Sulforaphene Suppresses Adipocyte Differentiation via Induction of Post-Translational Degradation of CCAAT/Enhancer Binding Protein Beta (C/EBPβ)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Adipocyte Differentiation of 3T3-L1 Pre-adipocytes
2.3. Isolation, Culture, and Adipocyte Differentiation of Human ASCs
2.4. Oil Red O Staining
2.5. Western Blot Assay
2.6. Quantitative Real Time (qRT) PCR
2.7. Statistical Analysis
3. Results
3.1. SFEN Exhibits Stronger Anti-adipogenic Effects than Other ITCs
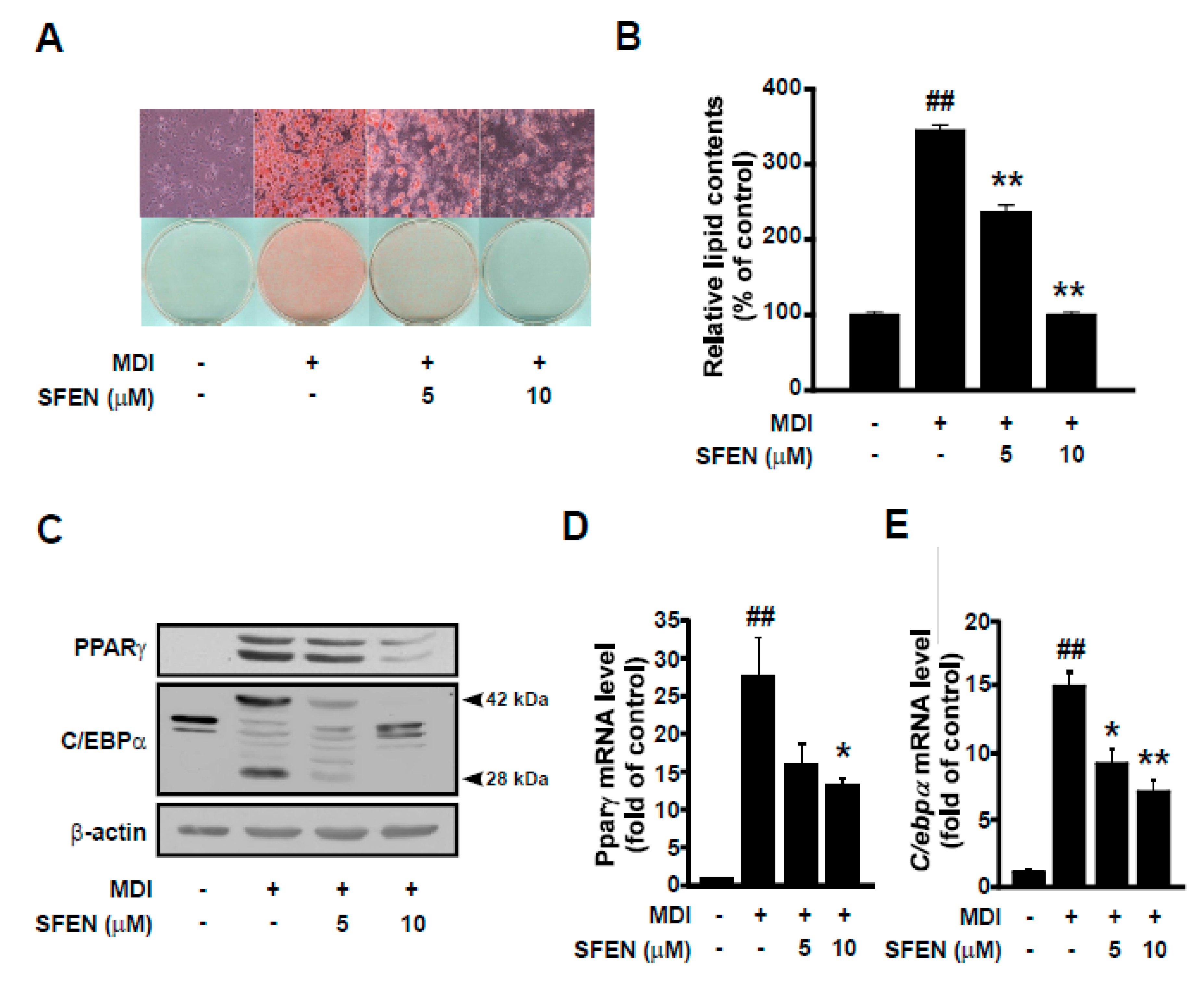
3.2. SFEN Decreases MDM-Induced PPARγ and C/EBPα Protein and mRNA Expression in a Dose-Dependent Manner
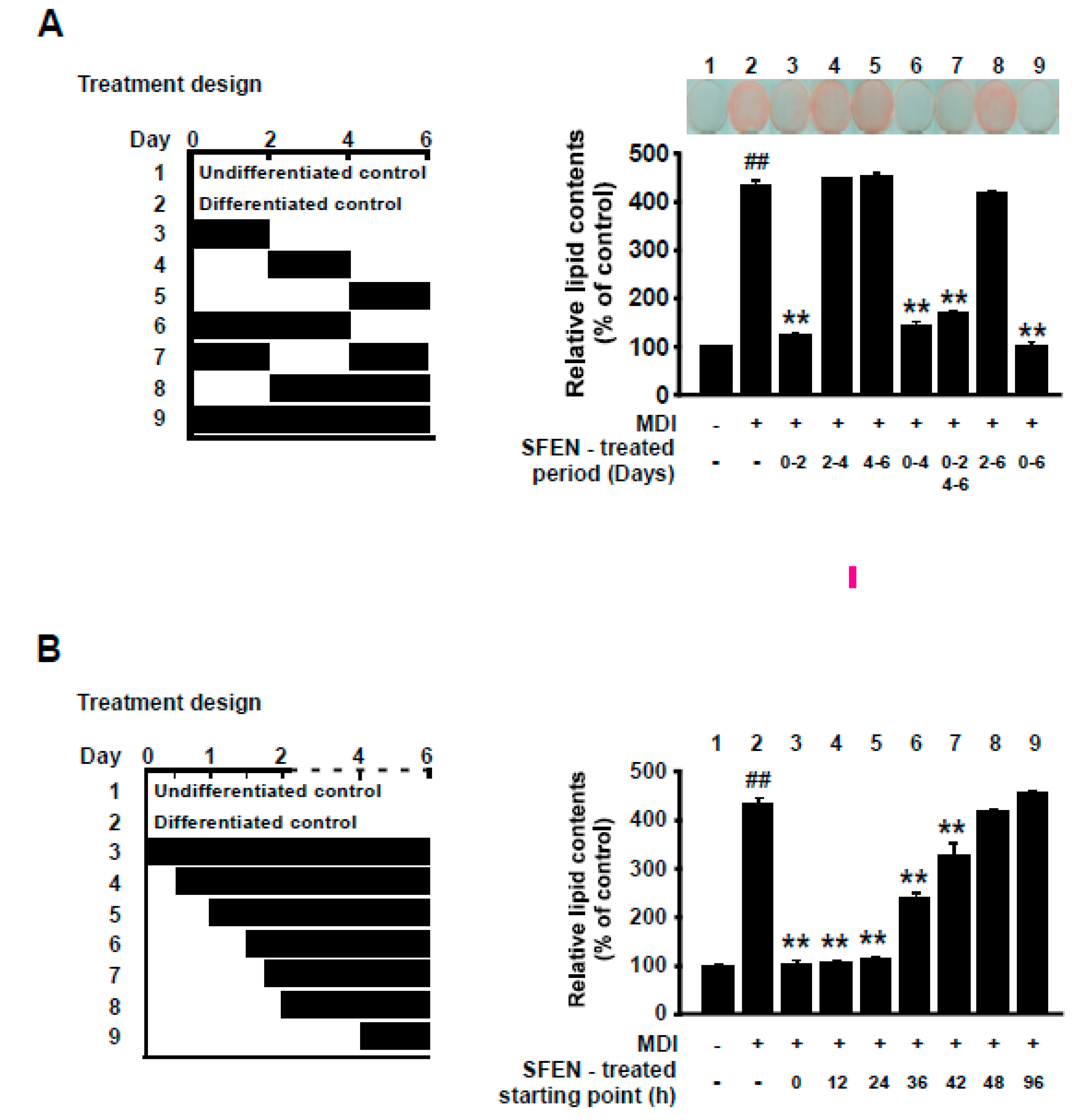
3.3. SFEN Exerts Anti-Adipogenic Effects at the Early Stage of Differentiation
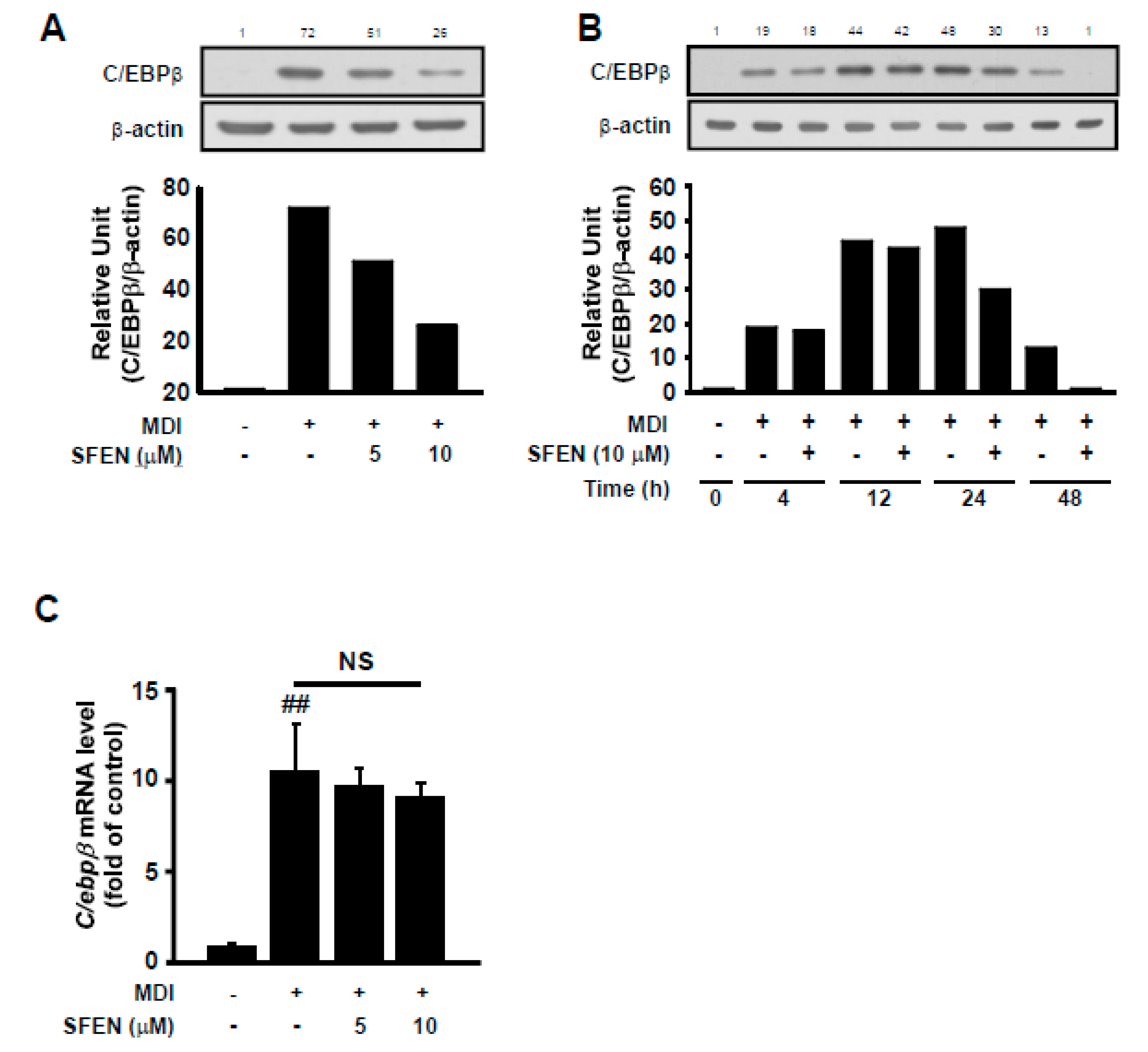
3.4. SFEN Reduces MDM-Induced Increases in C/EBPβ Protein Levels but Not mRNA Levels
3.5. SFEN Induces Post-Translational Degradation of C/EBPβ by Decreasing Its Stability
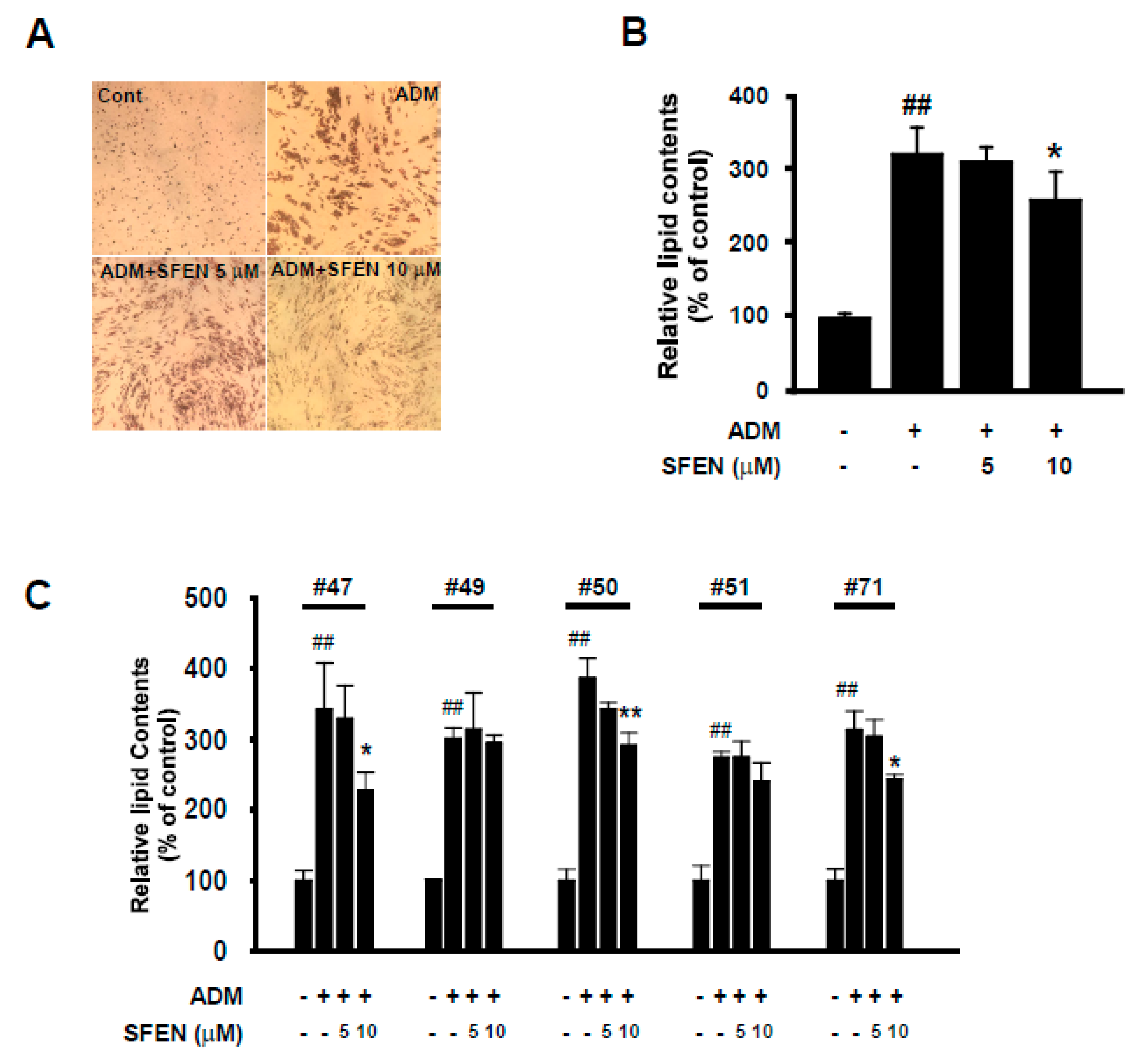
3.6. SFEN Suppresses Adipogenesis in Human ASCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AITC | allyl isothiocyanate |
| ALLN | n-acetyl-leu-leu-norleucinal |
| ASC | adipose tissue-derived stem cells |
| BCS | bovine calf serum |
| BITC | benzyl isothiocyanate |
| b-zip | basic leucine zipper domain |
| C/EBPα | CCAAT/enhancer-binding protein α |
| C/EBPβ | CCAAT/enhancer-binding protein β |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMEM-F12 | Dulbecco’s modified Eagle’s medium with Ham’s F12 |
| ERU | erucin |
| FBS | fetal bovine serum |
| GSK3β | glycogen synthase kinase 3β |
| HDM | human adipocyte differentiation medium |
| Ibe | iberin |
| IBMX | 3-isobutyl-1-methylxanthine |
| ITC | isothiocyanate |
| MACS | magnetic activated cell sorting system |
| MAPK | mitogen-activated protein kinase |
| MDM | mouse adipocyte differentiation medium |
| PBS | phosphate-buffered saline |
| PEITC | phenethyl isothiocyanate |
