Oils’ Impact on Comprehensive Fatty Acid Analysis and Their Metabolites in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Preparation of Serum
2.3. Fatty Acids Analysis in Serum
2.4. Estimation of Desaturases Activity
2.5. Lipoxygenase Metabolite Analysis
2.6. Statistical Analysis
3. Results
3.1. Fatty Acid Analysis in Serum
3.2. Estimation of Desaturase Activity
3.3. Lipoxygenase Metabolite Analysis
3.4. Growth of Rats Fed Various Oils
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kris-Etherton, P.; Fleming, J.; Harris, W.S. The debate about n-6 polyunsaturated fatty acid recommendations for cardiovascular health. J. Am. Diet. Assoc. 2010, 110, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Sinclair, A.J.; Zhao, F.; Li, D. Uncommon Fatty Acids and Cardiometabolic Health. Nutrients 2018, 10, 1559. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez-Hernández, E.; Nuñez-Valencia, C.; Villanueva, G.; Guevara, P.; De la Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: Randomised trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef]
- Raatz, S.K.; Conrad, Z.; Jahns, L.; Belury, M.A.; Picklo, M.J. Modeled replacement of traditional soybean and canola oil with high-oleic varieties increases monounsaturated fatty acid and reduces both saturated fatty acid and polyunsaturated fatty acid intake in the US adult population. Am. J. Clin. Nutr. 2018, 108, 594–602. [Google Scholar] [CrossRef]
- Ruiz-Nunez, B.; Dijck-Brouwer, D.A.; Muskiet, F.A. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Mursu, J.; Tuomainen, T.P.; Voutilainen, S. Dietary fatty acids and risk of coronary heart disease in men: The Kuopio Ischemic Heart Disease Risk Factor Study. Arter. Thromb. Vasc. Biol. 2014, 34, 2679–2687. [Google Scholar] [CrossRef]
- Te Morenga, L.; Montez, J.M. Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0186672. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Huth, P.J.; Fulgoni, V.L., 3rd; Larson, B.T. A systematic review of high-oleic vegetable oil substitutions for other fats and oils on cardiovascular disease risk factors: Implications for novel high-oleic soybean oils. Adv. Nutr. 2015, 6, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.; Young, J.; Lean, M.E.J.; Lara, J. Consumption of fish and vascular risk factors: A systematic review and meta-analysis of intervention studies. Atherosclerosis 2017, 266, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef]
- Shirooie, S.; Nabavi, S.F.; Dehpour, A.R.; Belwal, T.; Habtemariam, S.; Arguelles, S.; Sureda, A.; Daglia, M.; Tomczyk, M.; Sobarzo-Sanchez, E.; et al. Targeting mTORs by omega-3 fatty acids: A possible novel therapeutic strategy for neurodegeneration? Pharm. Res. 2018, 135, 37–48. [Google Scholar] [CrossRef]
- Aglago, E.K.; Huybrechts, I.; Murphy, N.; Casagrande, C.; Nicolas, G.; Pischon, T.; Fedirko, V.; Severi, G.; Boutron-Ruault, M.C.; Fournier, A.; et al. Consumption of Fish and Long-chain n-3 Polyunsaturated Fatty Acids Is Associated With Reduced Risk of Colorectal Cancer in a Large European Cohort. Clin. Gastroenterol. Hepatol. 2020, 18, 654–666. [Google Scholar] [CrossRef]
