Multivariate Analysis of Influence of Vitamin Intake on Vascular Function Parameters by Sex in the General Spanish Population: EVA Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Variables and Measuring Instruments: Vascular Function Parameters
2.3.1. Cardio-Ankle Vascular Index (CAVI) and Brachial-Ankle Pulse Wave Velocity (baPWV)
2.3.2. Carotid Femoral Pulse Wave Velocity (cfPWV)
2.3.3. Vitamin Intake
2.3.4. Physical Activity
2.4. Ethical Principles
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Subjects Included
3.2. Vitamin Intake by Sex and Age
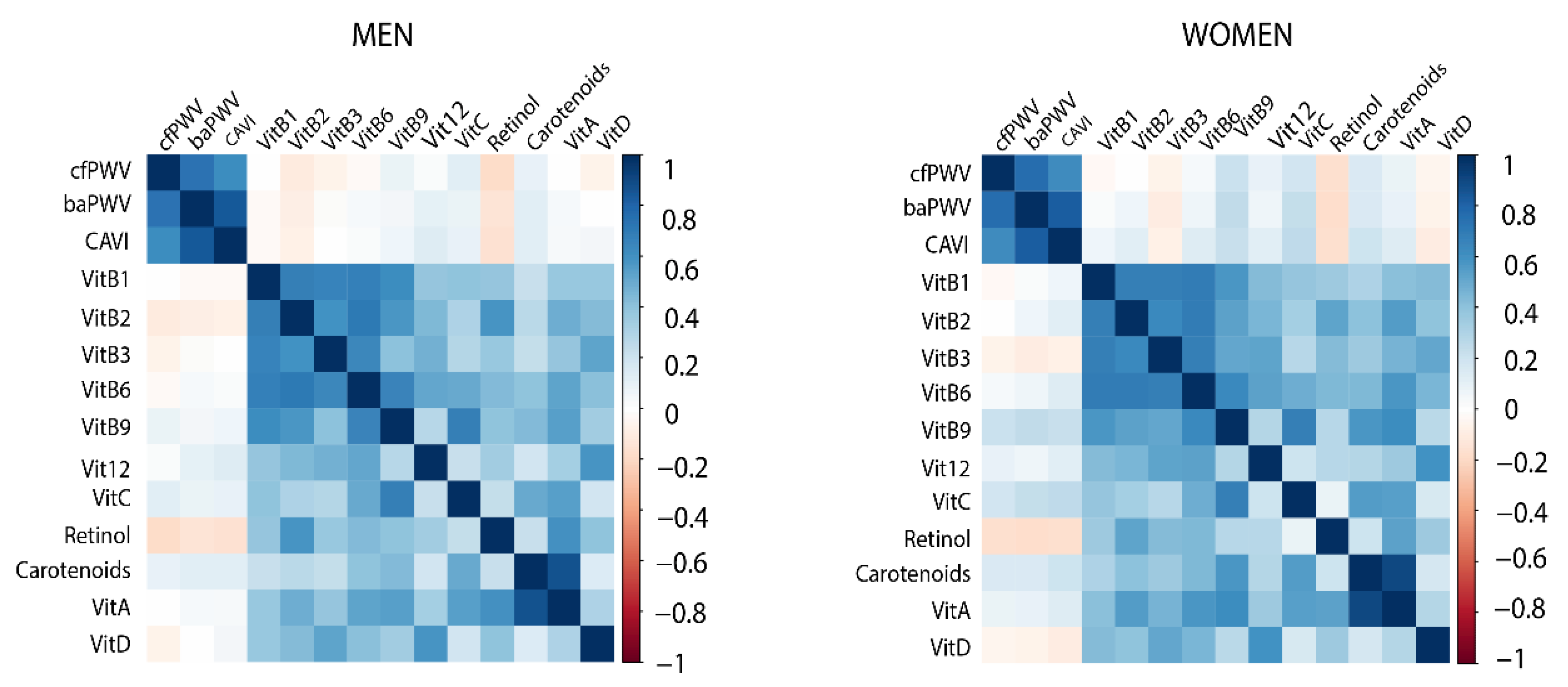
3.3. Association of Vitamins with Vascular Function Parameters
3.4. Multivariate Characterization of the Relationship between Vitamins and Vascular Function Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Virdis, A. Arterial Stiffness and Vascular Aging: From Pathophysiology to Treatment, with a Look at the Future. High Blood Press. Cardiovasc. Prev. 2018, 25, 135–136. [Google Scholar] [CrossRef] [Green Version]
- Satoh–Asahara, N.; Kotani, K.; Yamakage, H.; Yamada, T.; Araki, R.; Okajima, T.; Adachi, M.; Oishi, M.; Shimatsu, A. Cardio–ankle vascular index predicts for the incidence of cardiovascular events in obese patients: A multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis 2015, 242, 461–468. [Google Scholar] [CrossRef]
- Ben–Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta–analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Brachial–Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta–Analysis. Hypertension 2017, 69, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All–Cause Mortality with Arterial Stiffness A Systematic Review and Meta–Analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace–Raso, F.U.S.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid–femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.C.; Chuang, S.Y.; Lin, Y.P.; Chen, C.H. Brachial–ankle vs carotid–femoral pulse wave velocity as a determinant of cardiovascular structure and function. J. Hum. Hypertens. 2008, 22, 24–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Agnoletti, D.; Xu, Y.; Wang, J.-G.; Blacher, J.; Safar, M.E. Carotid–femoral pulse wave velocity in the elderly. J. Hypertens. 2014, 32, 1572–1576. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. Blood Press. 2013, 22, 193–278. [Google Scholar] [CrossRef] [Green Version]
- Shirai, K.; Hiruta, N.; Song, M.; Kurosu, T.; Suzuki, J.; Tomaru, T.; Miyashita, Y.; Saiki, A.; Takahashi, M.; Suzuki, K.; et al. Cardio–ankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, evidence and perspectives. J. Atheroscler. Thromb. 2011, 18, 924–938. [Google Scholar] [CrossRef] [Green Version]