| PPARγ | peroxisome proliferator-activated receptor γ |
| SFEN | sulforaphene |
| SFN | sulforaphane |
| SVF | stromal vascular fraction |
| UCP | uncoupling protein |
| VAT | visceral adipose tissue |
| WHR | waist hip ratio |
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; Leroith, D.; Karnieli, E. The metabolic syndrome--from insulin resistance to obesity and diabetes. Med. Clin. N. Am. 2011, 95, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [Green Version]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Siersbaek, R.; Nielsen, R.; Mandrup, S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol. Metab. Tem 2012, 23, 56–64. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [Green Version]
- Park, B.O.; Ahrends, R.; Teruel, M.N. Consecutive Positive Feedback Loops Create a Bistable Switch that Controls Preadipocyte-to-Adipocyte Conversion. Cell Rep. 2012, 2, 976–990. [Google Scholar] [CrossRef] [Green Version]
- Millward, C.A.; Heaney, J.D.; Sinasac, D.S.; Chu, E.C.; Bederman, I.R.; Gilge, D.A.; Previs, S.F.; Croniger, C.M. Mice with a Deletion in the Gene for CCAAT/Enhancer-Binding Protein β Are Protected Against Diet-Induced Obesity. Diabetes 2007, 56, 161. [Google Scholar] [CrossRef] [Green Version]
- van der Krieken, S.E.; Popeijus, H.E.; Mensink, R.P.; Plat, J. CCAAT/enhancer binding protein β in relation to ER stress, inflammation, and metabolic disturbances. Biomed Res. Int. 2015, 2015, 324815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christy, R.J.; Kaestner, K.H.; Geiman, D.E.; Lane, M.D. CCAAT/enhancer binding protein gene promoter: Binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 1991, 88, 2593–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajas, L.; Auboeuf, D.; Raspe, E.; Schoonjans, K.; Lefebvre, A.M.; Saladin, R.; Najib, J.; Laville, M.; Fruchart, J.C.; Deeb, S.; et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J. Biol. Chem. 1997, 272, 18779–18789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.Q.; Jiang, M.S.; Lane, M.D. Repressive effect of Sp1 on the C/EBPalpha gene promoter: Role in adipocyte differentiation. Mol. Cell. Biol. 1999, 19, 4855–4865. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.M.; Janssen, R.C.; Choudhury, M.; Baquero, K.C.; Aikens, R.M.; de la Houssaye, B.A.; Friedman, J.E. CCAAT/enhancer-binding protein beta (C/EBPbeta) expression regulates dietary-induced inflammation in macrophages and adipose tissue in mice. J. Biol. Chem. 2012, 287, 34349–34360. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.M.; Schroeder-Gloeckler, J.M.; Janssen, R.C.; Jiang, H.; Qadri, I.; Maclean, K.N.; Friedman, J.E. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology 2007, 45, 1108–1117. [Google Scholar] [CrossRef]
- Staiger, J.; Lueben, M.J.; Berrigan, D.; Malik, R.; Perkins, S.N.; Hursting, S.D.; Johnson, P.F. C/EBPbeta regulates body composition, energy balance-related hormones and tumor growth. Carcinogenesis 2009, 30, 832–840. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhauser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S219. [Google Scholar] [CrossRef]
- Dufour, V.; Alazzam, B.; Thepaut, M.; Ermel, G.; Baysse, C. Antimicrobial Activities of Isothiocyanates Against Campylobacter jejuni Isolates. Front. Cell. Infect. Microbiol. 2012, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.-F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Lee, H.; Im, S.W.; Jung, C.H.; Ha, T.Y. Allyl isothiocyanate ameliorates insulin resistance through the regulation of mitochondrial function. J. Nutr. Biochem. 2014, 25, 1026–1034. [Google Scholar] [CrossRef]
- Songsak, T.; Lockwood, G.B. Glucosinolates of seven medicinal plants from Thailand. Fitoterapia 2002, 73, 209–216. [Google Scholar] [CrossRef]
- Shishu; Kaur, I.P. Inhibition of cooked food-induced mutagenesis by dietary constituents: Comparison of two natural isothiocyanates. Food Chem. 2009, 112, 977–981. [Google Scholar] [CrossRef]
