Decanal Protects against UVB-Induced Photoaging in Human Dermal Fibroblasts via the cAMP Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. UV Irradiation
2.4. Cell Viability Assay
2.5. Procollagen Type I and Hyaluronic Acid Quantification
2.6. cAMP Assay
2.7. Western Blotting
2.8. Quantitative Reverse-Transcription PCR
2.9. Statistical Analysis
3. Results
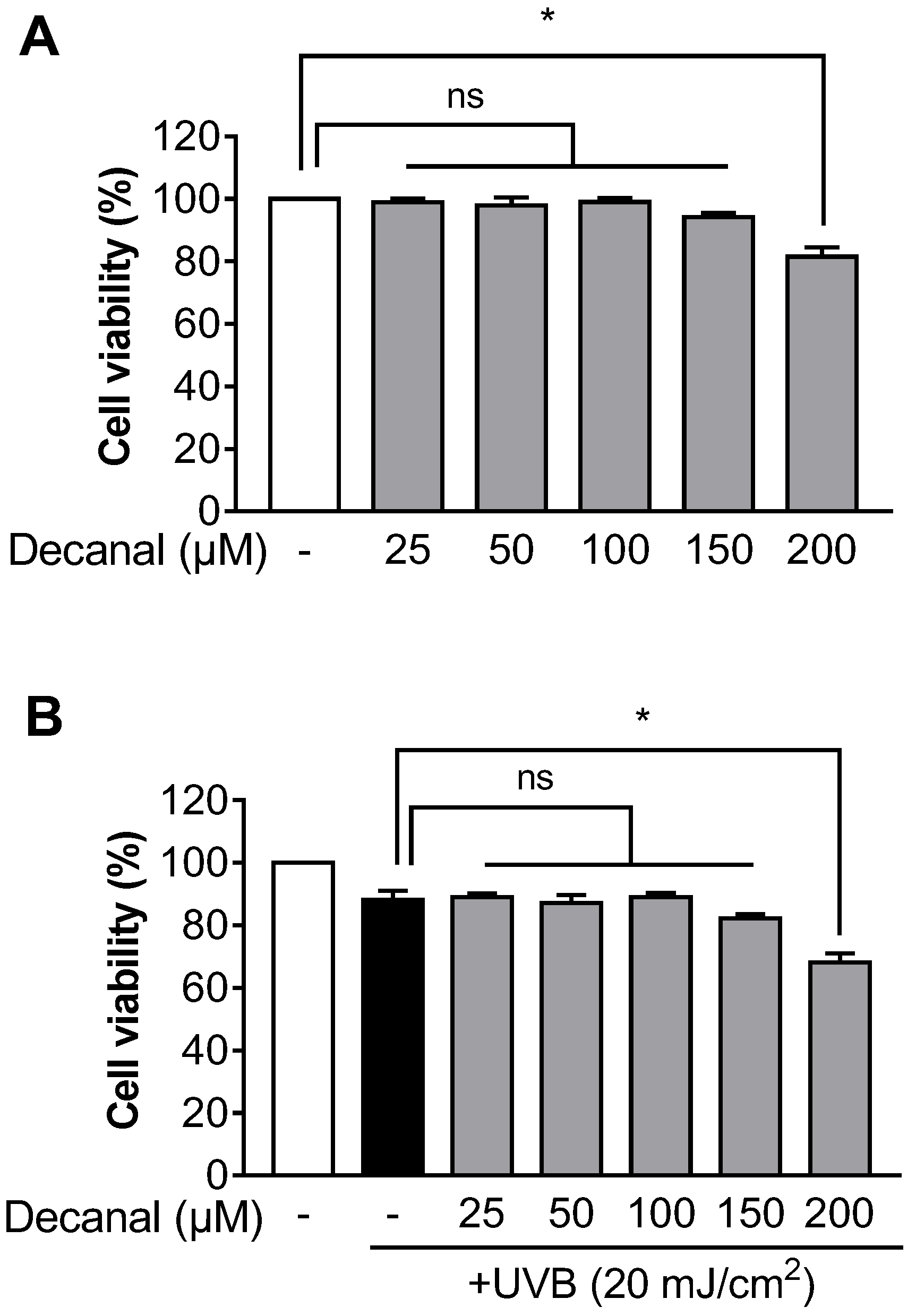
3.1. Decanal has Little Effect on Cell Viability in Hs68 Cells
3.2. Decanal Attenuates UVB-Induced Collagen Degradation in Hs68 Cells
3.3. Decanal Upregulated cAMP/PKA Signaling Pathway in Hs68 Cells
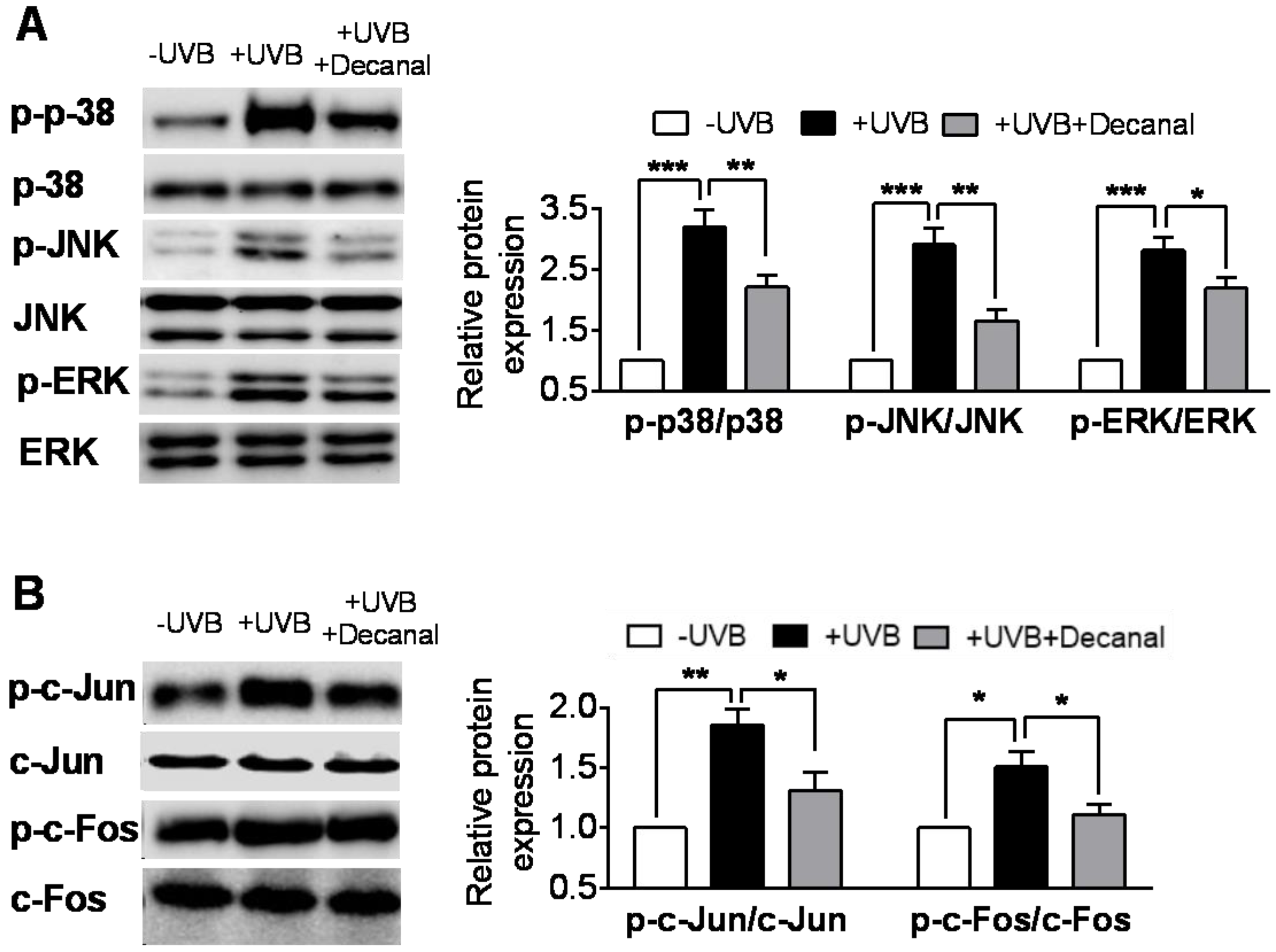
3.4. Decanal Inhibits UVB-Induced MAPK Pathway in Hs68 Cells
3.5. Decanal Promotes Collagen Synthesis Via cAMP Signaling Pathway
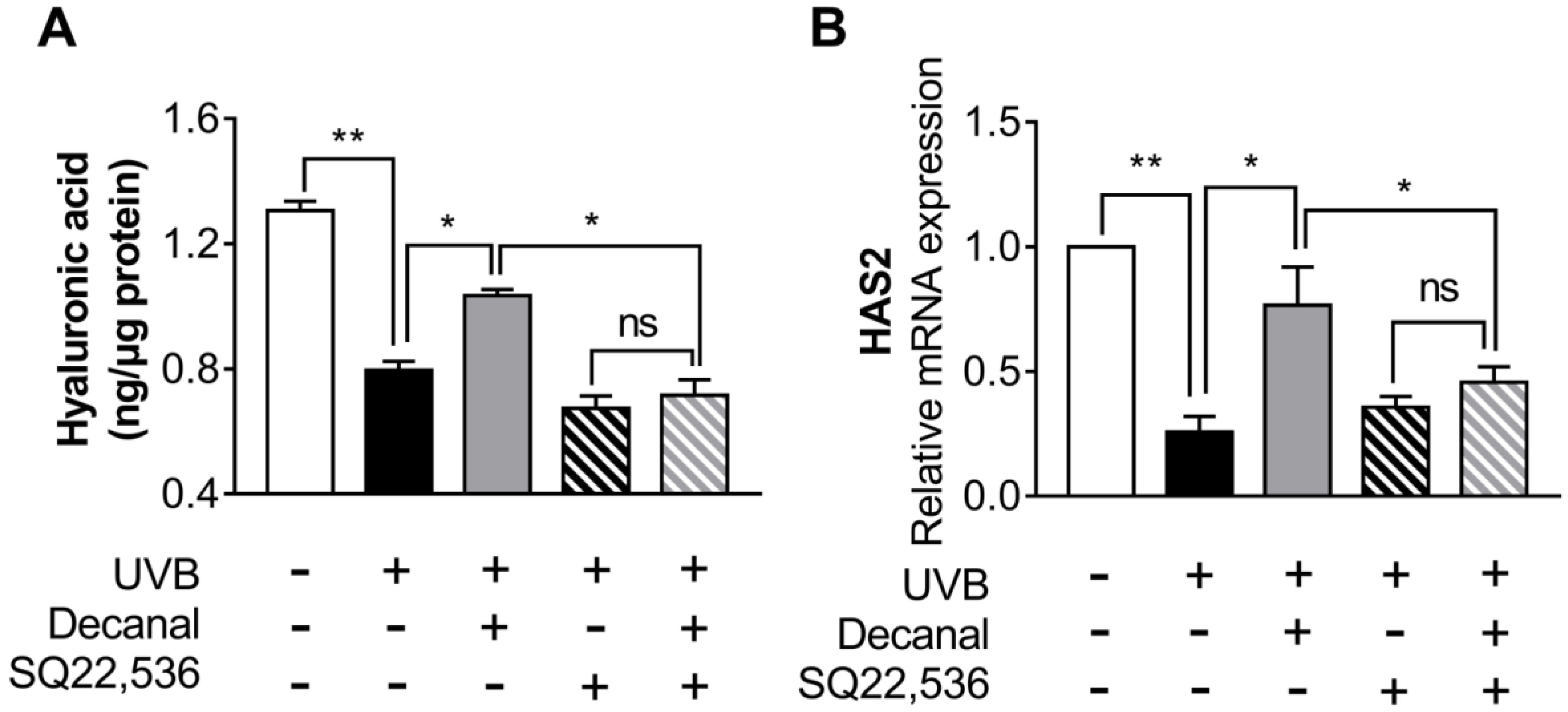
3.6. Decanal Enhances Hyaluronic Acid Synthesis Via cAMP Signaling Pathway
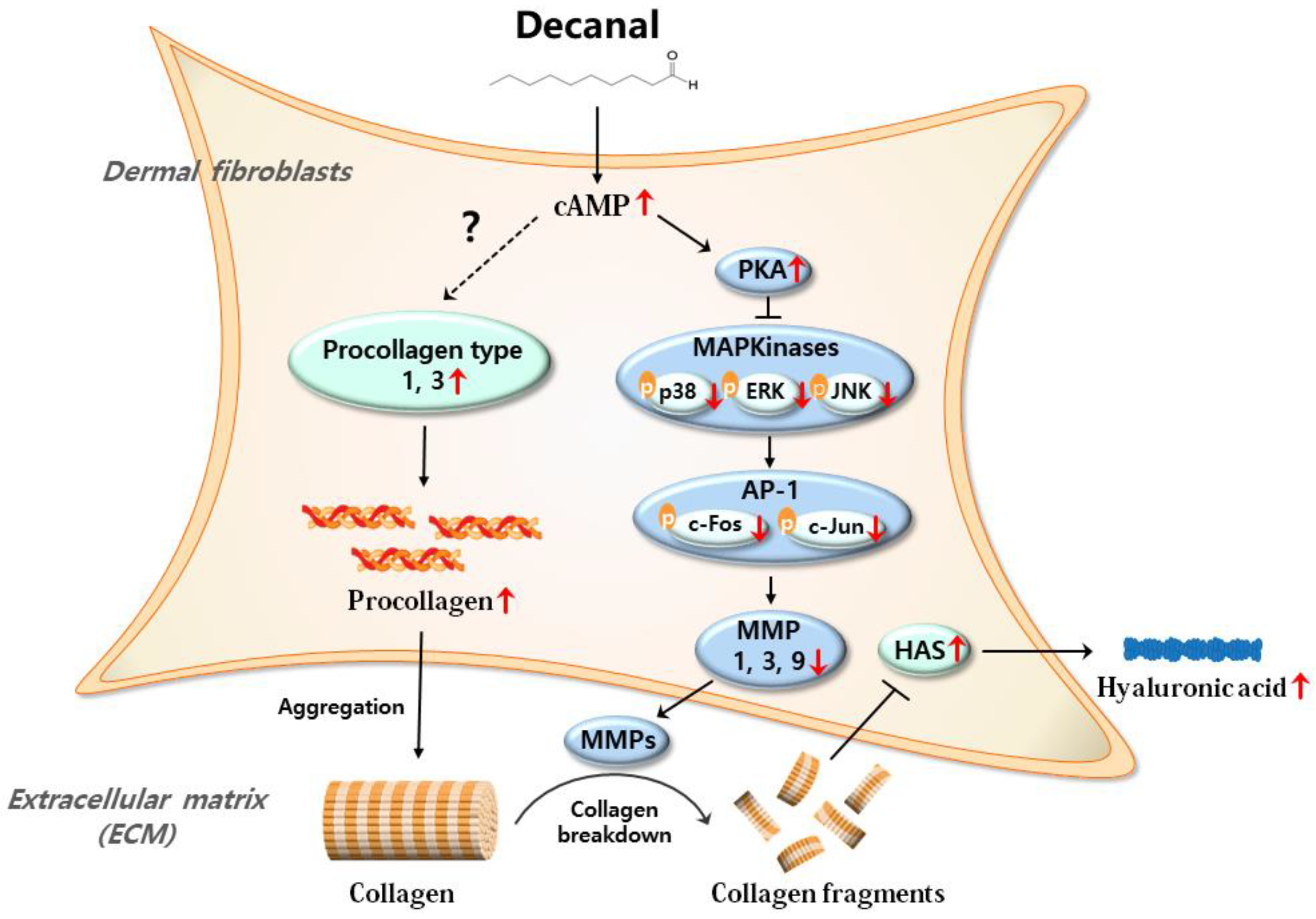
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. 2008, 7, s12–s16. [Google Scholar] [PubMed]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef]
- Chin, K.V.; Yang, W.L.; Ravatn, R.; Kita, T.; Reitman, E.; Vettori, D.; Cvijic, M.E.; Shin, M.; Iacono, L. Reinventing the wheel of cyclic AMP: Novel mechanisms of cAMP signaling. Ann. N. Y. Acad. Sci. 2002, 968, 49–64. [Google Scholar] [CrossRef]
- Beavo, J.; Bechtel, P.; Krebs, E. Activation of protein kinase by physiological concentrations of cyclic AMP. Proc. Natl. Acad. Sci. USA 1974, 71, 3580–3583. [Google Scholar] [CrossRef] [Green Version]
