Abstract
Aging is associated to cognitive decline, which can lead to loss of life quality, personal suffering, and ultimately neurodegenerative diseases. Neuroinflammation is one of the mechanisms explaining the loss of cognitive functions. Indeed, aging is associated to the activation of inflammatory signaling pathways, which can be targeted by specific nutrients with anti-inflammatory effects. Dietary n-3 polyunsaturated fatty acids (PUFAs) are particularly attractive as they are present in the brain, possess immunomodulatory properties, and are precursors of lipid derivates named specialized pro-resolving mediators (SPM). SPMs are crucially involved in the resolution of inflammation that is modified during aging, resulting in chronic inflammation. In this review, we first examine the effect of aging on neuroinflammation and then evaluate the potential beneficial effect of n-3 PUFA as precursors of bioactive derivates, particularly during aging, on the resolution of inflammation. Lastly, we highlight evidence supporting a role of n-3 PUFA during aging.
1. Introduction
Aging is a world concern as the elderly population tripled from 4% to 13% in the last century and is expected to grow sharply to reach 20% of the population in 2025 and 33% in 2050 [1]. Aging is associated to cognitive decline for 15–20% of the elderly >65 [2,3,4]. These cognitive alterations can lead to age-related disease such as neurodegenerative diseases. Alzheimer’s disease is the predominant one, affecting 24 million people in the world [5]. Thus, healthy aging constitutes a real economic challenge of the 21st century for the nations. The mechanisms explaining this process are still not fully elucidated, but neuroinflammation seems largely involved. Then, strategies to reduce and resolve neuroinflammation in a time-limited manner are encouraged. Recent studies suggest that nutrition, particularly fish oil, has promising anti-inflammatory effects. Fish oil contains n-3 long chain polyunsaturated fatty acids (LC-PUFAs), which are precursors of bioactive lipids called specialized pro-resolving mediators (SPMs) that largely contribute to this beneficial effect. Here, we review the effect of aging on neuroinflammation, in particular microglia activity and cognitive decline, and how n-3 LC-PUFAs and their derivates impact neuroinflammation, especially during aging. We discuss that nutrition, an environmental factor to which individuals are exposed throughout life, plays a key role to prevent or delay neuroinflammation during aging.
2. Aging and Neuroinflammation
Brain aging is associated to a chronic low-grade inflammation in the central nervous system (CNS) [6]. Microglial cells are the resident innate immune cells of the CNS and are involved in various physiological and pathophysiological functions [7,8]. These cells initiate the immune response when they recognize damage- (DAMPs) and pathogen-associated molecular patterns (PAMPs) thanks to their various pattern recognition receptors (PRRs), including toll-like receptors (TLRs) and nucleotide oligomerization domain (NOD)-like receptors [9]. They are strictly regulated by signals from the CNS [10] and with aging, they change their morphology, reduce their arborization, and decrease their mobility in human, non-human primates and rodents and then become senescent [11,12,13,14,15,16,17,18]. Indeed, aged microglia are “primed”, and are characterized by increased production of inflammatory markers, at baseline and in response to an immune stimulus, and by a decreased capacity to return to homeostasis [19,20,21]. Aged microglia also fail to degrade myelin fragments, resulting in the accumulation of lipofuscin granules, markers of microglial aging [22,23,24]. Thus, during aging, microglial functions change, resulting in increased immune age-related responses, driving the development of cognitive deficits, impaired synaptic plasticity and the progression of neurodegenerative pathologies [25,26]. These changes are mainly the result of age-induced defective mechanisms driving the inflammatory response [21,27].
During aging, under the basal condition, there is an increase in the expression of blood and brain levels of pro-inflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and interleukin-18 (IL-18), and a decrease in the expression of anti-inflammatory factors such as interleukin-10 (IL-10), interleukin-4 (IL-4), or brain derived neurotrophic factor (BDNF) [28,29]. Compared with young mice, aged mice have a higher expression of IL-6 in the hippocampus, cortex, and cerebellum [30,31], and a lower expression of IL-10 [32]. In aged microglial cells, there is a constant production of nuclear factor-kappa B (NFκB), a transcription factor involved in the activation of inflammatory pathways, leading to increased production of IL-6 [33]. Aged microglial cells from rodents produce more IL-1β and IL-6 than young ones [22,34,35,36]. Moreover, the serum level of IL-6 in elderly has been linked to the incidence of deficits in mobility and walking speed [37,38,39]. Markers of microglial activation are also increased during aging: major histocompatibility complex II (MHC II) [40,41], CD68 [42,43], caspase-1, as well as CD11b [44]. Indeed, in elderly without neurological pathologies, MHC II expression is related to increased brain IL-1β expression [45]. In the same way, ex vivo and in situ studies have shown that microglial cells of aged rats and mice display, compared with those of younger animals, a greater expression of MHC II, CD11b, and CD68—all markers of microglial cells’ activation [42,43]. The number of microglial cells expressing MHC II also increases with age in nonhuman primates [15] and in rats [18]. In the hippocampus, the number of microglial cells increases by 20% in aged mice compared with young adults [46].
The loss of homeostatic functions of microglia is a hallmark of unhealthy brain aging and neurodegenerative disorders [47]. Interestingly, recent studies using high-dimensional single-cell mapping or single cell RNAseq revealed that molecular signatures of microglia is altered with aging with some similar genes in rodents and humans [48,49,50]. The identification of aged-microglia subtypes allow to identify specific markers associated to unhealthy aging. Recent data pinpoint that mutations in triggering receptor expressed on myeloid cells 2 (Trem2) and colony stimulating factor 1 receptor (Csf1r) in microglia are responsible of neurodegenerative diseases, reinforcing the essential role of microglia in healthy aging. In elderly, the soluble form of Trem2 in the cerebrospinal fluid was associated to attenuated cognitive decline [51].
The increase in cytokine production in the blood and brain has been associated to age-related cognitive decline. IL-6 levels in the plasma of elderly have been positively correlated to cognitive decline, in particular to loss of speed of information processing [52,53,54]. This is in agreement with the fact that IL-6-deficient mice are protected from age-related decline of their cognitive performance following a bacterial endotoxin infection as compared with wild-type mice [55,56]. These mice also have less pro-inflammatory cytokines in the hippocampus. Moreover, in aged rodents, it is hippocampal IL-1β that is associated to impairment of learning and memory [57,58,59,60]. Pharmacological inhibition of IL-1β as well as its converting enzyme (ICE), which is essential for the release of IL-1β, has allowed to reduce memory impaiments induced by infection or stress in aged mice [61,62] and has improved the performance of aged rats [63]. Other studies have shown an increased expression of the NOD-like receptor protein 3 (NLRP3) in the hippocampus of aged mice, which regulates caspase-1 activation, and thus the maturation and secretion of IL-1β and IL-18 [64,65,66]. This NLRP3 activation by DAMPs as well as the production of reactive oxygen species (ROS) have been associated to age-related cognitive decline and neuropathological changes [67,68,69].
All these studies reveal that inflammation during aging characterized by microglial activation and pro-inflammatory cytokine production is partly responsible for age-related cognitive decline. Hence, reducing this low grade inflammation constitutes a good strategy to prevent age-related cognitive decline and the development of neurodegenerative pathologies.
3. N-3 PUFAs as Precursors of Lipid Mediators Involved in the Resolution of Inflammation
In the brain, the main n-3 LC-PUFA is docosahexaenoic acid (DHA), which represents 12–14% of total fatty acids in the brain [70,71,72,73,74,75] and has key-regulator functions in inflammation. Eicosapentaenoic acid (EPA) is the other n-3 LC-PUFAs of great importance, despite its low level in the brain because of its beta-oxidation [76]. Indeed, it is a precursor of many bioactive derivatives. N-3 LC-PUFAs can be synthesized from n-3 PUFA precursor alpha-linolenic acid (ALA), but the conversion rate is very low in humans [77,78] and becomes less efficient with aging [79,80]. Then, it is recommended to consume fish, which is the main dietary source of n-3 LC-PUFAs [80]. The absence of n-3 LC-PUFA consumption and/or a defect in their metabolism is responsible for increased neuroinflammation, leading to neurological disorders [81]. Indeed, numerous reviews have reported the powerful anti-inflammatory properties of n-3 LC-PUFAs [82,83,84,85,86].
Several mechanisms have been proposed to explain the immunomodulatory properties of n-3 LC-PUFAs. One of the most attractive is the synthesis of bioactive lipid mediators or oxylipins. These oxylipins are synthesized sequentially: first, those involved in the regulation of inflammation such as the eicosanoids (prostaglandins, leukotrienes, thromboxane), and then those involved in the resolution of inflammation called SPMs (resolvins, protectins, maresins). SPMs have both anti-inflammatory and pro-resolutive properties without immune suppression and induce a return to homeostasis [87,88,89,90]. They actively coordinate and finely tune the inflammatory response. They down-regulate the pro-inflammatory cytokines and up-regulate the anti-inflammatory cytokines, promote the phagocytosis of cellular debris and dead cells without immune suppression, reduce the concentration, and compete with pro-inflammatory oxylipins derived from n-6 PUFAs. Then, they underlie most of the beneficial effects attributed to their precursors [84,91,92,93]. Several enzymes are responsible for their synthesis: phospholipases A2 (PLA2s) for the release of fatty acids from the membranes, as well as cyclooxygenase (COX)-2, lipoxygenase (LOX), and cytochrome P450 monoxygenases (CYP450) [94]. They convert DHA and EPA into bioactive lipid mediators. In human serum, DHA- and EPA-derivates represent 30.7% and 25.9% of the identified SPMs, respectively [95,96]. These enzymes are expressed in the brain [97,98,99,100]. Following an inflammatory stimulus such as lipopolysaccharide (LPS), COX-2 is rapidly expressed in the hippocampus [100,101]. It was shown that COX-2 inhibition delays resolution of acute inflammation [102]. 15-LOX and 5-LOX are the most abundant LOX in the brain [97]. 15-LOX is both neurotoxic owing to the oxidative stress it generates [103] and neuroprotective owing to the SPMs it synthesizes [104,105]. Indeed, the impairment of 15-LOX activity (by gene deletion or pharmacological inhibition) reduces the SPM production in the brain and is associated to cognitive alterations [97]. CYP450 produces anti-inflammatory n-6 derived epoxides [106,107,108,109]. These enzymes have also been identified in brain cells such as microglia, astrocytes, oligodendrocytes, and neurons [110,111,112,113].