- Kanamoto, M.; Matsumoto, N.; Shiga, T.; Kunimoto, F.; Saito, S. Relationship between coronary artery stenosis and cardio–Ankle vascular index (CAVI) in patients undergoing cardiovascular surgery. J. Cardiovasc. Dis. Res. 2013, 4, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. In Proceedings of the National Academy of Sciences of the United States of America. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeid, R.; Hübner, U.; Bodis, M.; Graeber, S.; Geisel, J. Effect of adding B–vitamins to vitamin D and calcium supplementation on CpG methylation of epigenetic aging markers. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 411–417. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Migration. Nutrient Recommendations: Dietary Reference Intakes. Available online: https://ods.od.nih.gov/Health_Information/Dietary_Reference_Intakes.aspx (accessed on 15 January 2020).
- Gomez–Marcos, M.A.; Martinez–Salgado, C.; Gonzalez–Sarmiento, R.; Hernandez–Rivas, J.M.; Sanchez–Fernandez, P.L.; Recio–Rodriguez, J.I.; Rodriguez–Sanchez, E.; Garca–Ortiz, L. Association between different risk factors and vascular accelerated ageing (EVA study): Study protocol for a cross–sectional, descriptive observational study. BMJ Open 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A Novel Blood Pressure–independent Arterial Wall Stiffness Parameter; Cardio–Ankle Vascular Index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Recio–Rodriguez, J.I.; Rodriguez–Martin, C.; Gonzalez–Sanchez, J.; Rodriguez–Sanchez, E.; Martin–Borras, C.; Martínez–Vizcaino, V.; Arietaleanizbeaskoa, M.S.; Magdalena–Gonzalez, O.; Fernandez–Alonso, C.; Maderuelo–Fernandez, J.A.; et al. Evident smartphone app, a new method for the dietary record: Comparison with a food frequency questionnaire. J. Med. Int. Res. 2019, 21, 1–13. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2013, 310, 2191–2194. [Google Scholar]
- Ter–Braak, C.J. Canonical Correspondence Analysis: A New Eigenvector Technique for Multivariate Direct Gradient Analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.H. The Interpretation of Interaction in Contingency Tables. J. R. Stat. Soc. Ser. B (Methodol.) 2016, 25, 264–296. [Google Scholar] [CrossRef]
