Pituitary Glycoprotein Hormones in Human Milk before and after Pasteurization or Refrigeration
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Maternal Demographics
3.2. Comparison of Term and Preterm Breast Milk
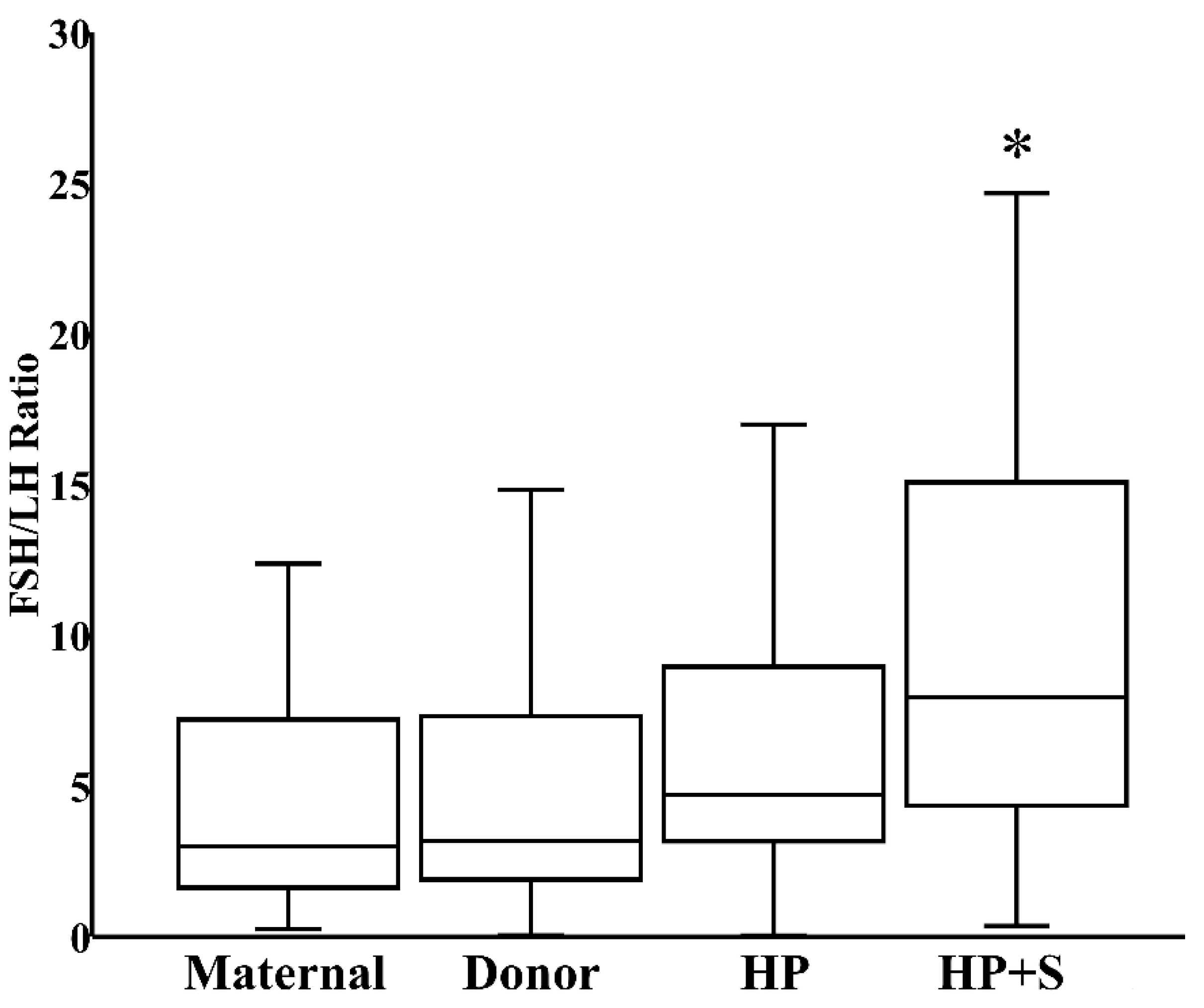
3.3. Effects of Pasteurization and Storage
3.4. Exploratory Analysis of Confounding Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blesa, M.; Sullivan, G.; Anblagan, D.; Telford, E.J.; Quigley, A.J.; Sparrow, S.A.; Serag, A.; Semple, S.I.; Bastin, M.E.; Boardman, J.P. Early breast milk exposure modifies brain connectivity in preterm infants. Neuroimage 2019, 184, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Pundir, S.; Mäkelä, J.; Nuora, A.; Junttila, N.; Wall, C.R.; Linderborg, K.; Cameron-Smith, D.; Lagström, H. Maternal influences on the glucocorticoid concentrations of human milk: The STEPS study. Clin. Nutr. 2019, 38, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, L.; Cofini, M.; Leonardi, A.; Penta, L.; Esposito, S. Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Front. Endocrinol. 2018, 9, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minabe, S.; Sato, M.; Inoue, N.; Watanabe, Y.; Magata, F.; Matsuda, F.; Uenoyama, Y.; Ozawa, H.; Tsukamura, H. Neonatal estrogen causes irreversible male infertility via specific suppressive action on hypothalamic Kiss1 neurons. Endocrinology 2019, 160, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Kaluarachchi, D.C.; Colaizy, T.T.; Pesce, L.M.; Tansey, M.; Klein, J.M. Congenital hypothyroidism with delayed thyroid-stimulating hormone elevation in premature infants born at less than 30 weeks gestation. J. Perinatol. 2017, 37, 277–282. [Google Scholar] [CrossRef]
- Scott, S.M.; Rose, S.R. Use of glucocorticoids for the fetus and preterm infant. Clin. Perinatol. 2018, 45, 93–102. [Google Scholar] [CrossRef]
- Vass, R.A.; Bell, E.F.; Colaizy, T.T.; Schmelzel, M.L.; Johnson, K.J.; Walker, J.R.; Ertl, T.; Roghair, R.D. Hormone levels in preterm and donor human milk before and after Holder pasteurization. Pediatr. Res. 2020. [Google Scholar] [CrossRef]
- Committee on Nutrition; Section on Breastfeeding; Committee on Fetus and Newborn. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017, 139, e20163440. [Google Scholar] [CrossRef] [Green Version]
- Piemontese, P.; Liotto, N.; Mallardi, D.; Roggero, P.; Puricelli, V.; Giannì, M.L.; Morniroli, D.; Tabasso, C.; Perrone, M.; Menis, C.; et al. The effect of human milk on modulating the quality of growth in preterm infants. Front. Pediatr. 2018, 6, 291. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Vass, R.A.; Kemeny, A.; Dergez, T.; Ertl, T.; Reglodi, D.; Jungling, A.; Tamas, A. Distribution of bioactive factors in human milk samples. Int. Breastfeed. J. 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, D.A. Thyroid function in premature infants. The hypothyroxinemia of prematurity. Clin. Perinatol. 1998, 25, 999–1014. [Google Scholar] [CrossRef]