3.1. DHA-derived SPMs
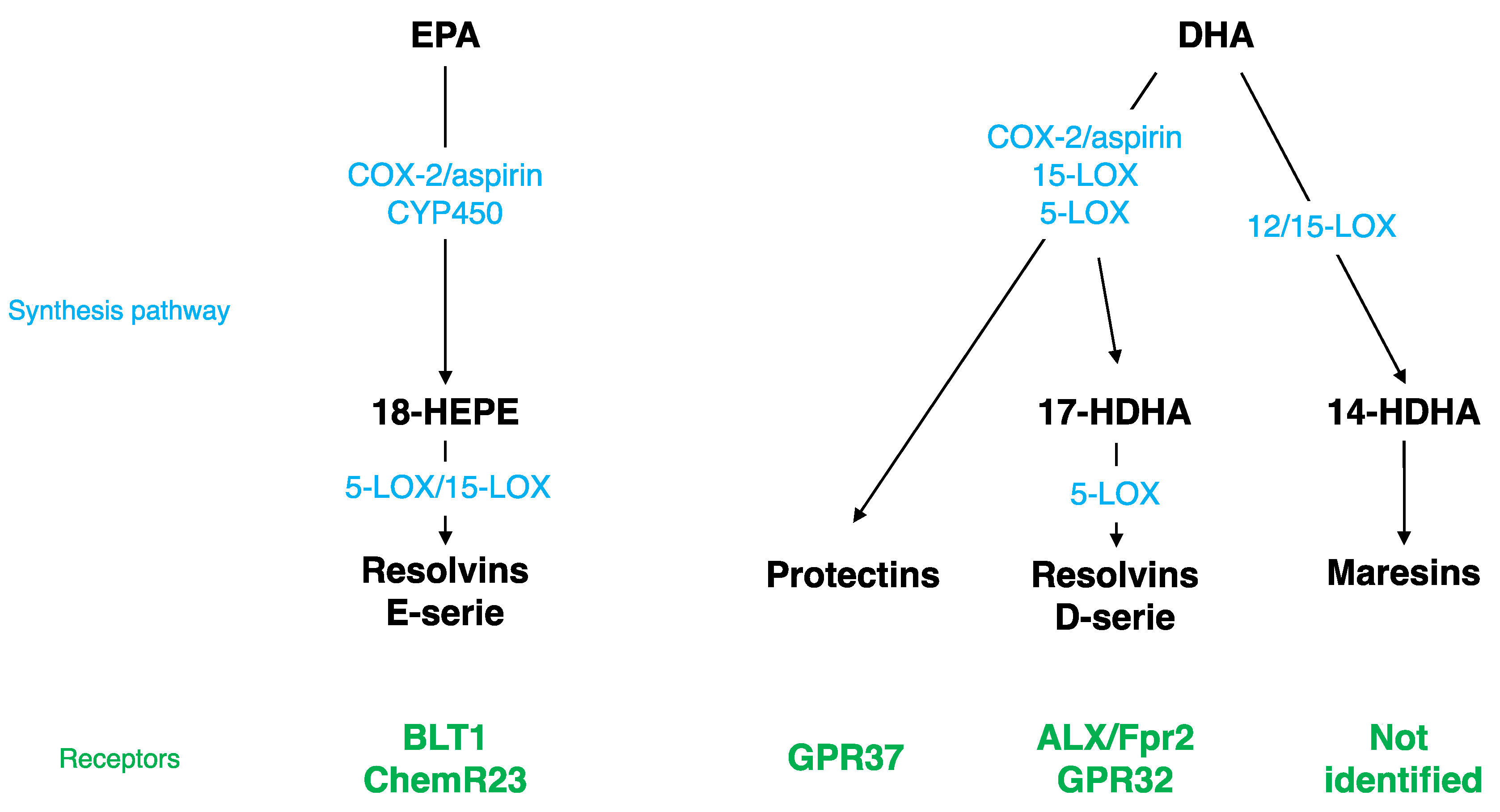
Different SPMs can be synthesized from DHA (Figure 1): monohydroxy DHA (17-HDHA) by acetylated COX-2, CYP450, and 15-LOX [114,115] and resolvin D1 (RvD1) via the production of 17-HDHA by 5-LOX [116,117]. These bioactive derivates have been mostly described at the periphery, but have also been detected in the brain. RvD1 was measured in mouse brain following cerebral ischemia [118]. Its level is modulated by a DHA intravenous injection [119] and during inflammation; it decreases at the beginning and then increases during the resolution phase [120]. RvD1 acts at picomolar range, but exerts its biological effects at nanomolar range [117,121]. The receptor of RvD1 is lipoxin A4 receptor/formyl peptide receptor 2 (ALX/FPR2) in rodents and G protein coupling receptor 32 (GPR32) in humans [116]. Several brain structures express ALX/FPR2: brainstem, spinal cord, hypothalamus, cortex, hippocampus, cerebellum, and striatum [122]. At the cellular level, these receptors are expressed in microglial cells [123], neurons [122,124], and astrocytes [125,126]. Via these receptors, RvD1 regulates micro-RNAs (miRNAs), which play a key role in modulating the expression of target genes such as inflammatory genes [123,125,127,128,129].
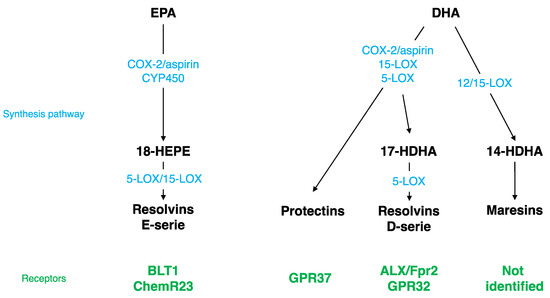
Figure 1.
Specialized Pro-resolving Mediator (SPM) synthesis pathway and receptors. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LOX, lipoxygenase; COX, cyclooxygenase; ALX/Fpr2, lipoxin A4 receptor/formyl peptide receptor 2; GPR32, G protein coupling receptor 32; BLT1, leukotriene B4 receptor; HDHA, monohydroxy DHA; CYP450, cytochrome P450 monoxygenases.
Other SPMs are derived from DHA: di-hydroxy-DHA termed protectin D1 (PD1) or neuroprotectin D1 (NPD1) when produced in the CNS by 5- and 15-LOX [130,131,132,133], and maresin 1-2 (MaR1-2) by 12/15-LOX [114,115,134]. NPD1, MaR1, and its precursor 14-HDHA were measured in the hippocampus [135]. The level of NPD1 and MaR1 decreases in the hippocampus of Alzheimer’s disease patients [136,137] and the level of NPD1 greatly increases following brain ischemia or acute central LPS injection [118,135]. NPD1 receptor has been identified only at the periphery in macrophages, but not in microglia [138], whereas the MaR1 receptor has not been identified yet [136]. NPD1 regulates NFκB, and then consequently pro-inflammatory gene expression [118,139,140]. MaR1 decreases pro-inflammatory signaling cascades and influences macrophages towards an M2 repair phenotype after cerebral ischemia or spinal cord injury [141,142,143].
3.2. EPA-derived SPMs
EPA is converted by acetylated COX-2 or CYP450 into 18R-HEPE, which is then metabolized into resolvins E1, E2, and E3 by 5- or 15-LOX (Figure 1) [114,144,145]. These derivates have been detected in the hippocampus [135,146,147]. RvE1 induces a decrease in LPS-induced pro-inflammatory cytokines’ (TNF-α, IL-6, IL-1β) gene expression in microglial cells by inhibiting the NFκB signaling pathway [123]. The receptors of RvE1 are a G protein coupling receptor, ChemR23, or chemokine like receptor 1 (CMKLR1) [144] and a leukotriene B4 receptor (BLT1) [148]. ChemR23 has been identified in the prefrontal cortex, hippocampus, and brainstem [149]. These receptors are also expressed in microglial cells [123,150], neurons [122,124], and astrocytes [126].
4. Role of Lipid Mediators in the Resolution of Inflammation
A large number of studies support the beneficial role of n-3 LC PUFAs in inflammation in human and animal models of acute and chronic inflammation, including in the brain (for recent reviews, see [82,83]). Here, we will review the biological roles at the brain level of RvD1 and RvE1, two distinct lipid mediators generated from the n-3 LC-PUFAs DHA and EPA, known for their powerful anti-inflammatory and pro-resolutive properties.
4.1. In Humans
The effect of RvD1 was mainly studied in Alzheimer’s and Parkinson’s patients (Table 1). In patients with dementia, the levels of RvD1 in cerebrospinal fluid are positively associated with the improvement of cognitive functions [126]. RvD1 promotes Aβ phagocytosis by macrophages isolated from Alzheimer’s patients, reducing the amyloid load [151,152]. Moreover, as cited in Krashia et al., endogenous RvD1 is decreased in patients diagnosed with early-Parkinson’s disease [153]. As a result, the decrease of RvD1 levels in Alzheimer’s and Parkinson’s disease patient’s brain could contribute to the disease development and progression. Conversely, an increased anti-inflammatory RvD1 activity has been reported in maniac and depressive patients, suggesting that RvD1 may serve to improve inflammatory imbalance [154].

Table 1.
Role of lipid mediators in the resolution of inflammation in humans.
The effect of RvE1 was reported in patients at the periphery (Table 1) [155,156,157], but not at the brain level. Hence, more studies are needed to develop this area.
4.2. In Animals
Several studies report that, in rodent models of inflammation, RvD1 and RvE1 display anti-inflammatory activities in the CNS (Table 2). Indeed, RvD1 reduces the activation of NFκB and the expression of pro-inflammatory factors such as IL-1β, IL-6, TNF-α, and iNOS in rats with hemorrhagic shock or in streptozotocin (STZ)-induced diabetic retinopathy [158,159]. RvD1 attenuates neuroinflammation through ALX-FPR2 receptor via miRNA in a neonatal hypoxia-ischemia rat pup model or in a remote damage model [125,160]. Moreover, RvD1 induces the polarization of macrophages and microglia toward an M2 phagocytic phenotype [161,162,163]. Chronic and early RvD1 administration in a rat model of Parkinson’s disease prevents central and peripheral inflammation, as well as neuronal dysfunction and motor deficits [153]. In addition, the precursors of RvD1, 17R-HDHA and 17S-HDHA, reduce the production of pro-inflammatory cytokines in the spinal cord and in the hippocampus [135,164].

Table 2.
Role of lipid mediators in the resolution of inflammation in animals.
RvE1 reduces the expression of pro-inflammatory cytokines IL-1β and IL-6 in the prefrontal cortex and decreases the neuropathological features of Aβ pathology in a murine model of Alzheimer’s disease [165]. Furthermore, repeated RvE1 administration moderates the activation of microglia by promoting ramified microglia following traumatic brain injury or peripheral brain injury [166].
The effect of RvD1 on neuroinflammation is associated to effects on cognition. Indeed, RvD1 prevents cognitive deficits. In a rodent model of systemic inflammation or traumatic brain injury, an intraperitoneal administration of 17R-RvD1 prevents cognitive decline [166,167]. Of note, higher levels of brain RvD1 in Fat-1 mice, owing to higher brain n-3 LC-PUFAs induced by genetic means, are linked to less cognitive deficits, a reduction in microglial activation, and in pro-inflammatory status following brain ischemia [168,169]. Conversely, lower levels of brain RvD1, owing to 15-LOX inhibition, are related to alterations in working memory and synaptic plasticity in rats [97].
RvD and RvE have been reported to prevent emotional behavior alterations in rodent models of mood disorders in the review of Furuyashiki et al. [170]. These SPMs have positive effects in LPS-induced or chronic stress-induced or post-myocardial infarct depression [164,171,172,173,174,175,176].