- Robinson, G.; Butcher, R.W.; Sutherland, E.W. Cyclic AMP. Ann. Rev. Biochem. 1968, 37, 149–174. [Google Scholar] [CrossRef]
- Park, C.-H.; Moon, Y.; Shin, C.M.; Chung, J.H. Cyclic AMP suppresses matrix metalloproteinase-1 expression through inhibition of MAPK and GSK-3β. J. Investig. Dermatol. 2010, 130, 2049–2056. [Google Scholar] [CrossRef] [Green Version]
- Perez-Aso, M.; Fernandez, P.; Mediero, A.; Chan, E.S.; Cronstein, B.N. Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 2014, 28, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.N.; Gil, C.H.; Kim, Y.R.; Shin, H.K.; Choi, B.T. Anti-photoaging properties of the phosphodiesterase 3 inhibitor cilostazol in ultraviolet B-irradiated hairless mice. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, T.; Park, J.; Moon, Y.; Kang, W.; Park, T. α-Ionone Protects Against UVB-Induced Photoaging in Human Dermal Fibroblasts. Molecules 2019, 24, 1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Park, T. Dietary Suberic Acid Protects Against UVB-Induced Skin Photoaging in Hairless Mice. Nutrients 2019, 11, 2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, P.A.; Figueiredo, A.C.; Lourenço, P.M.; Barroso, J.G.; Pedro, L.G.; Oliveira, M.M.; Schripsema, J.; Deans, S.G.; Scheffer, J.J. Hairy root cultures of Anethum graveolens (dill): Establishment, growth, time-course study of their essential oil and its comparison with parent plant oils. Biotechnol. Lett. 2002, 24, 1031–1036. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and biological activities of decanal, linalool, valencene, and octanal from sweet orange oil. J. Food Sci. 2012, 77, C1156–C1161. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Ye, B.; Shi, L.; Bai, X.; Lai, T. Effects of essential oil decanal on growth and transcriptome of the postharvest fungal pathogen Penicillium expansum. Postharvest Biol. Technol. 2018, 145, 203–212. [Google Scholar] [CrossRef]
- Dessinioti, C.; Antoniou, C.; Katsambas, A.; Stratigos, A.J. Basal cell carcinoma: What’s new under the sun. Photochem. Photobiol. 2010, 86, 481–491. [Google Scholar] [CrossRef]
- Lavker, R.; Veres, D.; Irwin, C.; Kaidbey, K. Quantitative assessment of cumulative damage from repetitive exposures to suberythemogenic doses of UVA in human skin. Photochem. Photobiol. 1995, 62, 348–352. [Google Scholar] [CrossRef]
- Hiraku, Y.; Ito, K.; Hirakawa, K.; Kawanishi, S. Photosensitized DNA damage and its protection via a novel mechanism. Photochem. Photobiol. 2007, 83, 205–212. [Google Scholar] [CrossRef]
- Pfau, R.; Hood, A.F.; Morison, W. Photoageing: The role of UVB, solar-simulated UVB, visible and psoralen UVA radiation. Br. J. Dermatol. 1986, 114, 319–327. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Short-term and long-term cellular and molecular events following UV irradiation of skin: Implications for molecular medicine. Exp. Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Freudenberger, T.; Zipper, P.; Melchior, A.; Grether-Beck, S.; Rabausch, B.; De Groot, J.; Twarock, S.; Hanenberg, H.; Homey, B. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007, 171, 1451–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawston, T.; Billington, C.; Cleaver, C.; Elliott, S.; Hui, W.; Koshy, P.; Shingleton, B.; Rowan, A. The regulation of MMPs and TIMPs in cartilage turnover. Ann. N. Y. Acad. Sci. 1999, 878, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.-Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biancheri, P.; MacDonald, T.T. Matrix Metalloproteinases. In Crohn’s Disease and Ulcerative Colitis; Springer: Berlin, Germany, 2017; pp. 135–140. [Google Scholar]
- Dzobo, K.; Leaner, V.D.; Parker, M.I. Absence of feedback regulation in the synthesis of COL1A1. Life Sci. 2014, 103, 25–33. [Google Scholar] [CrossRef]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Brömme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar] [CrossRef]
- Yokoyama, U.; Patel, H.H.; Lai, N.C.; Aroonsakool, N.; Roth, D.M.; Insel, P.A. The cyclic AMP effector Epac integrates pro-and anti-fibrotic signals. Proc. Natl. Acad. Sci. USA 2008, 105, 6386–6391. [Google Scholar] [CrossRef] [Green Version]
- Lipshtat, A.; Jayaraman, G.; He, J.C.; Iyengar, R. Design of versatile biochemical switches that respond to amplitude, duration, and spatial cues. Proc. Natl. Acad. Sci. USA 2010, 107, 1247–1252. [Google Scholar] [CrossRef] [Green Version]
- Ming, G.-L.; Song, H.-J.; Berninger, B.; Holt, C.E.; Tessier-Lavigne, M.; Poo, M.-M. cAMP-dependent growth cone guidance by netrin-1. Neuron 1997, 19, 1225–1235. [Google Scholar] [CrossRef] [Green Version]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J. Drugs Dermatol. 2011, 10, 990–1000. [Google Scholar] [PubMed]
- Kosaki, R.; Watanabe, K.; Yamaguchi, Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 1999, 59, 1141–1145. [Google Scholar] [PubMed]