- Zamani, S.A.; McClain, K.M.; Graubard, B.I.; Liao, L.M.; Abnet, C.C.; Cook, M.B.; Petrick, J.L. Dietary polyunsaturated fat intake in relation to head and neck, esophageal, and gastric cancer incidence in the NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Zheng, J.S.; Hu, X.J.; Zhao, Y.M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013, 346, f3706. [Google Scholar] [CrossRef]
- Dydjow-Bendek, D.; Zagozdzon, P. Total Dietary Fats, Fatty Acids, and Omega-3/Omega-6 Ratio as Risk Factors of Breast Cancer in the Polish Population-a Case-Control Study. In Vivo 2020, 34, 423–431. [Google Scholar] [CrossRef]
- Massey, K.A.; Nicolaou, A. Lipidomics of oxidized polyunsaturated fatty acids. Free Radic. Biol. Med. 2013, 59, 45–55. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Salajegheh, A. Angiogenesis in Health, Diseases and Malignancy. Anticancer Res. 2016, 36, 3226. [Google Scholar]
- Tang, K.; Honn, K.V. 12(S)-HETE in Cancer Metastasis. In Lipoxygenases and Their Metabolites: Biological Functions; Nigam, S., Pace-Asciak, C.R., Eds.; Springer US: Boston, MA, USA, 1999; pp. 181–191. [Google Scholar] [CrossRef]
- Avis, I.; Hong, S.H.; Martinez, A.; Moody, T.; Choi, Y.H.; Trepel, J.; Das, R.; Jett, M.; Mulshine, J.L. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J. 2001, 15, 2007–2009. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Jiang, L.; Wang, Y.; Yao, B.; Yang, S.; Zhang, B.; Zhang, M.Z. 12/15 Lipoxygenase regulation of colorectal tumorigenesis is determined by the relative tumor levels of its metabolite 12-HETE and 13-HODE in animal models. Oncotarget 2015, 6, 2879–2888. [Google Scholar] [CrossRef]
- Ghosh, J.; Myers, C.E. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 13182–13187. [Google Scholar] [CrossRef]
- Yuan, H.; Li, M.-Y.; Ma, L.T.; Hsin, M.K.Y.; Mok, T.S.K.; Underwood, M.J.; Chen, G.G. 15-Lipoxygenases and its metabolites 15(S)-HETE and 13(S)-HODE in the development of non-small cell lung cancer. Thorax 2010, 65, 321. [Google Scholar] [CrossRef]
- Gonzalez-Nunez, D.; Claria, J.; Rivera, F.; Poch, E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension 2001, 37, 334–338. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, C.H.; Kuang, H.Y.; Jiang, X.Y.; Liu, H.L.; Hua, W.F.; Liu, Z.J.; Zhou, H.; Sui, H.; Qi, R. 12S-hydroxyeicosatetraenoic acid levels link to coronary artery disease in Type 2 diabetic patients. J. Endocrinol. Investig. 2013, 36, 385–389. [Google Scholar] [CrossRef]
- Kowal, K.; Zukowski, S.; Kowal-Bielecka, O.; DuBuske, L.M.; Bodzenta-Lukaszyk, A. Concentrations of 15-HETE and PGE2 in Induced Sputum of Allergic Asthma Patients. J. Allergy Clin. Immunol. 2006, 117, S200. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Molto-Puigmarti, C.; Castellote, A.I.; Lopez-Sabater, M.C. Determination of conjugated linoleic acid in human plasma by fast gas chromatography. J. Chromatogr. A 2007, 1157, 422–429. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Guerendiain, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fito, M.; Salas-Salvado, J.; Martinez-Gonzalez, M.A.; Aros, F.; Lamuela-Raventos, R.M.; et al. Plasma fatty acid composition, estimated desaturase activities, and their relation with the metabolic syndrome in a population at high risk of cardiovascular disease. Clin. Nutr. 2014, 33, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Stawarska, A.; Bialek, A.; Tokarz, A. Heating of vegetable oils influences the activity of enzymes participating in arachidonic acid formation in Wistar rats. Nutr. Res. 2015, 35, 930–938. [Google Scholar] [CrossRef]