- Beevi, S.S.; Mangamoori, L.N.; Subathra, M.; Edula, J.R. Hexane extract of Raphanus sativus L. roots inhibits cell proliferation and induces apoptosis in human cancer cells by modulating genes related to apoptotic pathway. Plant Foods Hum. Nutr. (Dordr. Neth.) 2010, 65, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Seo, S.G.; Yang, H.; Yu, J.G.; Suk, S.J.; Jung, E.S.; Ji, H.; Kwon, J.Y.; Lee, H.J.; Lee, K.W. Anti-adipogenic effect of erucin in early stage of adipogenesis by regulating Ras activity in 3T3-L1 preadipocytes. J. Funct. Foods 2015, 19, 700–709. [Google Scholar] [CrossRef]
- Choi, K.-M.; Lee, Y.-S.; Kim, W.; Kim, S.J.; Shin, K.-O.; Yu, J.-Y.; Lee, M.K.; Lee, Y.-M.; Hong, J.T.; Yun, Y.-P.; et al. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J. Nutr. Biochem. 2014, 25, 201–207. [Google Scholar] [CrossRef]
- Chuang, W.-T.; Liu, Y.-T.; Huang, C.-S.; Lo, C.-W.; Yao, H.-T.; Chen, H.-W.; Lii, C.-K. Benzyl Isothiocyanate and Phenethyl Isothiocyanate Inhibit Adipogenesis and Hepatosteatosis in Mice with Obesity Induced by a High-Fat Diet. J. Agric. Food Chem. 2019, 67, 7136–7146. [Google Scholar] [CrossRef]
- Chen, J.; Bao, C.; Kim, J.T.; Cho, J.S.; Qiu, S.; Lee, H.J. Sulforaphene Inhibition of Adipogenesis via Hedgehog Signaling in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2018, 66, 11926–11934. [Google Scholar] [CrossRef]
- Kim, B.; Lee, B.; Kim, M.K.; Gong, S.P.; Park, N.H.; Chung, H.H.; Kim, H.S.; No, J.H.; Park, W.Y.; Park, A.K.; et al. Gene expression profiles of human subcutaneous and visceral adipose-derived stem cells. Cell Biochem. Funct. 2016, 34, 563–571. [Google Scholar] [CrossRef]
- Yang, H.; Seo, S.G.; Shin, S.H.; Min, S.; Kang, M.J.; Yoo, R.; Kwon, J.Y.; Yue, S.; Kim, K.H.; Cheng, J.X.; et al. 3,3’-Diindolylmethane suppresses high-fat diet-induced obesity through inhibiting adipogenesis of pre-adipocytes by targeting USP2 activity. Mol. Nutr. Food Res. 2017, 61, 1700119. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lo, C.W.; Chen, C.S.; Chen, Y.C.; Hsu, Y.A.; Huang, C.C.; Chang, C.Y.; Lin, C.J.; Lin, C.W.; Lin, H.J.; Liu, F.T.; et al. Allyl Isothiocyanate Ameliorates Obesity by Inhibiting Galectin-12. Mol. Nutr. Food Res. 2018, 62, e1700616. [Google Scholar] [CrossRef]