4.3. In Vitro
The effects of RvD1 and RvE1 were tested on different brain cells, highlighting their pro-resolutive properties (Table 3). In microglial cells, RvD1 enhances the effect of the anti-inflammatory cytokines IL-4, Arg1, and Ym1 and reduces the activation of microglia by decreasing CD11b expression, leading to a more anti-inflammatory phenotype of microglia [163,177,178]. Moreover, RvD1 reduces LPS-induced pro-inflammatory cytokine (TNF-α, IL-6, and IL-1β) gene expression in microglial BV2 cells by regulating miRNA expression [123]. It was also reported that RvD1 down-regulates Aβ-induced inflammation in human microglia [136]. RvD2 decreases the expression of toll like receptor 4 (TLR4, the receptor of LPS) following LPS treatment, and consequently its downstream signaling pathway NFκB [179]. RvE1 also reduces microglial activation and pro-inflammatory cytokine release in microglial cells [123,177]. In astrocytes, RvD1 decreases LPS-induced TNF-α production [164]. In neurons from spinal nods, RvD1 increases neurite outgrowth [180]. In PC12 neural cells, used as an in vitro model of Parkinson’s disease, RvD1 reduces TNF-α and IL-6 mRNA expression [181]. The anti-inflammatory properties of RvD1 were also tested in macrophage cells. RvD1 reduces the expression of pro-inflammatory markers (cytokines, PGE2) and increases anti-inflammatory cytokine IL-10 in murine macrophages stimulated by LPS [182]. RvD1 polarizes primary human macrophages toward a pro-resolutive phenotype through GPR32 receptor [183].

Table 3.
Role of lipid mediators in the resolution of inflammation in vitro.
5. Defects in Lipid Metabolism and Lipid Mediator Production during Aging
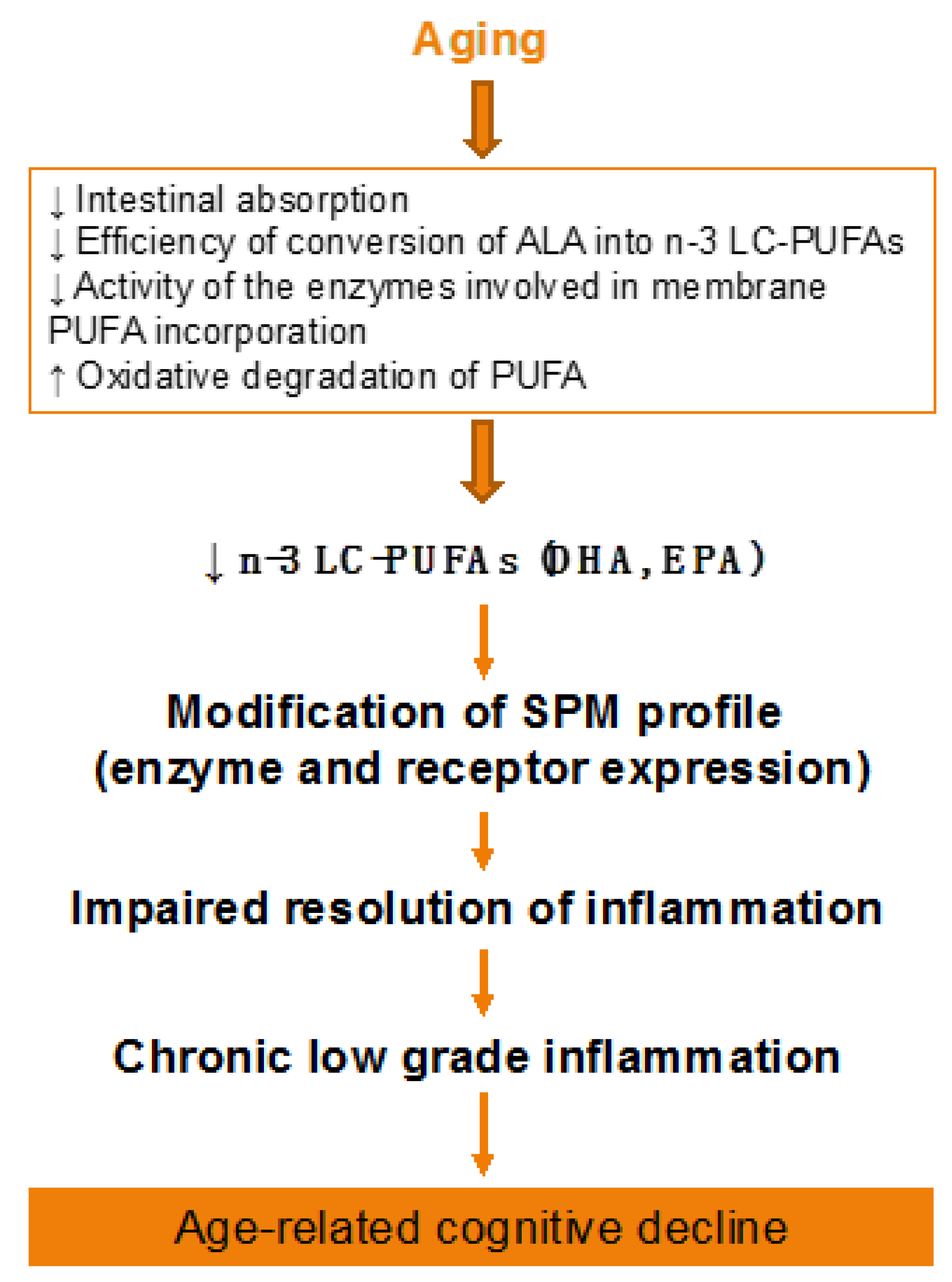
During aging, brain levels of n-3 LC-PUFAs decrease, although all brain structures are not affected in the same way [30,32,70,184]. This reduction was observed in human [185,186], especially in the cortex, the hippocampus, and the cerebellum [73,187,188,189], and in rodents [30,32,190,191], in particular in the hippocampus [191] and the cortex [73], which are key structures in mnesic processes. This decrease is mainly because of changes in lipid metabolism: alteration in the intestinal absorption of essential fatty acids [192,193,194]; impairment in the enzymes of phospholipid synthesis [195]; reduced conversion rates of the precursors into LC-PUFAs owing to reduced activity of the enzymes involved in their synthesis, in particular of Δ6 desaturase [186,196,197]; and modifications in the expression of the genes implicated in the metabolism of PUFAs. Indeed, single nucleotide polymorphisms (SNPs) in desaturase genes FADS1 (Δ5 desaturase), FADS2 (Δ6 desaturase), as well as ELOVL2 (elongase 2) are related to higher ALA and lower EPA plasma phospholipid levels with age, suggesting different rates of conversion [198]. Moreover, another possible reason of the decrease of n-3 LC-PUFAs in the membranes is their high propensity to oxidation to generate peroxidation products such as malonaldehyde (MDA), 4-hydroxy-2-nonenal (4-HNE), or 4-hydroxy-2-hexenal (4-HHE). Indeed, levels of MDA and 4-HNE are increased in aged brain of humans and rodents [199,200].
Aging-associated DHA metabolism disturbance could participate in cognitive decline (Figure 2). This has been demonstrated both in humans and animals. In elderly, decreased n-3 PUFA consumption associated to reduced erythrocyte DHA levels are inversely correlated with age-related cognitive decline [201,202,203]. In rats, a low-DHA dietary supply for one or more generations is related to alterations in cognitive function [204,205,206]. In addition, we showed in aged mice that an n-3 PUFA deficient diet impairs memory as well as neuroinflammation and synaptic plasticity [32,207,208,209,210]. Furthermore, the decrease in brain DHA content induced by a n-3 PUFA deficient diet increases vulnerability to inflammation, which trigger both synaptic and memory alteration [211,212]. On the contrary, a two-month n-3 LC-PUFA supplementation in aged mice (between 20 and 22 months old) reverses age-induced spatial memory deficits [30].
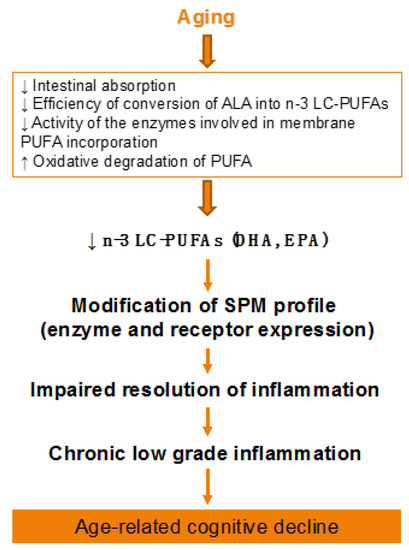
Figure 2.
Effect of aging on lipid metabolism. ALA, alpha-linolenic acid; PUFA, polyunsaturated fatty acid; SPM, specialized pro-resolving mediator.
Age-related alteration of n-3 PUFA metabolism contributes to reducing the n-3 LC-PUFA content in brain phospholipids. As n-3 LC-PUFAs are precursors of bioactive mediators involved in the resolution of inflammation, it may have consequences on SPM profile and production. Indeed, it was recently shown that blood oxylipin profile is altered in 45–64-year-old healthy men and women versus 19–28-year-old young people [213,214]. Moreover, Gangemi et al. (2005) demonstrated that aging is associated to a decrease in urinary LxA4/leukotriene, a ratio of anti-inflammatory/pro-inflammatory mediators synthesized from arachidonc acid and considered as an index of the endogenous anti-inflammatory potential [215]. Moreover, LxA4 is significantly lower in cerebrospinal fluid (CSF) of humans with Alzheimer’s disease as compared with humans with mild cognitive impairment or subjective cognitive impairment, with a positive correlation between CSF LxA4 and cognitive performance [126].
In animals, oxylipin profile modification was also reported with aging. Aged rodent brains display higher levels of TxB2, 6-keto-PGF1α, and PD1-like metabolites [214]. In a model of senescence-accelerated prone mice (SAMP8), the cortex contains higher levels of PGE2, TxB2, and 9,10-DiHOME and lower levels of 20-HETE and DHA-derived mediators (11-, 14-, and 20-HDoHE) [214]. However, when compared with same age senescent-accelerated mouse resistant 1 (SAMR1) mice, SAMP8 mice do not exhibit any difference in LXA4 or RvD1 levels, despite a greater degree of inflammation in SAMP8 mice [216]. Moreover, aged BalbC mice display higher levels of pro-inflammatory LTB4 and PGs, but lower anti-inflammatory RvD1 and MaR1 in peritoneal macrophages compared with young mice [217].
The modifications of oxylipin profile are linked to changes in the expression of the enzymes involved in oxylipin formation. In humans, the expression of PLA2 and LOX increases with aging in post-mortem brain [214]. Similar results were obtained in 70-year-old versus 41-year-old patients concerning PLA2 and CYP [214]. In Alzheimer’s disease patients, 15-LOX level is also increased in the hippocampus [126].
In animals, the expression of 5-LOX is increased with aging [214] whereas the expression of 12-LOX is decreased in nine-month-old SAMP8 mice [216].
The changes in oxylipin profile may have compensatory consequences on their receptors. Indeed, in humans, ALX/FPR2 and ChemR23 levels are higher in the hippocampus of Alzheimer’s disease patients as compared with controls [126]. A similar result was obtained for ALX/FPR2 in SAMP8, despite that its level is similar to the SAMR1 controls [216].
All these results suggest an altered resolution of inflammation during aging that may contribute to the age-related cognitive decline, as high inflammation is associated to altered cognition.