- Stawarska, A.; Bialek, A.; Tokarz, A. The type of dietary fat and dietary energy restriction affects the activity of the desaturases in the liver microsomes. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Frohberg, P.; Drutkowski, G.; Wobst, I. Monitoring eicosanoid biosynthesis via lipoxygenase and cyclooxygenase pathways in human whole blood by single HPLC run. J. Pharm Biomed. Anal. 2006, 41, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Bialek, A.; Jelinska, M.; Tokarz, A. Influence of maternal diet enrichment with conjugated linoleic acids on lipoxygenase metabolites of polyunsaturated fatty acids in serum of their offspring with 7,12-dimethylbenz a anthracene induced mammary tumors. Prostaglandins Other Lipid Mediat. 2015, 116, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Comba, A.; Maestri, D.M.; Berra, M.A.; Garcia, C.P.; Das, U.N.; Eynard, A.R.; Pasqualini, M.E. Effect of ω-3 and ω-9 fatty acid rich oils on lipoxygenases and cyclooxygenases enzymes and on the growth of a mammary adenocarcinoma model. Lipids Health Dis. 2010, 9, 112. [Google Scholar] [CrossRef]
- Jelinska, M.; Bialek, A.; Mojska, H.; Gielecinska, I.; Tokarz, A. Effect of conjugated linoleic acid mixture supplemented daily after carcinogen application on linoleic and arachidonic acid metabolites in rat serum and induced tumours. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1842, 2230–2236. [Google Scholar] [CrossRef]
- Oliveira-de-Lira, L.; Santos, E.M.C.; de Souza, R.F.; Matos, R.J.B.; Silva, M.C.D.; Oliveira, L.D.S.; Nascimento, T.G.D.; Schemly, P.; Souza, S.L. Supplementation-Dependent Effects of Vegetable Oils with Varying Fatty Acid Compositions on Anthropometric and Biochemical Parameters in Obese Women. Nutrients 2018, 10, 932. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Lottenberg, A.M.; Lavrador, M.S.F.; Afonso, M.S.; Machado, R.M. Chapter 24—Influence of Diet on Endothelial Dysfunction. In Endothelium and Cardiovascular Diseases; Da Luz, P.L., Libby, P., Chagas, A.C.P., Laurindo, F.R.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 341–362. [Google Scholar] [CrossRef]
- Lin, J.; Yang, R.; Tarr, P.T.; Wu, P.-H.; Handschin, C.; Li, S.; Yang, W.; Pei, L.; Uldry, M.; Tontonoz, P.; et al. Hyperlipidemic Effects of Dietary Saturated Fats Mediated through PGC-1β Coactivation of SREBP. Cell 2005, 120, 261–273. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; Matthan, N.R.; Lamon-Fava, S.; Lecker, J.L.; Lichtenstein, A.H. Reduction in dietary omega-6 polyunsaturated fatty acids: Eicosapentaenoic acid plus docosahexaenoic acid ratio minimizes atherosclerotic lesion formation and inflammatory response in the LDL receptor null mouse. Atherosclerosis 2009, 204, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Lee, H.J.; Park, H.Y.; Lee, S.H.; Jang, C.G.; Lee, S.Y. Effects of heme oxygenase system on the cyclooxygenase in the primary cultured hypothalamic cells. Arch. Pharm. Res. 2001, 24, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Pal, Y.P.; Pratap, A.P. Rice Bran Oil: A Versatile Source for Edible and Industrial Applications. J. Oleo Sci. 2017, 66, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Escrich, E.; Moral, R.; Solanas, M. Olive oil, an essential component of the Mediterranean diet, and breast cancer. Public Health Nutr. 2011, 14, 2323–2332. [Google Scholar] [CrossRef]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef]