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, E.J.; Kim, K.J.; Choi, J.; Jeon, H.J.; Seo, M.J.; Lee, B.Y. Ginsenoside Rg1 suppresses early stage of adipocyte development via activation of C/EBP homologous protein-10 in 3T3-L1 and attenuates fat accumulation in high fat diet-induced obese zebrafish. J. Ginseng. Res. 2017, 41, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, Y.-C.; Hsieh, P.-H.; Pan, M.-H.; Ho, C.-T. Cellular models for the evaluation of the antiobesity effect of selected phytochemicals from food and herbs. J. Food Drug Anal. 2017, 25, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef]
- Lund, P.; Pilgaard, L.; Duroux, M.; Fink, T.; Zachar, V. Effect of growth media and serum replacements on the proliferation and differentiation of adipose-derived stem cells. Cytotherapy 2009, 11, 189–197. [Google Scholar] [CrossRef]
- Storck, K.; Ell, J.; Regn, S.; Rittler-Ungetüm, B.; Mayer, H.; Schantz, T.; Müller, D.; Buchberger, M. Optimization of in vitro cultivation strategies for human adipocyte derived stem cells. Adipocyte 2015, 4, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Styner, M.; Sen, B.; Xie, Z.; Case, N.; Rubin, J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J. Cell. Biochem. 2010, 111, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.Y.; Hu, J.P.; Wu, M.M.; Wang, L.S.; Fang, N.Y. CCAAT/enhancer-binding protein CEBP-2 controls fat consumption and fatty acid desaturation in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2015, 468, 312–318. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Gronborg, M.; Huang, H.; Kim, J.W.; Otto, T.C.; Pandey, A.; Lane, M.D. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9766–9771. [Google Scholar] [CrossRef] [Green Version]