- van Wassenaer, A.G.; Kok, J.H.; Dekker, F.W.; de Vijlder, J.J. Thyroid function in very preterm infants: Influences of gestational age and disease. Pediatr. Res. 1997, 42, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Reuss, M.L.; Levinton, A.; Paneth, N.; Susser, M. Thyroxine values from newborn screening of 919 infants born before 29 week’s gestation. Am. J. Publ. Health 1997, 87, 1693–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Whetsell, M.; Klein, J.R. Local hormone networks and intestinal T cell homeostasis. Science 1997, 275, 1937–1939. [Google Scholar] [CrossRef]
- Andersson, A.M.; Toppari, J.; Haavisto, A.M.; Petersen, J.H.; Simell, T.; Simell, O.; Skakkebaek, N.E. Longitudinal reproductive hormone profiles in infants: Peak of inhibin B levels in infant boys exceeds levels in adult men. J. Clin. Endocrinol. Metab. 1998, 83, 675–681. [Google Scholar] [CrossRef]
- Schmidt, H.; Schwarz, H.P. Serum concentrations of LH and FSH in the healthy newborn. Eur. J. Endocrinol. 2000, 143, 213–215. [Google Scholar] [CrossRef] [Green Version]
- Hines, M.; Spencer, D.; Kung, K.T.; Browne, W.V.; Constantinescu, M.; Noorderhaven, R.M. The early postnatal period, mini-puberty, provides a window on the role of testosterone in human neurobehavioural development. Curr. Opin. Neurobiol. 2016, 38, 69–73. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Padmanabhan, V.; Baggiani, A.M.; Medri, G.; Marconi, A.M.; Pardi, G.; Beitins, I.Z. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: Circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J. Clin. Endocrinol. Metab. 1991, 73, 525–532. [Google Scholar]
- Clements, J.A.; Reyes, F.I.; Winter, J.S.; Faiman, C. Studies on human sexual development. III. Fetal pituitary and serum, and amniotic fluid concentrations of LH, CG, and FSH. J. Clin. Endocrinol. Metab. 1976, 42, 9–19. [Google Scholar] [CrossRef]
- Winter, J.S. Hypothalamic--pituitary function in the fetus and infant. Clin. Endocrinol. Metab. 1982, 11, 41–55. [Google Scholar] [CrossRef]
- Winter, J.S.D.; Faiman, C.; Hobson, W.C.; Prasad, A.V.; Reyes, F.I. Pituitary-gonadal relations in infancy. I. patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J. Clin. Endocrinol. Metab. 1975, 40, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kuiri-Hänninen, T.; Kallio, S.; Seuri, R.; Tyrväinen, E.; Liakka, A.; Tapanainen, J.; Sankilampi, U.; Dunkel, L. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J. Clin. Endocrinol. Metab. 2011, 96, 3432–3439. [Google Scholar] [CrossRef] [Green Version]
