulates endothelial palmitic acid transcytosis and promotes insulin resistance. Sci. Rep. 2017, 7, 44659. [Google Scholar] [CrossRef]







| Gene | Forward | Reverse | Product | Temperature |
|---|---|---|---|---|
| Leptin | GTCTTATGTTCAAGCAGTGCC | TGAAGCCCAGGAATGAAGT | 150 | 58 °C |
| Resistin | CCCACGGGATGAAGAACCTTT | CACGCTCACTTCCCCGACAT | 372 | 63 °C |
| MCP-1 | GGTGTCCCAAAGAAGCTGTAG | CTGAAGACCTTAGGGCAGATG | 161 | 61 °C |
| TNFα | GCTGAGCTCAAACCCTGGTA | CGGACTCCGCAAAGTCTAAG | 118 | 63 °C |
| Catalase | GCAGATACCTGTGAACTGTC | GTAGAATGTCCGCACCTGAG | 229 | 59 °C |
| GPX | GTCCACCGTGTATGCCTTCT | TCTGCAGATCGTTCATCTCG | 152 | 60 °C |
| SOD1 | TGGTGGTCCATGAGAAACAA | GTTTACTGCGCAATCCCAAT | 115 | 61 °C |
| GAPDH | TCCACCACCCTGTTGCTGTA | ACCACAGTCCATGCCATCAC | 452 | 63 °C |
| STD | STD+A | HFD | HFD+A | |
|---|---|---|---|---|
| Cholesterol (mg/dL) | 152.75 ± 3.19 | 153.36 ± 2.91 | 159.50 ± 5.19 | 165.64 ± 14.71 |
| Triglycerides (mg/dL) | 104.40 ± 14.78 | 131.20 ± 22.31 | 147.30 ± 36.29 | 155.00 ± 33.97 |
| Fasting glucose (mg/dL) | 109.20 ± 17.62 | 112.20 ± 14.31 | 132.70 ± 27.04 | 118.50 ± 27.86 |
| AUC of ITT | 11,014 ± 1512 | 10,549 ± 1,212 | 9,119 ± 1,723 | 11,134 ± 4,977 |
| AUC of OGTT * | 15,506 ± 2124 | 17,220 ± 2,099 | 21,458 ± 4,697 * | 17,618 ± 3,285 * |
| Body weight (g) | 26.33 ± 2.34 | 26.81 ± 1.94 | 41.20 ± 5.00 | 38.90 ± 4.94 |
| Epididymal adipose tissue weight (g) | 0.66 ± 0.14 | 0.78 ± 0.067 | 2.65 ± 0.60 | 2.48 ± 0.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Sánchez, M.A.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Viveros-Paredes, J.M.; Franco-Arroyo, N.N.; Godínez-Rubí, M.; Ortuño-Sahagun, D.; López-Roa, R.I. Alliin, An Allium sativum Nutraceutical, Reduces Metaflammation Markers in DIO Mice. Nutrients 2020, 12, 624. https://doi.org/10.3390/nu12030624
Sánchez-Sánchez MA, Zepeda-Morales ASM, Carrera-Quintanar L, Viveros-Paredes JM, Franco-Arroyo NN, Godínez-Rubí M, Ortuño-Sahagun D, López-Roa RI. Alliin, An Allium sativum Nutraceutical, Reduces Metaflammation Markers in DIO Mice. Nutrients. 2020; 12(3):624. https://doi.org/10.3390/nu12030624
Chicago/Turabian StyleSánchez-Sánchez, Marina A., Adelaida Sara Minia Zepeda-Morales, Lucrecia Carrera-Quintanar, Juan Manuel Viveros-Paredes, Noel Noé Franco-Arroyo, Marisol Godínez-Rubí, Daniel Ortuño-Sahagun, and Rocío Ivette López-Roa. 2020. "Alliin, An Allium sativum Nutraceutical, Reduces Metaflammation Markers in DIO Mice" Nutrients 12, no. 3: 624. https://doi.org/10.3390/nu12030624
APA StyleSánchez-Sánchez, M. A., Zepeda-Morales, A. S. M., Carrera-Quintanar, L., Viveros-Paredes, J. M., Franco-Arroyo, N. N., Godínez-Rubí, M., Ortuño-Sahagun, D., & López-Roa, R. I. (2020). Alliin, An Allium sativum Nutraceutical, Reduces Metaflammation Markers in DIO Mice. Nutrients, 12(3), 624. https://doi.org/10.3390/nu12030624