- Ron, D.; Habener, J.F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992, 6, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Tang, Q.Q.; Li, X.; Lane, M.D. Effect of phosphorylation and S-S bond-induced dimerization on DNA binding and transcriptional activation by C/EBPbeta. Proc. Natl. Acad. Sci. USA 2007, 104, 1800–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, T.; Ohoka, N.; Inoue, Y.; Hayashi, H.; Onozaki, K. C/EBP family transcription factors are degraded by the proteasome but stabilized by forming dimer. Oncogene 2003, 22, 1273–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Y.D.; Guo, L.; Huang, H.Y.; Zhu, H.; Huang, J.X.; Liu, Y.; Zhou, S.R.; Dang, Y.J.; Li, X.; et al. Protein inhibitor of activated STAT 1 (PIAS1) is identified as the SUMO E3 ligase of CCAAT/enhancer-binding protein beta (C/EBPbeta) during adipogenesis. Mol. Cell. Biol. 2013, 33, 4606–4617. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Yang, H.; Cao, P.; Menconi, M.; Chamberlain, C.; Petkova, V.; Hasselgren, P.O. Degradation of C/EBPbeta in cultured myotubes is calpain-dependent. J. Cell. Physiol. 2006, 208, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Li, S.F.; Qian, S.W.; Zhang, Y.Y.; Liu, Y.; Tang, Q.Q.; Li, X. Phosphorylation prevents C/EBPbeta from the calpain-dependent degradation. Biochem. Biophys. Res. Commun. 2012, 419, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Welm, A.L.; Timchenko, N.A.; Darlington, G.J. C/EBPalpha regulates generation of C/EBPbeta isoforms through activation of specific proteolytic cleavage. Mol. Cell. Biol. 1999, 19, 1695–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechner, S.; Mitterberger, M.C.; Mattesich, M.; Zwerschke, W. Role of C/EBPβ-LAP and C/EBPβ-LIP in early adipogenic differentiation of human white adipose-derived progenitors and at later stages in immature adipocytes. Differentiation 2013, 85, 20–31. [Google Scholar] [CrossRef]