- Cano–Ibáñez, N.; Gea, A.; Ruiz–Canela, M.; Corella, D.; Salas–Salvadó, J.; Schröder, H.; Navarrete–Muñoz, E.M.; Romaguera, D.; Martínez, J.A.; Barón–López, F.J.; et al. Diet quality and nutrient density in subjects with metabolic syndrome: Influence of socioeconomic status and lifestyle factors. A cross–sectional assessment in the PREDIMED–Plus study. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Skully, R.; Saleh, A.S. Aging and the Effects of Vitamins and Supplements. Clin. Geriatr. Med. 2011, 27, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Vitamins Associated with Brain Aging, Mild Cognitive Impairment, and Alzheimer Disease: Biomarkers, Epidemiological and Experimental Evidence, Plausible Mechanisms, and Knowledge Gaps. Adv. Nutr. Int. Rev. J. 2017, 8, 958–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Kim, M.K.; Jung, S.; Shin, J.; Choi, B.Y. The cross–sectional relationships of dietary and serum vitamin D with cardiometabolic risk factors: Metabolic components, subclinical atherosclerosis, and arterial stiffness. Nutrition 2016, 32, 1048–1056. [Google Scholar] [CrossRef]
- Van Dijk, S.C.; Enneman, A.W.; Van Meurs, J.; Swart, K.M.A.; Ham, A.H.; Van Wijngaarden, J.P.; Brouwer–Brolsma, E.M.; Van der Zwaluw, N.L.; Van Schoor, N.M.; Dhonukshe–Rutten, R.A.M.; et al. B–vitamin levels and genetics of hyperhomocysteinemia are not associated with arterial stiffness. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, S.C.; Enneman, A.W.; Swart, K.M.A.; Van Wijngaarden, J.P.; Ham, A.C.; Brouwer–Brolsma, E.M.; Van Der Zwaluw, N.L.; Blom, H.J.; Feskens, E.J.; Geleijnse, J.M.; et al. Effects of 2–year vitamin B12 and folic acid supplementation in hyperhomocysteinemic elderly on arterial stiffness and cardiovascular outcomes within the B–PROOF trial. J. Hypertens. 2015, 33, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Jung, S.; Kim, M.K.; Choi, B.Y.; Shin, M.H.; Shin, D.H.; Lee, Y.H.; Chun, B.Y.; Hong, K.W.; Hwang, J.Y. Habitual dietary intake of β–carotene, vitamin C, folate, or vitamin E may interact with single nucleotide polymorphisms on brachial–ankle pulse wave velocity in healthy adults. Eur. J. Nutr. 2016, 55, 855–866. [Google Scholar] [CrossRef]

| Overall (501) | Men (249) | Women (252) | p-value | |
|---|---|---|---|---|
| Age, years | 55.9 (45.0–66.4) | 55.9 (45.2–66.4) | 56.1 (44.9–66.0) | 0.79 |
| Marital status, n (%) | 0.016 | |||
| Single | 107 (21.4) | 54 (21.7) | 53 (21.0) | |
| Married | 331 (66.1) | 175 (70.3) | 156 (61.9) | |
| Widowed | 31 (6.2) | 12 (4.8) | 20 (7.9) | |
| Divorced/separated | 32 (6.4) | 8 (3.2) | 23 (9.2) | |
| Educational level, n (%) | 0.49 | |||
| Primary school | 134 (26.7) | 66 (26.5) | 68 (27.0) | |
| Secondary school | 180 (35.9) | 94 (37.8) | 86 (34.1) | |
| Tertiary school | 18 (3.6) | 6 (2.4) | 12 (4.8) | |
| College university | 169 (33.7) | 83 (33.3) | 86 (34.1) | |