- Röck, K.; Grandoch, M.; Majora, M.; Krutmann, J.; Fischer, J.W. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB) new insights into mechanisms of matrix remodeling. J. Biol. Chem. 2011, 286, 18268–18276. [Google Scholar] [CrossRef] [Green Version]
- Röck, K.; Tigges, J.; Sass, S.; Schütze, A.; Florea, A.-M.; Fender, A.C.; Theis, F.J.; Krutmann, J.; Boege, F.; Fritsche, E. miR-23a-3p causes cellular senescence by targeting hyaluronan synthase 2: Possible implication for skin aging. J. Investig. Dermatol. 2015, 135, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Sanz, G.; Schlegel, C.; Pernollet, J.-C.; Briand, L. Comparison of odorant specificity of two human olfactory receptors from different phylogenetic classes and evidence for antagonism. Chem. Sens. 2005, 30, 69–80. [Google Scholar] [CrossRef]
- Adipietro, K.A.; Mainland, J.D.; Matsunami, H. Functional evolution of mammalian odorant receptors. PLoS Genet. 2012, 8, e1002821. [Google Scholar] [CrossRef] [Green Version]
- Selbie, L.A.; Hill, S.J. G protein-coupled-receptor cross-talk: The fine-tuning of multiple receptor-signalling pathways. Trends Pharm. Sci. 1998, 19, 87–93. [Google Scholar] [CrossRef]
- Thomsen, W.; Frazer, J.; Unett, D. Functional assays for screening GPCR targets. Curr. Opin. Biotechnol. 2005, 16, 655–665. [Google Scholar] [CrossRef]
- Scharffetter-Kochanek, K.; Wlaschek, M.; Brenneisen, P.; Schauen, M.; Blaudschun, R.; Wenk, J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol. Chem. 1997, 378, 1247–1258. [Google Scholar] [PubMed]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef]