- Li, Y.; Bevilacqua, E.; Chiribau, C.B.; Majumder, M.; Wang, C.; Croniger, C.M.; Snider, M.D.; Johnson, P.F.; Hatzoglou, M. Differential control of the CCAAT/enhancer-binding protein beta (C/EBPbeta) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J. Biol. Chem. 2008, 283, 22443–22456. [Google Scholar] [CrossRef] [Green Version]
- Hungness, E.S.; Robb, B.W.; Luo, G.-J.; Pritts, T.A.; Hershko, D.D.; Hasselgren, P.-O. Proteasome Inhibitors Activate the Transcription Factors C/EBP-β and δ in Human Intestinal Epithelial Cells. Biochem. Biophys. Res. Commun. 2002, 290, 469–474. [Google Scholar] [CrossRef]
- Patel, Y.M.; Lane, M.D. Mitotic Clonal Expansion during Preadipocyte Differentiation: Calpain-mediated Turnover of p27. J. Biol. Chem. 2000, 275, 17653–17660. [Google Scholar] [CrossRef] [Green Version]
- Mi, L.; Di Pasqua, A.J.; Chung, F.L. Proteins as binding targets of isothiocyanates in cancer prevention. Carcinogenesis 2011, 32, 1405–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedel, A.; Lömsziegler-Heitbrock, H.W. The C/EBP Family of Transcription Factors. Immunobiology 1995, 193, 171–185. [Google Scholar] [CrossRef]
- Lamy, E.; Scholtes, C.; Herz, C.; Mersch-Sundermann, V. Pharmacokinetics and pharmacodynamics of isothiocyanates. Drug Metab. Rev. 2011, 43, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Posner, G.H.; Cho, C.-G.; Green, J.V.; Zhang, Y.; Talalay, P. Design and synthesis of bifunctional isothiocyanate analogs of sulforaphane: Correlation between structure and potency as inducers of anticarcinogenic detoxication enzymes. J. Med. Chem. 1994, 37, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Tianjie, J.; Gandao, Z.; Xijun, W. Effects of volatile sulfur compounds on hydrogenation of Chinese rapeseed oil. Nanjing Huagong Xueyuan Xuebao 1994, 4. [Google Scholar]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells (Dayt. Ohio) 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Challa, T.D.; Straub, L.G.; Balaz, M.; Kiehlmann, E.; Donze, O.; Rudofsky, G.; Ukropec, J.; Ukropcova, B.; Wolfrum, C. Regulation of De Novo Adipocyte Differentiation Through Cross Talk Between Adipocytes and Preadipocytes. Diabetes 2015, 64, 4075–4087. [Google Scholar] [CrossRef] [Green Version]