6. Evidence Supporting a Role of Dietary n-3 PUFAs during Aging
Bioactive nutrients such as n-3 PUFAs constitute an interesting potential way to prevent or delay neuroinflammation that occurs during aging. Here, we will focus on dietary n-3 PUFAs because they modify the levels of brain n-3 LC-PUFAs [83,84,218] that are both anti-inflammatory and pro-resolutive and prevent cognitive decline associated to aging.
Evidence in humans (Table 4) and animals (Table 5) supports a powerful role of n-3 LC-PUFAs in the regulation of both inflammatory pathways, and in fine, in the resolution of inflammation, including in the brain (recently reviewed in [83]). Here, we will focus on dietary supplementation using n-3 LC PUFAs during aging. Barberger-Gateau highlighted in elderly that the more they eat n-3 PUFAs, the less they are subjected to cognitive decline [219]. Similarly, Tan et al. showed in the Framingham Study participants that lower erythrocyte DHA levels are related to cognitive impairment [220]. Moreover, in a prospective observational study, baseline dietary DHA intake levels at 70 years old are positively correlated with a better declarative memory test performance at the age of 75 in a healthy population [221]. Dietary supplementation with n-3 PUFAs conducted in humans has been motivated by observational studies showing the link between dietary consumption of DHA and improved cognitive function and/or reduced cognitive decline in the elderly. Indeed, fish oil consumption, leading to increased levels of DHA in erythrocytes, has been associated with better cognitive performance in elderly [222] and with a lower risk of developing neurological disorders [223,224,225]. DHA dietary supply is associated to better performance and speed in a verbal learning test in a cohort of 45–70-year-old healthy individuals [226] and to improved mini mental state examination (MMSE) scores, used to evaluate cognitive functions and memory abilities, in a cohort of elderly of 75-year-olds [227]. Yurko-Mauro et al. have shown in a systematic meta-analysis that DHA intake improves episodic, working and semantic memories [228]. More recently, McNamara et al. have revealed that fish oil consumption decreases self-reported inefficiencies in everyday functioning as well as improves cognition in elderly with cognitive complaints [229]. Moreover, circulating n-3 PUFAs (including DHA) have been negatively associated to the level of cytokines [230,231,232].

Table 4.
Evidence supporting a role of dietary n-3 PUFAs during aging in humans.

Table 5.
Evidence supporting a role of dietary n-3 PUFAs during aging in animals.
Beneficial effects of n-3 LC-PUFAs have also been found in animals. Administration of a DHA/EPA diet to aged mice protects against neuroinflammation and cognitive impairment [30] and improves spatial cognition and learning ability and memory [233,234]. Interventional studies in aged rodents have demonstrated that the ingestion of a fish oil-enriched diet decreases the ex vivo production of IL-1β, TNF-α, and IL-6 by monocytes and macrophages [235,236,237]. Moreover, circulating concentrations of IL-1β, TNF-α, and IL-6 following LPS injections are lower in rats and mice fed a fish oil-enriched diet [238,239,240]. Furthermore, age-related brain expression of pro-inflammatory cytokines in rodents is reduced with high levels of DHA [241].
In addition, it is possible to modulate oxylipin profile via dietary interventions. Indeed, as reviewed by Caligiuri et al. in human blood, the oxylipin profile is changed towards a less inflammatory profile after n-3 LC-PUFA consumption [214]. We found that in mice treated with LPS, a brain n-3 LC-PUFA increase by dietary supplementation promotes the synthesis of n-3 PUFA derived SPMs and decreases n-6 PUFA-derived SPMs displaying an anti-inflammatory profile [100]. Moreover, increased plasmatic pro-inflammatory oxylipins in elderly is reversed by dietary n-3 PUFA (alpha-linolenic acid, the precursor of n-3 LC-PUFAs) [213]. The OmegAD study revealed that Alzheimer’s disease patients treated with n-3 PUFAs preserve their RvD1 levels as compared with placebo-treated patients [242]. In aged rats, n-3 LC-PUFA supplementation increases DHA-derived oxylipins in the cortex and improves the reference memory-related ability learning [243].
The modification of SPM levels in blood and brain cells of aged human and rodents is accompanied by some modification of the expression of their enzymes involved in their synthesis. 15-LOX mRNA expression increases in n-3 LC-PUFA supplemented group and decreases in n-3 LC-PUFA deficient diet [100,244,245]. 15-LOX generates both 15-HETEs that inhibit NFκB [103] as well as RvD1 that contributes to the preservation of cognitive performance [97].
These results suggest that dietary habits may be essential regulators of oxylipin profile and reinforce the importance of the recommendation of n-3 PUFA rich diet.
7. Conclusions
In conclusion, aging is characterized by low-grade neuroinflammation, in particular, activation of microglial cells and increase in the production of brain pro-inflammatory factors, such as cytokines. This neuroinflammation is associated with cognitive decline (15–20% of the >65-year-old elderly), which affects life quality and has a major economic and social impact. In this context, it is a priority to find strategies to delay the evolution towards neurodegenerative diseases. n-3 LC-PUFAs and their bioactive lipid derivates (SPMs) are promising as they reduce and resolve inflammation. SPMs are modulated by aging and dietary means reinforcing the importance of nutrition in the regulation of inflammation. Changes in dietary n-3 PUFA balance should have dramatic consequences in brain PUFA metabolism, and finally in the response to neuroinflammation particularly during aging. More studies are needed to confirm the role of SPMs in age-related changes, with this field being yet in emergence, and to investigate the interest to combine different oxylipins to potentiate their beneficial effects during aging. The clinical form (encapsulated SPMs or more stable-SPM analogues), the doses, and the way of administration should also be defined.
Author Contributions
All authors (C.J., A.-L.D., M.C., V.P., S.L.) contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Programme FUI, projet BrainBooster, grant number DOS0049628/00.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Camfield, D.A.; Owen, L.; Scholey, A.B.; Pipingas, A.; Stough, C. Dairy constituents and neurocognitive health in ageing. Br. J. Nutr. 2011, 106, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Kuh, D.; Bewick, M.; Standberg, T.; Farrell, J.; Pengelly, R.; Joel, M.E.; Rodriguez Mañas, L.; Mercier, J.; Bringer, J.; et al. Operational Definition of Active and Healthy Ageing (AHA): A Conceptual Framework. J. Nutr. Health Aging 2015, 19, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.D.; Du, K.; Rendeiro, C.; Wang, L.; Wu, Q.; Rubakhin, S.S.; Vazhappilly, R.; Baxter, J.H.; Sweedler, J.V.; Rhodes, J.S. A unique combination of micronutrients rejuvenates cognitive performance in aged mice. Behav. Brain Res. 2017, 320, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.A.; Barnes, C.A. The neurobiology of memory changes in normal aging. Exp. Gerontol. 2003, 38, 61–69. [Google Scholar] [CrossRef]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Di Benedetto, S.; Müller, L.; Wenger, E.; Düzel, S.; Pawelec, G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci. Biobehav. Rev. 2017, 75, 114–128. [Google Scholar] [CrossRef]
- Spittau, B. Aging Microglia-Phenotypes, Functions and Implications for Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 194. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W.G.M. Neuronal “On” and “Off” signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef]
- Streit, W.J. Microglial senescence: Does the brain’s immune system have an expiration date? Trends Neurosci. 2006, 29, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.S.; Ma, J.; Jegathees, T.; Goldsbury, C. Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol. 2017, 27, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S.T. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflammation 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hefendehl, J.K.; Neher, J.J.; Sühs, R.B.; Kohsaka, S.; Skodras, A.; Jucker, M. Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell 2014, 13, 60–69. [Google Scholar] [CrossRef]
- Sheffield, L.G.; Berman, N.E. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol. Aging 1998, 19, 47–55. [Google Scholar] [CrossRef]
- Sloane, J.A.; Hollander, W.; Moss, M.B.; Rosene, D.L.; Abraham, C.R. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol. Aging 1999, 20, 395–405. [Google Scholar] [CrossRef]
- Perry, V.H.; Matyszak, M.K.; Fearn, S. Altered antigen expression of microglia in the aged rodent CNS. Glia 1993, 7, 60–67. [Google Scholar] [CrossRef]
- Ogura, K.; Ogawa, M.; Yoshida, M. Effects of ageing on microglia in the normal rat brain: Immunohistochemical observations. NeuroReport 1994, 5, 1224–1226. [Google Scholar] [CrossRef]
- Domínguez-González, M.; Puigpinós, M.; Jové, M.; Naudi, A.; Portero-Otín, M.; Pamplona, R.; Ferrer, I. Regional vulnerability to lipoxidative damage and inflammation in normal human brain aging. Exp. Gerontol. 2018, 111, 218–228. [Google Scholar] [CrossRef]
- Esiri, M.M. Ageing and the brain. J. Pathol. 2007, 211, 181–187. [Google Scholar] [CrossRef]
- Matt, S.M.; Johnson, R.W. Neuro-immune dysfunction during brain aging: New insights in microglial cell regulation. Curr. Opin. Pharmacol. 2016, 26, 96–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sierra, A.; Gottfried-Blackmore, A.C.; McEwen, B.S.; Bulloch, K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007, 55, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Eichhoff, G.; Busche, M.A.; Garaschuk, O. In vivo calcium imaging of the aging and diseased brain. Eur. J. Nucl. Med. Mol. Imaging 2008, 35 (Suppl. S1), S99–S106. [Google Scholar] [CrossRef] [PubMed]
- Safaiyan, S.; Kannaiyan, N.; Snaidero, N.; Brioschi, S.; Biber, K.; Yona, S.; Edinger, A.L.; Jung, S.; Rossner, M.J.; Simons, M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016, 19, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Muccigrosso, M.M.; Godbout, J.P. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 2015, 96, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Braak, H.; Xue, Q.-S.; Bechmann, I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2009, 118, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Andraka, J.M.; Sharma, N.; Marchalant, Y. Can krill oil be of use for counteracting neuroinflammatory processes induced by high fat diet and aging? Neurosci. Res. 2019. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Tichauer, J.E.; Eugenín, J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J. Neurochem. 2010, 112, 1099–1114. [Google Scholar] [CrossRef]