- Weaver, L.; Laker, M.; Nelson, R. Intestinal permeability in the newborn. Arch. Dis. Child. 1984, 59, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, S.; Shinohara, K.; Yamashiro, Y.; Walker, W.A.; Sanderson, I.R. Growth factors in breast milk and their effect on gastrointestinal development. Zhonghua Minguo Xiao Er Ke Yi Xue Hui Za Zhi 1997, 38, 332–337. [Google Scholar] [PubMed]
- Tessaro, I.; Modina, S.C.; Franciosi, F.; Sivelli, G.; Terzaghi, L.; Lodde, V.; Luciano, A.M. Effect of oral administration of low-dose follicle stimulating hormone on hyperandrogenized mice as a model of polycystic ovary syndrome. J. Ovarian Res. 2015, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukacs, H.; Hiatt, E.S.; Lei, Z.M.; Rao, C.V. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm. Behav. 1995, 29, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Lukacs, H.; Hiatt, E.S.; Reid, K.H.; Iyer, V.; Rao, C.V. Administration of human chorionic gonadotropin affects sleep-wake phases and other associated behaviors in cycling female rats. Brain Res. 1994, 654, 181–190. [Google Scholar] [CrossRef]
- McCormick, J.B. Gonadotrophin in urine and spinal fluid; quantitative studies for chorionic moles and choriocarcinomas. Obstet. Gynecol. 1954, 3, 58–66. [Google Scholar]
- Vesell, M.; Goldman, D. Friedman test on spinal fluid in cases of hydatidiform mole and pregnancy. Am. J. Obstet. Gynecol. 1941, 42, 272–275. [Google Scholar] [CrossRef]
- Petrusz, P. Localization and sites of action of gonadotropins in brain. In Anatomical Neuroendocrinology; Stumpf, W.E., Grant, L.D., Eds.; Karger: Basel, Switzerland, 1975; pp. 176–184. [Google Scholar]
- Wilson, A.C.; Salamat, M.S.; Haasl, R.J.; Roche, K.M.; Karande, A.; Meethal, S.V.; Terasawa, E.; Bowen, R.L.; Atwood, C.S. Human neurons express type I GnRH receptor and respond to GnRH I by increasing luteinizing hormone expression. J. Endocrinol. 2006, 191, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Wang, L.; Wu, C.; Shi, H.; Zhou, Z.; Montgomery, S.; Cao, Y. Sex hormones, gonadotropins, and sex hormone-binding globulin in infants fed breast milk, cow milk formula, or soy formula. Sci. Rep. 2017, 7, 4332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adgent, M.A.; Umbach, D.M.; Zemel, B.S.; Kelly, A.; Schall, J.I.; Ford, E.G.; James, K.; Darge, K.; Botelho, J.C.; Vesper, H.W.; et al. A longitudinal study of estrogen-responsive tissues and hormone concentrations in infants fed soy formula. J. Clin. Endocrinol. Metab. 2018, 103, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Starzyk, J.; Wójcik, M.; Wojtyś, J.; Tomasik, P.; Mitkowska, Z.; Pietrzyk, J.J. Ovarian hyperstimulation syndrome in newborns--a case presentation and literature review. Horm. Res. 2009, 71, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.G.; Parsons, T.F. Glycoprotein hormones: Structure and function. Annu. Rev. Biochem. 