| Alcohol consumption | <0.001 | |||
| Teetotaler | 250 (49.9) | 93 (37.3) | 157 (62.3) | |
| Low risk | 201 (40.1) | 122 (49.0) | 79 (31.3) | |
| Moderate consumption | 37 (7.4) | 25 (10.0) | 12 (4.8) | |
| Risk consumption | 13 (2.6) | 9 (3.6) | 4 (1.6) | |
| Current smoker | 0.32 | |||
| Yes | 90 (18.0) | 49 (19.7) | 41 (16.3) | |
| No | 411 (82.0) | 200 (80.3) | 211 (83.7) | |
| Physical activity | <0.001 | |||
| Low | 87 (17.4) | 32 (12.9) | 55 (21.8) | |
| Moderate | 285 (56.9) | 124 (49.8) | 161 (63.9) | |
| Vigorous | 129 (25.7) | 93 (37.3) | 36 (14.3) | |
| BMI | <0.001 | |||
| Underweight ≤18.5 | 7 (1.4) | 1 (0.4) | 6 (2.4) | |
| Normal weight = 18.5–24.9 | 174 (34.9) | 71 (28.6) | 103 (41.2) | |
| Overweight = 25–29.9 | 223 (4.8) | 134 (54.1) | 89 (35.6) | |
| Obesity >30 | 94 (18.8) | 42 (16.9) | 52 (20.8) | |
| cfPWV, (m/s) | 6.1 (5.1–7.3) | 6.3 (5.3–8.1) | 5.8 (5.0–7.0) | 0.001 |
| baPWV, (m/s) | 12.4 (10.9–14.4) | 12.7 (11.2–14.6) | 12.1 (10.4–14.2) | 0.008 |
| CAVI | 7.9 (6.9–9) | 8.2 (6.9–9.2) | 7.7 (6.9–8.8) | 0.021 |
| Antidiabetic drugs | 35 (7.0) | 23 (65.7) | 12 (34.3) | 0.049 |
| Antihypertensive drugs | 96 (19.2) | 50 (52.1) | 46 (47.9) | 0.604 |
| Lipid–lowering drugs | 102 (20.4) | 49 (48.0) | 53 (52.0) | 0.707 |
| Type 2 Diabetes | 38 (7.6) | 26 (68.4) | 12 (31.6) | 0.016 |
| Hypertension | 147 (29.3) | 82 (32.9) | 65 (25.8) | <0.001 |
| Dyslipidemia | 326 (65.1) | 162 (49.7) | 164 (50.3) | 0.905 |
| Men (n = 249) | p-value a | Women (n = 252) | p-value b | p-value c | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age Ranges | Years 1 ≤ 50 n = 99 | 51 < Years 2 ≤ 70 n = 100 | Years 3 > 70 n = 50 | Years 1 ≤ 50 n = 100 | 51 < Years 2 ≤ 70 n = 102 | Years 3 > 70 n = 50 | |||
| FAT–SOLUBLE VITAMINS | |||||||||
| Vitamin A (ug/day) | 951.91 (673.90–1324.01) | 1022.90 (806.41–1308.50) | 1104.35 (704.51–1539.73) | 0.263 | 987.21 (750.23–1249.03) | 1209.43 (894.12– 1667.48) | 1074.08 (788.76–1327.50) | 0.003 * | 0.086 |
| Carotenoids (ug/day) | 2942.00 (1822.68–4476.31) | 3387.48 (2464.94–5215.30) | 3896.81 (2048.01–5651.49) | 0.035 *# | 3026.76 (2032.06–4564.49) | 4749.78 (2957.60–6611.49) | 4048.52 (2366.93–5497.64) | <0.01 * | 0.034 |
| Retinol (ug/day) | 353.20 (261.18–471.66) | 359.17 (225.60–494.01) | 312.33 (224.05–385.93) | 0.238 | 364.75 (261.29–502.47) | 335.18 (247.60–444.28) | 291.69 (210.52–402.65) | 0.009 # | 0.997 |
| Vitamin D (ug/day) | 5.60 (3.51–8.12) | 5.94 (4.04–8.42) | 5.94 (4.50–7.99) | 0.701 | 5.95 (3.92–8.39) | 5.34 (4.07–7.80) | 5.13 (3.59–7.73) | 0.585 | 0.635 |
| WATER–SOLUBLE VITAMINS | |||||||||