- Cribbs, D.H.; Berchtold, N.C.; Perreau, V.; Coleman, P.D.; Rogers, J.; Tenner, A.J.; Cotman, C.W. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. J. Neuroinflammation 2012, 9, 179. [Google Scholar] [CrossRef]
- Labrousse, V.F.; Nadjar, A.; Joffre, C.; Costes, L.; Aubert, A.; Grégoire, S.; Bretillon, L.; Layé, S. Short-Term Long Chain Omega3 Diet Protects from Neuroinflammatory Processes and Memory Impairment in Aged Mice. PLoS ONE 2012, 7, e36861. [Google Scholar] [CrossRef]
- Ye, S.M.; Johnson, R.W. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation 2001, 9, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Moranis, A.; Delpech, J.-C.; De Smedt-Peyrusse, V.; Aubert, A.; Guesnet, P.; Lavialle, M.; Joffre, C.; Layé, S. Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain Behav. Immun. 2012, 26, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.M.; Johnson, R.W. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappaB. J. Neuroimmunol. 2001, 117, 87–96. [Google Scholar] [CrossRef]
- Rozovsky, I.; Finch, C.E.; Morgan, T.E. Age-related activation of microglia and astrocytes: In vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol. Aging 1998, 19, 97–103. [Google Scholar] [CrossRef]
- Ye, S.M.; Johnson, R.W. Increased interleukin-6 expression by microglia from brain of aged mice. J. Neuroimmunol. 1999, 93, 139–148. [Google Scholar] [CrossRef]
- Yu, W.H.; Go, L.; Guinn, B.A.; Fraser, P.E.; Westaway, D.; McLaurin, J. Phenotypic and functional changes in glial cells as a function of age. Neurobiol. Aging 2002, 23, 105–115. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc. 1999, 47, 639–646. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cavazzini, C.; Corsi, A.; Bartali, B.; Russo, C.R.; Lauretani, F.; Ferrucci, L.; Cavazzini, C.; Corsi, A.M.; Bartali, B.; et al. Biomarkers of frailty in older persons. J. Endocrinol. Investig. 2002, 25, 10–15. [Google Scholar]
- Henry, C.J.; Huang, Y.; Wynne, A.M.; Godbout, J.P. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 2009, 23, 309–317. [Google Scholar] [CrossRef]
- VanGuilder, H.D.; Bixler, G.V.; Brucklacher, R.M.; Farley, J.A.; Yan, H.; Warrington, J.P.; Sonntag, W.E.; Freeman, W.M. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J. Neuroinflammation 2011, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.M.; Patel, N.V.; Patel, N.K.; Wei, M.; Morgan, T.E.; de Beer, M.C.; de Villiers, W.J.S.; Finch, C.E. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neurosci. Lett. 2005, 390, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.; Nally, R.; Nolan, Y.; McCartney, Y.; Linden, J.; Lynch, M.A. The age-related attenuation in long-term potentiation is associated with microglial activation. J. Neurochem. 2006, 99, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Harry, G.J. Microglia during development and aging. Pharmacol. Ther. 2013, 139, 313–326. [Google Scholar] [CrossRef]
- Sheng, J.G.; Mrak, R.E.; Griffin, W.S. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol. 1998, 95, 229–234. [Google Scholar] [CrossRef]
- Mouton, P.R.; Long, J.M.; Lei, D.-L.; Howard, V.; Jucker, M.; Calhoun, M.E.; Ingram, D.K. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002, 956, 30–35. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581. [Google Scholar] [CrossRef]
- Mrdjen, D.; Hartmann, F.J.; Becher, B. High Dimensional Cytometry of Central Nervous System Leukocytes During Neuroinflammation. Methods Mol. Biol. 2017, 1559, 321–332. [Google Scholar]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271. [Google Scholar] [CrossRef]
- Sankowski, R.; Böttcher, C.; Masuda, T.; Geirsdottir, L.; Sagar; Sindram, E.; Seredenina, T.; Muhs, A.; Scheiwe, C.; Shah, M.J.; et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat. Neurosci. 2019, 22, 2098–2110. [Google Scholar] [CrossRef]
- Ewers, M.; Franzmeier, N.; Suárez-Calvet, M.; Morenas-Rodriguez, E.; Caballero, M.A.A.; Kleinberger, G.; Piccio, L.; Cruchaga, C.; Deming, Y.; Dichgans, M.; et al. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci. Transl. Med. 2019, 11, eaav6221. [Google Scholar] [CrossRef] [PubMed]
- Rafnsson, S.B.; Deary, I.J.; Smith, F.B.; Whiteman, M.C.; Rumley, A.; Lowe, G.D.O.; Fowkes, F.G.R. Cognitive decline and markers of inflammation and hemostasis: The Edinburgh Artery Study. J. Am. Geriatr. Soc. 2007, 55, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.D.; Huang, M.-H.; Albert, M.; Harris, T.; Rowe, J.W.; Seeman, T.E. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 2002, 59, 371–378. [Google Scholar] [CrossRef]
- Schram, M.T.; Euser, S.M.; de Craen, A.J.M.; Witteman, J.C.; Frölich, M.; Hofman, A.; Jolles, J.; Breteler, M.M.B.; Westendorp, R.G.J. Systemic markers of inflammation and cognitive decline in old age. J. Am. Geriatr. Soc. 2007, 55, 708–716. [Google Scholar] [CrossRef]
- Braida, D.; Sacerdote, P.; Panerai, A.E.; Bianchi, M.; Aloisi, A.M.; Iosuè, S.; Sala, M. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav. Brain Res. 2004, 153, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Sparkman, N.L.; Buchanan, J.B.; Heyen, J.R.R.; Chen, J.; Beverly, J.L.; Johnson, R.W. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J. Neurosci. 2006, 26, 10709–10716. [Google Scholar] [CrossRef] [PubMed]
- Sparkman, N.L.; Johnson, R.W. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation 2008, 15, 323–330. [Google Scholar] [CrossRef]
- Buchanan, J.B.; Sparkman, N.L.; Chen, J.; Johnson, R.W. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology 2008, 33, 755–765. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Higgins, E.A.; Biedenkapp, J.C.; Sprunger, D.B.; Wright-Hardesty, K.J.; Watkins, L.R.; Rudy, J.W.; Maier, S.F. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol. Aging 2006, 27, 723–732. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Frank, M.G.; Hein, A.M.; Higgins, E.A.; Watkins, L.R.; Rudy, J.W.; Maier, S.F. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav. Immun. 2009, 23, 46–54. [Google Scholar] [CrossRef]
- Gibertini, M.; Newton, C.; Friedman, H.; Klein, T.W. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav. Immun. 1995, 9, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.R.; Kumagawa, K.; Fleshner, M.; Watkins, L.R.; Maier, S.F.; Rudy, J.W. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav. Immun. 1998, 12, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Gemma, C.; Fister, M.; Hudson, C.; Bickford, P.C. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur. J. Neurosci. 2005, 22, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Zheng, Y.; Wang, S. Inflammasome and Cancer. Exp. Suppl. 2018, 108, 281–302. [Google Scholar]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013, 18, 519–532. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Joffre, C.; Grégoire, S.; De Smedt, V.; Acar, N.; Bretillon, L.; Nadjar, A.; Layé, S. Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot. Essent. Fatty Acids 2016, 114, 1–10. [Google Scholar] [CrossRef]
- Carrie, I.; Clement, M.; de Javel, D.; Frances, H.; Bourre, J.M. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000, 41, 465–472. [Google Scholar] [PubMed]
- Chung, W.L.; Chen, J.J.; Su, H.M. Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J. Nutr. 2008, 138, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Little, S.J.; Lynch, M.A.; Manku, M.; Nicolaou, A. Docosahexaenoic acid-induced changes in phospholipids in cortex of young and aged rats: A lipidomic analysis. Prostaglandins Leukot Essent Fatty Acids 2007, 77, 155–162. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Carlson, S.E. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 2006, 75, 329–349. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.; Chen, Z.Y. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br. J. Nutr. 2005, 94, 544–550. [Google Scholar] [CrossRef]
- Chen, C.T.; Bazinet, R.P. beta-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot Essent Fatty Acids 2015, 92, 33–40. [Google Scholar] [CrossRef]
- Kidd, P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: Clinical findings and structural-functional synergies with cell membrane phospholipids. Altern. Med. Rev. 2007, 12, 207–227. [Google Scholar]
- Plourde, M.; Cunnane, S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Nichols, P.D.; Petrie, J.; Singh, S. Long-chain omega-3 oils-an update on sustainable sources. Nutrients 2010, 2, 572–585. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Laye, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-inflammatory effects of omega-3 fatty acids in the brain: Physiological mechanisms and relevance to pharmacology. Pharmacol. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Headland, S.E.; Norling, L.V. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Massey, K.A.; Nicolaou, A. Lipidomics of oxidized polyunsaturated fatty acids. Free Radic. Biol. Med. 2013, 59, 45–55. [Google Scholar] [CrossRef]
- Colas, R.A.; Shinohara, M.; Dalli, J.; Chiang, N.; Serhan, C.N. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 2014, 307, C39–C54. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol. Aspects Med. 2018, 64, 1–17. [Google Scholar] [CrossRef]
- Shalini, S.-M.; Ho, C.F.-Y.; Ng, Y.-K.; Tong, J.-X.; Ong, E.-S.; Herr, D.R.; Dawe, G.S.; Ong, W.-Y. Distribution of Alox15 in the Rat Brain and Its Role in Prefrontal Cortical Resolvin D1 Formation and Spatial Working Memory. Mol. Neurobiol. 2018, 55, 1537–1550. [Google Scholar] [CrossRef]
- Nadjar, A.; Tridon, V.; May, M.J.; Ghosh, S.; Dantzer, R.; Amedee, T.; Parnet, P. NFkappaB activates in vivo the synthesis of inducible Cox-2 in the brain. J. Cereb. Blood Flow Metab. 2005, 25, 1047–1059. [Google Scholar] [CrossRef]
- Navarro-Mabarak, C.; Camacho-Carranza, R.; Espinosa-Aguirre, J.J. Cytochrome P450 in the central nervous system as a therapeutic target in neurodegenerative diseases. Drug Metab. Rev. 2018, 50, 95–108. [Google Scholar] [CrossRef]
- Rey, C.; Delpech, J.C.; Madore, C.; Nadjar, A.; Greenhalgh, A.D.; Amadieu, C.; Aubert, A.; Pallet, V.; Vaysse, C.; Layé, S.; et al. Dietary n-3 long chain PUFA supplementation promotes a pro-resolving oxylipin profile in the brain. Brain Behav. Immun. 2019, 76, 17–27. [Google Scholar] [CrossRef]