- Czernichow, S.; Kengne, A.-P.; Huxley, R.R.; Batty, G.D.; de Galan, B.; Grobbee, D.; Pillai, A.; Zoungas, S.; Marre, M.; Woodward, M.; et al. Comparison of waist-to-hip ratio and other obesity indices as predictors of cardiovascular disease risk in people with type-2 diabetes: A prospective cohort study from ADVANCE. Eur. J. Cardiovasc. Prev. Rehabil. 2012, 18, 312–319. [Google Scholar] [CrossRef]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjonneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef] [Green Version]



| Donor No. | Sex | WHR | BMI (kg/m2) | Age (Years Old) |
|---|---|---|---|---|
| #47 | Female | 0.98 | 24.4 | 71 |
| #49 | Female | 0.91 | 21.6 | 50 |
| #50 | Female | 0.94 | 23.0 | 54 |
| #51 | Female | 0.85 | 36.6 | 45 |
| #71 | Female | 0.94 | 23.7 | 58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Kang, M.J.; Hur, G.; Lee, T.K.; Park, I.S.; Seo, S.G.; Yu, J.G.; Song, Y.S.; Park, J.H.Y.; Lee, K.W. Sulforaphene Suppresses Adipocyte Differentiation via Induction of Post-Translational Degradation of CCAAT/Enhancer Binding Protein Beta (C/EBPβ). Nutrients 2020, 12, 758. https://doi.org/10.3390/nu12030758
Yang H, Kang MJ, Hur G, Lee TK, Park IS, Seo SG, Yu JG, Song YS, Park JHY, Lee KW. Sulforaphene Suppresses Adipocyte Differentiation via Induction of Post-Translational Degradation of CCAAT/Enhancer Binding Protein Beta (C/EBPβ). Nutrients. 2020; 12(3):758. https://doi.org/10.3390/nu12030758
Chicago/Turabian StyleYang, Hee, Min Jeong Kang, Gihyun Hur, Tae Kyung Lee, In Sil Park, Sang Gwon Seo, Jae Gak Yu, Yong Sang Song, Jung Han Yoon Park, and Ki Won Lee. 2020. "Sulforaphene Suppresses Adipocyte Differentiation via Induction of Post-Translational Degradation of CCAAT/Enhancer Binding Protein Beta (C/EBPβ)" Nutrients 12, no. 3: 758. https://doi.org/10.3390/nu12030758