| Vitamin C (mg/day) | 1 61.07 ± 92.11 | 166.48 ±78.07 | 192.90 ± 89.00 | 0.104 | 154.83 ± 69.70 | 201.09 ± 84.63 | 198.76 ±70.30 | <0.01 *# | 0.094 |
| Vitamin B1 (mg/day) | 1.62 (1.21–2.20) | 1.61 (1.28–2.04) | 1.71 (1.45–2.07) | 0.450 | 1.47 (1.14–1.87) | 1.57 (1.23–1.91) | 1.55 (1.32–1.86) | 0.429 | 0.015 |
| Vitamin B2 (mg/day) | 1.79 (1.42 –2.32) | 1.71 (1.36–2.17) | 1.83 (1.67–2.21) | 0.404 | 1.64 (1.35–2.09) | 1.84 (1.47–2.23) | 1.81 (1.56–2.09) | 0.107 | 0.254 |
| Vitamin B3 (mg/day) | 39.79 ± 13.01 | 40.14 ± 9.18 | 41.19 ± 9.45 | 0.768 | 38.13 ± 11.54 | 37.57 ± 11.36 | 35.14 ± 9.64 | 0.294 | 0.004 |
| Vitamin B6 (mg/day) | 2.46 (1.91–3.20) | 2.55 (2.00–3.04) | 2.66 (2.14–3.30) | 0.504 | 2.30 (1.87–2.73) | 2.50 (2.01–3.11) | 2.35 (2.10–2.98) | 0.169 | 0.128 |
| Vitamin B9 (ug/day) | 305.43 ± 103.36 | 319.08 ± 100.19 | 358.59 ± 107.39 | 0.015# | 295.65 ± 108.08 | 364.23 ± 130.92 | 354.40 ± 85.32 | <0.01 *# | 0.185 |
| Vitamin B12 (ug/day) | 8.45 (5.97–11.84) | 8.94 (6.85–13.63) | 10.14 (7.35–16.99) | 0.050 | 7.40 (5.61–9.10) | 8.83 (6.36–13.09) | 7.84 (6.27–10.98) | 0.016 * | 0.004 |
| Men (n = 249) | Women (n = 252) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Years 1 ≤ 50 (n = 99) | 51 < Years 2 ≤ 70 (n = 100) | Years 3 > 70 (n = 50) | p-value | Years 1 ≤ 50 (n = 100) | 51 < Years 2 ≤ 70 (n = 102) | Years 3 > 70 (n = 50) | p-value | ||
| FAT–SOLUBLE VITAMINS | FAT–SOLUBLE VITAMINS | ||||||||
| Vitamin A (ug/day) DRI: 900 | 52 (54.5) | 60 (60.0) | 29 (58.0) | 0.489 | Vitamin A DRI:700 | 75 (75.0) | 85 (83.3) | 41 (82.0) | 0.271 |
| Vitamin D (ug/day) DRI: 15,15,20 | 2 (2.0) | 3 (3.0) | 0 (0.0) | 0.470 | Vitamin D DRI:15,15,20 | 5 (5.0) | 5 (4.9) | 1 (2.0) | 0.239 |
| WATER–SOLUBLE VITAMINS | WATER–SOLUBLE VITAMINS | ||||||||
| Vitamin C (mg/day) DRI: 90 | 73 (73.7) | 83 (83.0) | 40 (80.0) | 0.160 | Vitamin C DRI:75 | 88 (88.0) | 95 (93.2) | 48 (96.0) | 0.109 |
| Vitamin B1 (mg/day) DRI: 1.2 | 72 (72.7) | 76 (76.0) | 44 (88.0) | 0.074 | Vitamin B1 DRI:1.1 | 77 (77.0) | 84 (82.3) | 41 (82.0) | 0.547 |
| Vitamin B2 (mg/day) DRI: 1.3 | 82 (82.8) | 77 (77.0) | 42 (84.0) | 0.490 | Vitamin B2 DRI:1.1 | 88 (88.0) | 94 (92.2) | 47 (94.0) | 0.291 |
| Vitamin B3 (mg/day) DRI: 16 | 95 (95.6) | 95 (95.0) | 48 (96.0) | 0.935 | Vitamin B3 DRI:14 | 99 (99.0) | 100 (98.0) | 49 (98.0) | 0.479 |
| Vitamin B6 (mg/day) DRI: 1.3,1.7,1.7 | 94 (94.9) | 94 (94.0) | 45 (93.8) | 0.951 | Vitamin B6 DRI:1.3,1.5,1.5 | 93 (93.0) | 95 (93.2) | 44 (88.0) | 0.505 |
| Vitamin B9 (ug/day) DRI: 400 | 16 (16.2) | 22 (22.0) | 16 (32.0) | 0.084 | Vitamin B9 DRI:400 | 18 (18.0) | 34 (33.3) | 35 (70.0) | 0.043 |