- Czapski, G.A.; Gajkowska, B.; Strosznajder, J.B. Systemic administration of lipopolysaccharide induces molecular and morphological alterations in the hippocampus. Brain Res. 2010, 1356, 85–94. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, Y.W.; Han, J.; Liang, H.; Wang, N.; Cheng, Y. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARgamma: A possible neuroprotective effect in ischemic brain. J. Lipid Res. 2015, 56, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Pratico, D.; Zhukareva, V.; Yao, Y.; Uryu, K.; Funk, C.D.; Lawson, J.A.; Trojanowski, J.Q.; Lee, V.M. 12/15-lipoxygenase is increased in Alzheimer’s disease: Possible involvement in brain oxidative stress. Am. J. Pathol. 2004, 164, 1655–1662. [Google Scholar] [CrossRef]
- Yigitkanli, K.; Zheng, Y.; Pekcec, A.; Lo, E.H.; van Leyen, K. Increased 12/15-Lipoxygenase Leads to Widespread Brain Injury Following Global Cerebral Ischemia. Transl. Stroke Res. 2017, 8, 194–202. [Google Scholar] [CrossRef]
- Bystrom, J.; Wray, J.A.; Sugden, M.C.; Holness, M.J.; Swales, K.E.; Warner, T.D.; Edin, M.L.; Zeldin, D.C.; Gilroy, D.W.; Bishop-Bailey, D. Endogenous epoxygenases are modulators of monocyte/macrophage activity. PLoS ONE 2011, 6, e26591. [Google Scholar] [CrossRef]
- Fleming, I. Cytochrome P450-dependent eicosanoid production and crosstalk. Curr. Opin. Lipidol. 2011, 22, 403–409. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Edin, M.L.; De Maeyer, R.P.; Bystrom, J.; Newson, J.; Lih, F.B.; Stables, M.; Zeldin, D.C.; Bishop-Bailey, D. CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. USA 2016, 113, E3240–E3249. [Google Scholar] [CrossRef]
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120431. [Google Scholar] [CrossRef]
- Levi, G.; Minghetti, L.; Aloisi, F. Regulation of prostanoid synthesis in microglial cells and effects of prostaglandin E2 on microglial functions. Biochimie 1998, 80, 899–904. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Modulation of inflammation in brain: A matter of fat. J. Neurochem. 2007, 101, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.P.; Gehlhaus, M.; Knoth, R.; Volk, B. Expression and function of cytochrome p450 in brain drug metabolism. Curr. Drug Metab. 2007, 8, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Volk, B.; Hettmannsperger, U.; Papp, T.; Amelizad, Z.; Oesch, F.; Knoth, R. Mapping of phenytoin-inducible cytochrome P450 immunoreactivity in the mouse central nervous system. Neuroscience 1991, 42, 215–235. [Google Scholar] [CrossRef]
- Barden, A.E.; Mas, E.; Mori, T.A. n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr. Opin. Lipidol. 2016, 27, 26–32. [Google Scholar] [CrossRef]
- Halade, G.V.; Black, L.M.; Verma, M.K. Paradigm shift - Metabolic transformation of docosahexaenoic and eicosapentaenoic acids to bioactives exemplify the promise of fatty acid drug discovery. Biotechnol. Adv. 2018, 36, 935–953. [Google Scholar] [CrossRef]
- Recchiuti, A. Resolvin D1 and its GPCRs in resolution circuits of inflammation. Prostaglandins Other Lipid Mediat. 2013, 107, 64–76. [Google Scholar] [CrossRef]
- Sun, Y.P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar] [CrossRef]
- Marcheselli, V.L.; Hong, S.; Lukiw, W.J.; Tian, X.H.; Gronert, K.; Musto, A.; Hardy, M.; Gimenez, J.M.; Chiang, N.; Serhan, C.N.; et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003, 278, 43807–43817. [Google Scholar] [CrossRef]
- Mulik, R.S.; Bing, C.; Ladouceur-Wodzak, M.; Munaweera, I.; Chopra, R.; Corbin, I.R. Localized delivery of low-density lipoprotein docosahexaenoic acid nanoparticles to the rat brain using focused ultrasound. Biomaterials 2016, 83, 257–268. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Z.P.; Gui, P.; Xia, W.; Xia, Z.; Zhang, X.C.; Deng, Q.Z.; Xuan, W.; Marie, C.; Wang, L.L.; et al. Endogenous expression pattern of resolvin D1 in a rat model of self-resolution of lipopolysaccharide-induced acute respiratory distress syndrome and inflammation. Int. Immunopharmacol. 2014, 23, 247–253. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef]
- Ho, C.F.-Y.; Ismail, N.B.; Koh, J.K.-Z.; Gunaseelan, S.; Low, Y.-H.; Ng, Y.-K.; Chua, J.J.-E.; Ong, W.-Y. Localisation of Formyl-Peptide Receptor 2 in the Rat Central Nervous System and Its Role in Axonal and Dendritic Outgrowth. Neurochem. Res. 2018, 43, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Nadjar, A.; Buaud, B.; Vaysse, C.; Aubert, A.; Pallet, V.; Laye, S.; Joffre, C. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav. Immun. 2016, 55, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010, 16, 592–597, 1p following 597. [Google Scholar] [CrossRef]
- Bisicchia, E.; Sasso, V.; Catanzaro, G.; Leuti, A.; Besharat, Z.M.; Chiacchiarini, M.; Molinari, M.; Ferretti, E.; Viscomi, M.T.; Chiurchiù, V. Resolvin D1 Halts Remote Neuroinflammation and Improves Functional Recovery after Focal Brain Damage Via ALX/FPR2 Receptor-Regulated MicroRNAs. Mol. Neurobiol. 2018, 55, 6894–6905. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, M.; Hjorth, E.; Cortes-Toro, V.; Eyjolfsdottir, H.; Graff, C.; Nennesmo, I.; Palmblad, J.; Eriksdotter, M.; Sambamurti, K.; et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement 2015, 11, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Fredman, G.; Serhan, C.N. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem. J. 2011, 437, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Fredman, G.; Serhan, C.N. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 2012, 180, 2018–2027. [Google Scholar] [CrossRef]
- Recchiuti, A.; Krishnamoorthy, S.; Fredman, G.; Chiang, N.; Serhan, C.N. MicroRNAs in resolution of acute inflammation: Identification of novel resolvin D1-miRNA circuits. FASEB J. 2011, 25, 544–560. [Google Scholar] [CrossRef]
- Aursnes, M.; Tungen, J.E.; Vik, A.; Colas, R.; Cheng, C.-Y.C.; Dalli, J.; Serhan, C.N.; Hansen, T.V. Total synthesis of the lipid mediator PD1n-3 DPA: Configurational assignments and anti-inflammatory and pro-resolving actions. J. Nat. Prod. 2014, 77, 910–916. [Google Scholar] [CrossRef]
- Doyle, R.; Sadlier, D.M.; Godson, C. Pro-resolving lipid mediators: Agents of anti-ageing? Semin. Immunol. 2018, 40, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.L.; Serhan, C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 2003, 278, 14677–14687. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O. Bioactive metabolites of docosahexaenoic acid. Biochimie 2017, 136, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef]
- Orr, S.K.; Palumbo, S.; Bosetti, F.; Mount, H.T.; Kang, J.X.; Greenwood, C.E.; Ma, D.W.; Serhan, C.N.; Bazinet, R.P. Unesterified docosahexaenoic acid is protective in neuroinflammation. J. Neurochem. 2013, 127, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, X.; Hjorth, E.; Colas, R.A.; Schroeder, L.; Granholm, A.C.; Serhan, C.N.; Schultzberg, M. Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Abeta42 Phagocytosis. Mol. Neurobiol. 2016, 53, 2733–2749. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Cui, J.G.; Marcheselli, V.L.; Bodker, M.; Botkjaer, A.; Gotlinger, K.; Serhan, C.N.; Bazan, N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Investig. 2005, 115, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Caterina, M.J. Accelerating the reversal of inflammatory pain with NPD1 and its receptor GPR37. J. Clin. Investig. 2018, 128, 3246–3249. [Google Scholar] [CrossRef]
- Bazan, N.G.; Eady, T.N.; Khoutorova, L.; Atkins, K.D.; Hong, S.; Lu, Y.; Zhang, C.; Jun, B.; Obenaus, A.; Fredman, G.; et al. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp. Neurol. 2012, 236, 122–130. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, J.; Chen, F.; Lin, Y. Neuroprotectin D1 attenuates brain damage induced by transient middle cerebral artery occlusion in rats through TRPC6/CREB pathways. Mol. Med. Rep. 2013, 8, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Xian, W.; Wu, Y.; Xiong, W.; Li, L.; Li, T.; Pan, S.; Song, L.; Hu, L.; Pei, L.; Yao, S.; et al. The pro-resolving lipid mediator Maresin 1 protects against cerebral ischemia/reperfusion injury by attenuating the pro-inflammatory response. Biochem. Biophys. Res. Commun. 2016, 472, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Xian, W.; Li, T.; Li, L.; Hu, L.; Cao, J. Maresin 1 attenuates the inflammatory response and mitochondrial damage in mice with cerebral ischemia/reperfusion in a SIRT1-dependent manner. Brain Res. 2019, 1711, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Francos-Quijorna, I.; Santos-Nogueira, E.; Gronert, K.; Sullivan, A.B.; Kopp, M.A.; Brommer, B.; David, S.; Schwab, J.M.; López-Vales, R. Maresin 1 Promotes Inflammatory Resolution, Neuroprotection, and Functional Neurological Recovery After Spinal Cord Injury. J. Neurosci. 2017, 37, 11731–11743. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T.; Arita, M.; Omori, K.; Recchiuti, A.; Van Dyke, T.E.; Serhan, C.N. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem. 2010, 285, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Arita, M.; Matsueda, S.; Iwamoto, R.; Fujihara, T.; Nakanishi, H.; Taguchi, R.; Masuda, K.; Sasaki, K.; Urabe, D.; et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012, 287, 10525–10534. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Liu, Z.; Bazinet, R.P. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: An intracerebroventricular study. J. Neurochem. 2011, 116, 363–373. [Google Scholar] [CrossRef]
- Siegert, E.; Paul, F.; Rothe, M.; Weylandt, K.H. The effect of omega-3 fatty acids on central nervous system remyelination in fat-1 mice. BMC Neurosci. 2017, 18, 19. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef]
- Guo, X.; Fu, Y.; Xu, Y.; Weng, S.; Liu, D.; Cui, D.; Yu, S.; Liu, X.; Jiang, K.; Dong, Y. Chronic mild restraint stress rats decreased CMKLR1 expression in distinct brain region. Neurosci. Lett. 2012, 524, 25–29. [Google Scholar] [CrossRef]
- Graham, K.L.; Zabel, B.A.; Loghavi, S.; Zuniga, L.A.; Ho, P.P.; Sobel, R.A.; Butcher, E.C. Chemokine-like receptor-1 expression by central nervous system-infiltrating leukocytes and involvement in a model of autoimmune demyelinating disease. J. Immunol. 2009, 183, 6717–6723. [Google Scholar] [CrossRef]
- Famenini, S.; Rigali, E.A.; Olivera-Perez, H.M.; Dang, J.; Chang, M.T.; Halder, R.; Rao, R.V.; Pellegrini, M.; Porter, V.; Bredesen, D.; et al. Increased intermediate M1-M2 macrophage polarization and improved cognition in mild cognitive impairment patients on omega-3 supplementation. FASEB J. 2017, 31, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Mizwicki, M.T.; Liu, G.; Fiala, M.; Magpantay, L.; Sayre, J.; Siani, A.; Mahanian, M.; Weitzman, R.; Hayden, E.Y.; Rosenthal, M.J.; et al. 1alpha,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-beta phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimers Dis. 2013, 34, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945. [Google Scholar] [CrossRef] [PubMed]