| Type | Gene Description | Sequences (5′→3) |
|---|---|---|
| Human | Collagen type I alpha 1 chain (COL1A1) | F: ACATGTTCAGCTTTGTGGACC |
| R: TGTACGCAGGTGATTGGTGG | ||
| Collagen type I alpha 2 chain (COL1A2) | F: CGGACTTTGTTGCTGCTTGC | |
| R: CAGCAAAGTTCCCACCGAGA | ||
| Collagen type III alpha 1 chain (COL3A1) | F: TCGAGGCAGTGATGGTCAAC | |
| R: AGGTCCAACTTCACCCTTAGCA | ||
| Matrix metalloproteinase 1 (MMP1) | F: AGGGGAGATCATCGGGACAACTC | |
| R: GAGAGTCCAAGAGAATGGCCGA | ||
| Matrix metalloproteinase 3 (MMP3) | F: GCATTCAGTCCCTCTATGGACCTC | |
| R: GGGATTTGCGCCAAAAGTGC | ||
| Matrix metalloproteinase 9 (MMP9) | F: GACGCAGACATCGTCATCCA | |
| R: AAACCGAGTTGGAACCACGAC | ||
| Hyaluronic acid synthase 2 (HAS2) | F: GAGCAGCCCATTGAACCAGA | |
| R: AGGAAGCGCAGAATTGGGAG | ||
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F: GACAGTCAGCCGCATCTTCT | |
| R: GCGCCCAATACGACCAAATC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Choi, D.; Park, T. Decanal Protects against UVB-Induced Photoaging in Human Dermal Fibroblasts via the cAMP Pathway. Nutrients 2020, 12, 1214. https://doi.org/10.3390/nu12051214
Kang W, Choi D, Park T. Decanal Protects against UVB-Induced Photoaging in Human Dermal Fibroblasts via the cAMP Pathway. Nutrients. 2020; 12(5):1214. https://doi.org/10.3390/nu12051214
Chicago/Turabian StyleKang, Wesuk, Dabin Choi, and Taesun Park. 2020. "Decanal Protects against UVB-Induced Photoaging in Human Dermal Fibroblasts via the cAMP Pathway" Nutrients 12, no. 5: 1214. https://doi.org/10.3390/nu12051214