- Berbert, A.A.; Kondo, C.R.M.; Almendra, C.L.; Matsuo, T.; Dichi, I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition 2005, 21, 131–136. [Google Scholar] [CrossRef]
- Goncalves-de-Albuquerque, C.F.; Medeiros-de-Moraes, I.M.; Oliveira, F.M.; Burth, P.; Bozza, P.T.; Castro Faria, M.V.; Silva, A.R.; Castro-Faria-Neto, H.C. Omega-9 Oleic Acid Induces Fatty Acid Oxidation and Decreases Organ Dysfunction and Mortality in Experimental Sepsis. PLoS ONE 2016, 11, e0153607. [Google Scholar] [CrossRef]
- Medeiros-de-Moraes, I.M.; Goncalves-de-Albuquerque, C.F.; Kurz, A.R.M.; Oliveira, F.M.J.; de Abreu, V.H.P.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 Oleic Acid, the Main Compound of Olive Oil, Mitigates Inflammation during Experimental Sepsis. Oxid. Med. Cell. Longev. 2018, 2018, 6053492. [Google Scholar] [CrossRef]
- Ayerza, R.; Coates, W. Ground chia seed and chia oil effects on plasma lipids and fatty acids in the rat. Nutr. Res. 2005, 25, 995–1003. [Google Scholar] [CrossRef]
- Engler, M.M.; Engler, M.B.; Kroetz, D.L.; Boswell, K.D.B.; Neeley, E.; Krassner, S.M. The effects of a diet rich in docosahexaenoic acid on organ and vascular fatty acid composition in spontaneously hypertensive rats. Prostaglandins Leukot. Essent. Fat. Acids 1999, 61, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Smink, W.; Gerrits, W.J.; Gloaguen, M.; Ruiter, A.; van Baal, J. Linoleic and alpha-linolenic acid as precursor and inhibitor for the synthesis of long-chain polyunsaturated fatty acids in liver and brain of growing pigs. Animal 2012, 6, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Emken, E.A.; Adlof, R.O.; Gulley, R.M. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta 1994, 1213, 277–288. [Google Scholar] [CrossRef]
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Gordon Bell, J.; Raynard, R.S.; Sargent, J.R. The effect of dietary linoleic acid on the fatty acid composition of individual phospholipid and lipoxygenase products from gills and leucocytes of Atlantic salmon (Salmo salar). Lipids 1991, 26, 445–450. [Google Scholar] [CrossRef]
- Miller, C.C.; Ziboh, V.A.; Wong, T.; Fletcher, M.P. Dietary supplementation with oils rich in (n-3) and (n-6) fatty acids influences in vivo levels of epidermal lipoxygenase products in guinea pigs. J. Nutr. 1990, 120, 36–44. [Google Scholar] [CrossRef]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef]
- Gulliksson, M.; Brunnstrom, A.; Johannesson, M.; Backman, L.; Nilsson, G.; Harvima, I.; Dahlen, B.; Kumlin, M.; Claesson, H.E. Expression of 15-lipoxygenase type-1 in human mast cells. Biochim. Biophys. Acta 2007, 1771, 1156–1165. [Google Scholar] [CrossRef]
- Ferdouse, A.; Leng, S.; Winter, T.; Aukema, H.M. Dietary n-6 and n-3 PUFA alter the free oxylipin profile differently in male and female rat hearts. Br. J. Nutr. 2019, 122, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Schmidt, S.; Kressel, G.; Dong, H.; Willenberg, I.; Hammock, B.D.; Hahn, A.; Schebb, N.H. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 19–29. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Alegakis, A.K.; Fragkiadaki, P.; Papakonstantinou, E.; Tsilimidos, G.; Geraci, F.; Sarandi, E.; Nikitovic, D.; Spandidos, D.A.; Tsatsakis, A. Application of metabolomics part II: Focus on fatty acids and their metabolites in healthy adults. Int. J. Mol. Med. 2019, 43, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef]