| Vitamin B12 (ug/day) DRI: 2.4 | 95 (96.0) | 95 (95.0) | 48 (96.0) | 0.935 | Vitamin B12 DRI:2.4 | 98 (8.0) | 98 (96.1) | 48 (96.0) | 0.782 |
| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
| β | (95% CI) | p | β | (95% CI) | p | ||
| cfPWV | cfPWV | ||||||
| Age | 0.105 | 0.090; 0.120 | <0.001 | Age | 0.084 | 0.071; 0.097 | <0.001 |
| Retinol (ug/day) | 0.001 | 0.000; 0.001 | 0.023 | Vitamin B2 (mg/day) | −0.475 | −0.828; −0.121 | 0.009 |
| Vitamin B1 (mg/day) | 0.433 | −0.061; 0.926 | 0.085 | Vitamin B3 (mg/day) | 0.023 | 0.003; 0.043 | 0.027 |
| Vitamin B2 (mg/day) | −0.620 | −1.183; −0.058 | 0.031 | ||||
| baPWV | baPWV | ||||||
| Age | 0.120 | 0.104; 0.136 | <0.001 | Age | 0.159 | 0.143; 0.174 | <0.001 |
| Vitamin B3 (mg/day) | −0.023 | −0.050; 0.003 | 0.086 | Vitamin B1 (mg/day) | −0.523 | −1.018; −0.028 | 0.038 |
| Vitamin B6 (mg/day) | 0.384 | 0.038; 0.730 | 0.030 | Vitamin B3 (mg/day) | 0.024 | −0.002; 0.050 | 0.072 |
| Vitamin B9 (ug/day) | −0.003 | −0.006; 0.000 | 0.032 | Vitamin B12 | −0.019 | −0.038; 0.000 | 0.046 |
| CAVI | CAVI | ||||||
| Age | 0.077 | 0.067; 0.086 | <0.001 | Age | 0.069 | 0.061; 0.078 | <0.001 |
| Vitamin A (ug/day) | 0.001 | 0.000; 0.001 | 0.073 | Vitamin D (ug/day) | −0.046 | −0.080; −0.012 | 0.007 |
| Vitamin B6 (mg/day) | 0.123 | −0.016; 0.262 | 0.083 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patino-Alonso, M.C.; Gómez Sánchez, M.; Gómez Sánchez, L.; Alonso-Domínguez, R.; Sánchez-Aguadero, N.; Sánchez Salgado, B.; Rodríguez Sánchez, E.; García Ortiz, L.; Gómez-Marcos, M.A. Multivariate Analysis of Influence of Vitamin Intake on Vascular Function Parameters by Sex in the General Spanish Population: EVA Study. Nutrients 2020, 12, 643. https://doi.org/10.3390/nu12030643
Patino-Alonso MC, Gómez Sánchez M, Gómez Sánchez L, Alonso-Domínguez R, Sánchez-Aguadero N, Sánchez Salgado B, Rodríguez Sánchez E, García Ortiz L, Gómez-Marcos MA. Multivariate Analysis of Influence of Vitamin Intake on Vascular Function Parameters by Sex in the General Spanish Population: EVA Study. Nutrients. 2020; 12(3):643. https://doi.org/10.3390/nu12030643
Chicago/Turabian StylePatino-Alonso, Maria C, Marta Gómez Sánchez, Leticia Gómez Sánchez, Rosario Alonso-Domínguez, Natalia Sánchez-Aguadero, Benigna Sánchez Salgado, Emiliano Rodríguez Sánchez, Luis García Ortiz, and Manuel A Gómez-Marcos. 2020. "Multivariate Analysis of Influence of Vitamin Intake on Vascular Function Parameters by Sex in the General Spanish Population: EVA Study" Nutrients 12, no. 3: 643. https://doi.org/10.3390/nu12030643