1981, 50, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Oberkotter, L.V.; Tenore, A.; Pasquariello, P.S.; Zavod, W. A thyroxine-binding protein in human breast milk similar to serum thyroxine-binding globulin. J. Clin. Endocrinol. Metab. 1983, 57, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Unger, S.; O′Connor, D.L.; Stone, D.; Harvey, S.; Clandinin, M.T.; Field, C.J. Effect of pasteurization on selected immune components of donated human breast milk. J. Perinatol. 2011, 31, 593–598. [Google Scholar] [CrossRef] [Green Version]
- de Waard, M.; Mank, E.; van Dijk, K.; Schoonderwoerd, A.; van Goudoever, J.B. Holder-pasteurized human donor milk: How long can it be preserved? J. Pediatr. Gastroenterol. Nutr. 2018, 66, 479–483. [Google Scholar] [CrossRef]
- Hanna, N.; Ahmed, K.; Anwar, M.; Petrova, A.; Hiatt, M.; Hegyi, T. Effect on storage on breast milk antioxidant activity. Arch. Dis Child. Fetal Neonatal Ed. 2004, 89, F518–F520. [Google Scholar] [CrossRef] [Green Version]
- Bertino, E.; Giribaldi, M.; Baro, C.; Giancotti, V.; Pazzi, M.; Peila, C.; Tonetto, P.; Arslanoglu, S.; Moro, G.E.; Cavallarin, L.; et al. Effect of prolonged refrigeration on the lipid profile, lipase activity, and oxidative status of human milk. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 390–396. [Google Scholar] [CrossRef]
- Marinković, V.; Ranković-Janevski, M.; Spasić, S.; Nikolić-Kokić, A.; Lugonja, N.; Djurović, D.; Miletić, S.; Vrvić, M.M.; Spasojević, I. Antioxidative activity of colostrum and human milk: Effects of pasteurization and storage. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 901–906. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vass, R.A.; Roghair, R.D.; Bell, E.F.; Colaizy, T.T.; Johnson, K.J.; Schmelzel, M.L.; Walker, J.R.; Ertl, T. Pituitary Glycoprotein Hormones in Human Milk before and after Pasteurization or Refrigeration. Nutrients 2020, 12, 687. https://doi.org/10.3390/nu12030687
Vass RA, Roghair RD, Bell EF, Colaizy TT, Johnson KJ, Schmelzel ML, Walker JR, Ertl T. Pituitary Glycoprotein Hormones in Human Milk before and after Pasteurization or Refrigeration. Nutrients. 2020; 12(3):687. https://doi.org/10.3390/nu12030687
Chicago/Turabian StyleVass, Réka A., Robert D. Roghair, Edward F. Bell, Tarah T. Colaizy, Karen J. Johnson, Mendi L. Schmelzel, Jacky R. Walker, and Tibor Ertl. 2020. "Pituitary Glycoprotein Hormones in Human Milk before and after Pasteurization or Refrigeration" Nutrients 12, no. 3: 687. https://doi.org/10.3390/nu12030687
APA StyleVass, R. A., Roghair, R. D., Bell, E. F., Colaizy, T. T., Johnson, K. J., Schmelzel, M. L., Walker, J. R., & Ertl, T. (2020). Pituitary Glycoprotein Hormones in Human Milk before and after Pasteurization or Refrigeration. Nutrients, 12(3), 687. https://doi.org/10.3390/nu12030687