- Kok Kendirlioglu, B.; Unalan Ozpercin, P.; Yuksel Oksuz, O.; Sozen, S.; Cihnioglu, R.; Kalelioglu, T.; Ilnem, M.C.; Karamustafalioglu, N. Resolvin D1 as a novel anti-inflammatory marker in manic, depressive and euthymic states of bipolar disorder. Nord. J. Psychiatry 2020, 74, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Immunonutrition suppresses acute inflammatory responses through modulation of resolvin E1 in patients undergoing major hepatobiliary resection. Surgery 2016, 160, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Hiram, R.; Rizcallah, E.; Marouan, S.; Sirois, C.; Sirois, M.; Morin, C.; Fortin, S.; Rousseau, E. Resolvin E1 normalizes contractility, Ca2+ sensitivity and smooth muscle cell migration rate in TNF-α- and IL-6-pretreated human pulmonary arteries. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L776–L788. [Google Scholar] [CrossRef] [PubMed]
- Gyurko, R.; Van Dyke, T.E. The role of polyunsaturated ω-3 fatty acid eicosapentaenoic acid-derived resolvin E1 (RvE1) in bone preservation. Crit. Rev. Immunol. 2014, 34, 347–357. [Google Scholar] [CrossRef]
- Sordi, R.; Chiazza, F.; Collotta, D.; Migliaretti, G.; Colas, R.A.; Vulliamy, P.; Brohi, K.; Dalli, J.; Collino, M.; Thiemermann, C. Resolvin D1 Attenuates the Organ Injury Associated With Experimental Hemorrhagic Shock. Ann. Surg. 2019. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, F.; Wang, W.; Wang, H.; Zhang, X. Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: Possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol. Vis. 2017, 23, 242–250. [Google Scholar]
- Liu, W.; Huang, J.; Doycheva, D.; Gamdzyk, M.; Tang, J.; Zhang, J.H. RvD1binding with FPR2 attenuates inflammation via Rac1/NOX2 pathway after neonatal hypoxic-ischemic injury in rats. Exp. Neurol. 2019, 320, 112982. [Google Scholar] [CrossRef]
- Rossi, S.; Di Filippo, C.; Gesualdo, C.; Potenza, N.; Russo, A.; Trotta, M.C.; Zippo, M.V.; Maisto, R.; Ferraraccio, F.; Simonelli, F.; et al. Protection from endotoxic uveitis by intravitreal Resolvin D1: Involvement of lymphocytes, miRNAs, ubiquitin-proteasome, and M1/M2 macrophages. Mediat. Inflamm. 2015, 2015, 149381. [Google Scholar] [CrossRef]
- Titos, E.; Rius, B.; Gonzalez-Periz, A.; Lopez-Vicario, C.; Moran-Salvador, E.; Martinez-Clemente, M.; Arroyo, V.; Claria, J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 2011, 187, 5408–5418. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Y.; Wang, Y.; Wu, J.; Song, L.; Xian, W.; Yuan, S.; Pei, L.; Shang, Y. Resolvin D1 promotes the interleukin-4-induced alternative activation in BV-2 microglial cells. J. Neuroinflammation 2014, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoaty, S.; Wigerblad, G.; Bas, D.B.; Codeluppi, S.; Fernandez-Zafra, T.; El-Awady el, S.; Moustafa, Y.; Abdelhamid Ael, D.; Brodin, E.; Svensson, C.I. Spinal actions of lipoxin A4 and 17(R)-resolvin D1 attenuate inflammation-induced mechanical hypersensitivity and spinal TNF release. PLoS ONE 2013, 8, e75543. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, A.; Aytan, N.; Palaska, I.; Stephens, D.; Crabtree, L.; Benincasa, C.; Jenkins, B.G.; Carreras, I.; Dedeoglu, A. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp. Neurol. 2018, 300, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.L.; Rowe, R.K.; Ellis, T.W.; Yee, N.S.; O’Hara, B.F.; Adelson, P.D.; Lifshitz, J. Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse. Brain Behav. Immun. 2015, 47, 131–140. [Google Scholar] [CrossRef]
- Terrando, N.; Gomez-Galan, M.; Yang, T.; Carlstrom, M.; Gustavsson, D.; Harding, R.E.; Lindskog, M.; Eriksson, L.I. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013, 27, 3564–3571. [Google Scholar] [CrossRef]
- Delpech, J.-C.; Madore, C.; Joffre, C.; Aubert, A.; Kang, J.X.; Nadjar, A.; Layé, S. Transgenic increase in n-3/n-6 fatty acid ratio protects against cognitive deficits induced by an immune challenge through decrease of neuroinflammation. Neuropsychopharmacology 2015, 40, 525–536. [Google Scholar] [CrossRef]
- Luo, C.; Ren, H.; Wan, J.B.; Yao, X.; Zhang, X.; He, C.; So, K.F.; Kang, J.X.; Pei, Z.; Su, H. Enriched endogenous omega-3 fatty acids in mice protect against global ischemia injury. J. Lipid Res. 2014, 55, 1288–1297. [Google Scholar] [CrossRef]
- Furuyashiki, T.; Akiyama, S.; Kitaoka, S. Roles of multiple lipid mediators in stress and depression. Int. Immunol. 2019, 579–587. [Google Scholar] [CrossRef]
- Deyama, S.; Ishikawa, Y.; Yoshikawa, K.; Shimoda, K.; Ide, S.; Satoh, M.; Minami, M. Resolvin D1 and D2 Reverse Lipopolysaccharide-Induced Depression-Like Behaviors Through the mTORC1 Signaling Pathway. Int. J. Neuropsychopharmacol 2017, 20, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Shimoda, K.; Suzuki, H.; Ishikawa, Y.; Ishimura, K.; Fukuda, H.; Hitora-Imamura, N.; Ide, S.; Satoh, M.; Kaneda, K.; et al. Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology (Berl.) 2018, 235, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Shimoda, K.; Ikeda, H.; Fukuda, H.; Shuto, S.; Minami, M. Resolvin E3 attenuates lipopolysaccharide-induced depression-like behavior in mice. J. Pharmacol. Sci. 2018, 138, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.P.; Sperotto, N.D.; Maciel, I.S.; Leite, C.E.; Souza, A.H.; Campos, M.M. Effects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology 2014, 86, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.; Bernier, J.; Godbout, R.; Rousseau, G. Resolvin D1, a metabolite of omega-3 polyunsaturated fatty acid, decreases post-myocardial infarct depression. Mar. Drugs 2014, 12, 5396–5407. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Deyama, S.; Shimoda, K.; Yoshikawa, K.; Ide, S.; Satoh, M.; Minami, M. Rapid and sustained antidepressant effects of resolvin D1 and D2 in a chronic unpredictable stress model. Behav. Brain Res. 2017, 332, 233–236. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Bertz, T.; Ji, R.R. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J. Neuroimmune Pharmacol. 2013, 8, 37–41. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.; Schultzberg, M.; Hjorth, E. Differential regulation of resolution in inflammation induced by amyloid-β42 and lipopolysaccharides in human microglia. J. Alzheimers Dis. 2015, 43, 1237–1250. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Zhang, R.; Qiao, S.; Fan, J. Resolvin D2 recovers neural injury by suppressing inflammatory mediators expression in lipopolysaccharide-induced Parkinson’s disease rat model. Biochem. Biophys. Res. Commun. 2015, 460, 799–805. [Google Scholar] [CrossRef]
- Shevalye, H.; Yorek, M.S.; Coppey, L.J.; Holmes, A.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J. Neurophysiol. 2015, 114, 199–208. [Google Scholar] [CrossRef]
- Xu, J.; Gao, X.; Yang, C.; Chen, L.; Chen, Z. Resolvin D1 Attenuates Mpp+-Induced Parkinson Disease via Inhibiting Inflammation in PC12 Cells. Med. Sci. Monit. 2017, 23, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Benabdoun, H.A.; Kulbay, M.; Rondon, E.-P.; Vallières, F.; Shi, Q.; Fernandes, J.; Fahmi, H.; Benderdour, M. In vitro and in vivo assessment of the proresolutive and antiresorptive actions of resolvin D1: Relevance to arthritis. Arthritis Res. Ther. 2019, 21, 72. [Google Scholar] [CrossRef]
- Schmid, M.; Gemperle, C.; Rimann, N.; Hersberger, M. Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32. J. Immunol. 2016, 196, 3429–3437. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Able, J.; Jandacek, R.; Rider, T.; Tso, P. Inbred C57BL/6J and DBA/2J mouse strains exhibit constitutive differences in regional brain fatty acid composition. Lipids 2009, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 1991, 26, 421–425. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Liu, Y.; Jandacek, R.; Rider, T.; Tso, P. The aging human orbitofrontal cortex: Decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 293–304. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Högyes, E.; Kitajka, K.; Puskás, L.G.; Zvara, A.; Hackler, L.; Nyakas, C.; Penke, Z.; Farkas, T. Modification by docosahexaenoic acid of age-induced alterations in gene expression and molecular composition of rat brain phospholipids. Proc. Natl. Acad. Sci. USA 2003, 100, 11321–11326. [Google Scholar] [CrossRef]
- Dyall, S.C.; Michael, G.J.; Whelpton, R.; Scott, A.G.; Michael-Titus, A.T. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol. Aging 2007, 28, 424–439. [Google Scholar] [CrossRef]
- Latour, A.; Grintal, B.; Champeil-Potokar, G.; Hennebelle, M.; Lavialle, M.; Dutar, P.; Potier, B.; Billard, J.-M.; Vancassel, S.; Denis, I. Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat hippocampal CA1. Aging Cell 2013, 12, 76–84. [Google Scholar] [CrossRef]
- Arranz, L.; Naudí, A.; De la Fuente, M.; Pamplona, R. Exceptionally old mice are highly resistant to lipoxidation-derived molecular damage. Age (Dordr.) 2013, 35, 621–635. [Google Scholar] [CrossRef]
- Favrelière, S.; Perault, M.C.; Huguet, F.; De Javel, D.; Bertrand, N.; Piriou, A.; Durand, G. DHA-enriched phospholipid diets modulate age-related alterations in rat hippocampus. Neurobiol. Aging 2003, 24, 233–243. [Google Scholar] [CrossRef]
- Drozdowski, L.; Thomson, A.B.R. Aging and the intestine. World J. Gastroenterol. 2006, 12, 7578–7584. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-T.; So, P.-W.; Parkinson, J.R.; Yu, W.S.; Hankir, M.; Herlihy, A.H.; Goldstone, A.P.; Frost, G.S.; Wasserfall, C.; Bell, J.D. The combined effects on neuronal activation and blood-brain barrier permeability of time and n-3 polyunsaturated fatty acids in mice, as measured in vivo using MEMRI. Neuroimage 2010, 50, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, S.; Rabinovitz, S.; Mostofsky, D.I. Essential fatty acids and the brain: From infancy to aging. Neurobiol. Aging 2005, 26 (Suppl. S1), 98–102. [Google Scholar] [CrossRef]
- Ilincheta de Boschero, M.G.; Roque, M.E.; Salvador, G.A.; Giusto, N.M. Alternative pathways for phospholipid synthesis in different brain areas during aging. Exp. Gerontol. 2000, 35, 653–668. [Google Scholar] [CrossRef]
- Bourre, J.M.; Piciotti, M. Delta-6 desaturation of alpha-linolenic acid in brain and liver during development and aging in the mouse. Neurosci. Lett. 1992, 141, 65–68. [Google Scholar] [CrossRef]
- Kumar, V.B.; Vyas, K.; Buddhiraju, M.; Alshaher, M.; Flood, J.F.; Morley, J.E. Changes in membrane fatty acids and delta-9 desaturase in senescence accelerated (SAMP8) mouse hippocampus with aging. Life Sci. 1999, 65, 1657–1662. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Tanaka, T.; Tang, W.; Manichaikul, A.; Foy, M.; Kabagambe, E.K.; Nettleton, J.A.; King, I.B.; Weng, L.-C.; Bhattacharya, S.; et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: A meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011, 7, e1002193. [Google Scholar] [CrossRef]
- Cini, M.; Moretti, A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiol. Aging 1995, 16, 53–57. [Google Scholar] [CrossRef]
- Dei, R.; Takeda, A.; Niwa, H.; Li, M.; Nakagomi, Y.; Watanabe, M.; Inagaki, T.; Washimi, Y.; Yasuda, Y.; Horie, K.; et al. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer’s disease. Acta Neuropathol. 2002, 104, 113–122. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Kaufman, J.S.; Satia, J.A.; Rosamond, W.; Folsom, A.R. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: The Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr. 2007, 85, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Heude, B.; Ducimetière, P.; Berr, C. EVA Study Cognitive decline and fatty acid composition of erythrocyte membranes—The EVA Study. Am. J. Clin. Nutr. 2003, 77, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Colacicco, A.M.; D’Introno, A.; Capurso, C.; Torres, F.; Rizzo, C.; Capurso, A.; Panza, F. Dietary intake of unsaturated fatty acids and age-related cognitive decline: A 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol. Aging 2006, 27, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Hayakawa, S.; Wada, S. Effect of age on the modification of brain polyunsaturated fatty acids and enzyme activities by fish oil diet in rats. Mech. Ageing Dev. 1989, 50, 17–25. [Google Scholar] [PubMed]
- Lim, S.Y.; Suzuki, H. Intakes of dietary docosahexaenoic acid ethyl ester and egg phosphatidylcholine improve maze-learning ability in young and old mice. J. Nutr. 2000, 130, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Catalan, J.; Moriguchi, T.; Slotnick, B.; Murthy, M.; Greiner, R.S.; Salem, N. Cognitive deficits in docosahexaenoic acid-deficient rats. Behav. Neurosci. 2002, 116, 1022–1031. [Google Scholar] [CrossRef]
- Lafourcade, M.; Larrieu, T.; Mato, S.; Duffaud, A.; Sepers, M.; Matias, I.; De Smedt-Peyrusse, V.; Labrousse, V.F.; Bretillon, L.; Matute, C.; et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011, 14, 345–350. [Google Scholar] [CrossRef]
- Thomazeau, A.; Bosch-Bouju, C.; Manzoni, O.; Layé, S. Nutritional n-3 PUFA Deficiency Abolishes Endocannabinoid Gating of Hippocampal Long-Term Potentiation. Cereb. Cortex 2017, 27, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Bara, A.; Larrieu, T.; Lassalle, O.; Joffre, C.; Layé, S.; Manzoni, O.J. Amplification of mGlu5-Endocannabinoid Signaling Rescues Behavioral and Synaptic Deficits in a Mouse Model of Adolescent and Adult Dietary Polyunsaturated Fatty Acid Imbalance. J. Neurosci. 2017, 37, 6851–6868. [Google Scholar] [CrossRef]
- Larrieu, T.; Hilal, M.L.; Hilal, L.M.; Fourrier, C.; De Smedt-Peyrusse, V.; Sans, N.N.S.; Capuron, L.; Layé, S. Nutritional omega-3 modulates neuronal morphology in the prefrontal cortex along with depression-related behaviour through corticosterone secretion. Transl. Psychiatry 2014, 4, e437. [Google Scholar] [CrossRef]
- Mingam, R.; Moranis, A.; Bluthé, R.-M.; De Smedt-Peyrusse, V.; Kelley, K.W.; Guesnet, P.; Lavialle, M.; Dantzer, R.; Layé, S. Uncoupling of interleukin-6 from its signalling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. Eur. J. Neurosci. 2008, 28, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Delpech, J.-C.; Thomazeau, A.; Madore, C.; Bosch-Bouju, C.; Larrieu, T.; Lacabanne, C.; Remus-Borel, J.; Aubert, A.; Joffre, C.; Nadjar, A.; et al. Dietary n-3 PUFAs Deficiency Increases Vulnerability to Inflammation-Induced Spatial Memory Impairment. Neuropsychopharmacology 2015, 40, 2774–2787. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, S.P.B.; Aukema, H.M.; Ravandi, A.; Pierce, G.N. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp. Gerontol. 2014, 59, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, S.P.B.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary modulation of oxylipins in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H903–H918. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Pescara, L.; D’Urbano, E.; Basile, G.; Nicita-Mauro, V.; Davì, G.; Romano, M. Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp. Gerontol. 2005, 40, 612–614. [Google Scholar] [CrossRef]
- Wang, X.; Puerta, E.; Cedazo-Minguez, A.; Hjorth, E.; Schultzberg, M. Insufficient resolution response in the hippocampus of a senescence-accelerated mouse model--SAMP8. J. Mol. Neurosci. 2015, 55, 396–405. [Google Scholar] [CrossRef]
- Arnardottir, H.H.; Dalli, J.; Norling, L.V.; Colas, R.A.; Perretti, M.; Serhan, C.N. Resolvin D3 Is Dysregulated in Arthritis and Reduces Arthritic Inflammation. J. Immunol. 2016, 197, 2362–2368. [Google Scholar] [CrossRef]
- Joffre, C. Polyunsaturated fatty acid metabolism in the brain and brain cells. In Feed Your Mind. How Does Nutrition Modulate Brain Function Throughout Life; IntechOpen: London, UK, 2019; pp. 13–36. [Google Scholar]
- Barberger-Gateau, P. Association between Mediterranean Diet and late-life cognition. JAMA 2009, 302, 2433, author reply 2433. [Google Scholar] [CrossRef]
- Tan, Z.S.; Harris, W.S.; Beiser, A.S.; Au, R.; Himali, J.J.; Debette, S.; Pikula, A.; Decarli, C.; Wolf, P.A.; Vasan, R.S.; et al. Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology 2012, 78, 658–664. [Google Scholar] [CrossRef]
- Titova, O.E.; Sjögren, P.; Brooks, S.J.; Kullberg, J.; Ax, E.; Kilander, L.; Riserus, U.; Cederholm, T.; Larsson, E.-M.; Johansson, L.; et al. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordr.) 2013, 35, 1495–1505. [Google Scholar] [CrossRef]
- Whalley, L.J.; Fox, H.C.; Wahle, K.W.; Starr, J.M.; Deary, I.J. Cognitive aging, childhood intelligence, and the use of food supplements: Possible involvement of n-3 fatty acids. Am. J. Clin. Nutr. 2004, 80, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Raffaitin, C.; Letenneur, L.; Berr, C.; Tzourio, C.; Dartigues, J.F.; Alpérovitch, A. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology 2007, 69, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Grodstein, F.; van Rooij, F.J.A.; Hofman, A.; Rosner, B.; Stampfer, M.J.; Witteman, J.C.M.; Breteler, M.M.B. Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. Am. J. Clin. Nutr. 2009, 90, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; van Boxtel, M.P.J.; Ocké, M.; Verschuren, W.M.M.; Kromhout, D.; Launer, L.J. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004, 62, 275–280. [Google Scholar] [CrossRef]
- González, S.; Huerta, J.M.; Fernández, S.; Patterson, A.M.; Lasheras, C. The relationship between dietary lipids and cognitive performance in an elderly population. Int. J. Food Sci. Nutr. 2010, 61, 217–225. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef]
- McNamara, R.K.; Kalt, W.; Shidler, M.D.; McDonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging 2018, 64, 147–156. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef]
- Alfano, C.M.; Imayama, I.; Neuhouser, M.L.; Kiecolt-Glaser, J.K.; Smith, A.W.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, inflammation, and ω-3 and ω-6 fatty acid intake among breast cancer survivors. J. Clin. Oncol. 2012, 30, 1280–1287. [Google Scholar] [CrossRef]
- Farzaneh-Far, R.; Harris, W.S.; Garg, S.; Na, B.; Whooley, M.A. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis 2009, 205, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Gamoh, S.; Hashimoto, M.; Hossain, S.; Masumura, S. Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Petursdottir, A.L.; Farr, S.A.; Morley, J.E.; Banks, W.A.; Skuladottir, G.V. Effect of dietary n-3 polyunsaturated fatty acids on brain lipid fatty acid composition, learning ability, and memory of senescence-accelerated mouse. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Sun, D.; Rahman, M.; Fernandes, G. Different ratios of eicosapentaenoic and docosahexaenoic omega-3 fatty acids in commercial fish oils differentially alter pro-inflammatory cytokines in peritoneal macrophages from C57BL/6 female mice. J. Nutr. Biochem. 2007, 18, 23–30. [Google Scholar] [CrossRef]
- Jia, Q.; Zhou, H.-R.; Shi, Y.; Pestka, J.J. Docosahexaenoic acid consumption inhibits deoxynivalenol-induced CREB/ATF1 activation and IL-6 gene transcription in mouse macrophages. J. Nutr. 2006, 136, 366–372. [Google Scholar] [CrossRef][Green Version]
- Yaqoob, P.; Calder, P. Effects of dietary lipid manipulation upon inflammatory mediator production by murine macrophages. Cell. Immunol. 1995, 163, 120–128. [Google Scholar] [CrossRef]
- Sadeghi, S.; Wallace, F.A.; Calder, P.C. Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology 1999, 96, 404–410. [Google Scholar] [CrossRef]
- Vreden, S.G.; Blok, W.L.; Sauerwein, R.W.; Oettinger, M.C.; Verhave, J.P.; Meuwissen, J.E.; Van der Meer, J.W.; Van den Broek, M.F. Inhibition of Plasmodium berghei liver schizont development and reduction of cytokine production capacity in rats by dietary fish oil supplementation. Am. J. Trop. Med. Hyg. 1995, 53, 206–210. [Google Scholar] [CrossRef][Green Version]
- Miguelez, M.; Anisman, H.; Weber, J.-M.; Merali, Z. Effects of acute or chronic omega-3 and omega-6 polyunsaturated fatty acid treatment on behavioral, neuroendocrine and cytokine changes elicited by exogenous interleukin-1beta challenge. J. Neuroimmunol. 2006, 181, 19–28. [Google Scholar] [CrossRef]
- Minogue, A.M.; Lynch, A.M.; Loane, D.J.; Herron, C.E.; Lynch, M.A. Modulation of amyloid-beta-induced and age-associated changes in rat hippocampus by eicosapentaenoic acid. J. Neurochem. 2007, 103, 914–926. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, R.; Yao, C.; Wu, Q.; Christelle, M.; Xie, W.; Zhang, X.; Sun, W.; Wang, H.; Yao, S. Effects of resolvin D1 on inflammatory responses and oxidative stress of lipopolysaccharide-induced acute lung injury in mice. Chin. Med. J. (Engl.) 2014, 127, 803–809. [Google Scholar]
- Hashimoto, M.; Katakura, M.; Tanabe, Y.; Al Mamun, A.; Inoue, T.; Hossain, S.; Arita, M.; Shido, O. n-3 fatty acids effectively improve the reference memory-related learning ability associated with increased brain docosahexaenoic acid-derived docosanoids in aged rats. Biochim. Biophys. Acta 2015, 1851, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S.; Ertley, R.N.; DeMar, J.C., Jr.; Rapoport, S.I.; Bazinet, R.P.; Lee, H.J. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol. Psychiatry 2007, 12, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Rao, J.S.; Rapoport, S.I.; Igarashi, M. Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain. Biochim. Biophys. Acta 2011, 1811, 111–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).