- Sakamoto, A.; Saotome, M.; Iguchi, K.; Maekawa, Y. Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Heart Failure: Current Understanding for Basic to Clinical Relevance. Int. J. Mol. Sci. 2019, 20, 4025. [Google Scholar] [CrossRef]
- Pomponi, M.; Loria, G.; Salvati, S.; Di Biase, A.; Conte, G.; Villella, C.; Righino, E.; Ciciarelli, C.; Bria, P.; La Torre, G.; et al. DHA effects in Parkinson disease depression. Basal Ganglia 2014, 4, 61–66. [Google Scholar] [CrossRef]
- Canhada, S.; Castro, K.; Perry, I.S.; Luft, V.C. Omega-3 fatty acids’ supplementation in Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2018, 21, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Launer, L.J.; Ott, A.; Witteman, J.C.; Hofman, A.; Breteler, M.M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997, 42, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Zandi, P.P.; Tucker, K.L.; Fitzpatrick, A.L.; Kuller, L.H.; Fried, L.P.; Burke, G.L.; Carlson, M.C. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology 2005, 65, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Tirinato, L.; Pagliari, F.; Limongi, T.; Marini, M.; Falqui, A.; Seco, J.; Candeloro, P.; Liberale, C.; Di Fabrizio, E. An Overview of Lipid Droplets in Cancer and Cancer Stem Cells. Stem Cells Int. 2017, 2017, 1656053. [Google Scholar] [CrossRef]
- Bozza, P.T.; Viola, J.P. Lipid droplets in inflammation and cancer. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 243–250. [Google Scholar] [CrossRef] [PubMed]




| Protein (g) | 210.0 | ||
| Fat (g) | 39.2 | ||
| Fiber (g) | 43.2 | ||
| Starch (g) | 300.0 | ||
| Ash (g) | 55.0 | ||
| Vitamin A (IU) | 15,000 | Vitamin B6 (mg) | 17.0 |
| Lysine (g) | 14.5 | Histidine (g) | 6.0 |
| Vitamin D3 (IU) | 1000 | Vitamin B12 (µg) | 80.0 |
| Methionine (g) | 4.1 | Arginine (g) | 13.0 |
| Vitamin E (mg) | 90.0 | Pantothenate (mg) | 30.0 |
| Tryptophan (g) | 3.0 | Phenylalanine (g) | 10.0 |
| Vitamin K3 (mg) | 3.0 | Folic acid (mg) | 5.0 |
| Threonine (g) | 7.4 | Tyrosine (g) | 7.8 |
| Vitamin B1 (mg) | 21.0 | Nicotinic acid (mg) | 133.0 |
| Isoleucine (g) | 17.5 | Choline (mg) | 2750.0 |
| Vitamin B2 (mg) | 16.0 | Biotin (mg) | 0.4 |
| Valine (g) | 11.0 | ||
| Calcium (g) | 10.0 | Potassium (g) | 9.4 |
| Iron (mg) | 250.0 | Cobalt (mg) | 2.0 |
| Phosphorus total (g) | 8.17 | Sodium (g) | 2.2 |
| Manganese (mg) | 100.0 | Iodine (mg) | 1.0 |
| Phosphorus saturated (g) | 4.5 | Chlorine (g) | 2.5 |
| Zinc (mg) | 76.9 | Selenium (mg) | 0.5 |
| Magnesium (g) | 3.0 | Sulfur (g) | 1.9 |
| Copper (mg) | 21.3 | ||
| Fatty Acid | Olive Oil | Linseed Oil | Sunflower Oil | Sesame Oil | Carotino Oil | Rice Oil |
|---|---|---|---|---|---|---|
| Myristic acid (C14:0) | 0.02 | nd | 0.06 | 0.02 | 0.35 | 0.37 |
| Palmitic acid (C16:0) | 10.92 | 5.44 | 5.97 | 10.00 | 13.59 | 19.88 |
| Stearic acid (C18:0) | 2.58 | 2.86 | 4.91 | 5.21 | 2.50 | 2.07 |
| Oleic acid (C18:1 n-9) | 74.46 | 11.29 | 25.40 | 36.27 | 52.94 | 40.90 |
| Linoleic acid (C18:2 n-6) | 5.94 | 15.47 | 59.91 | 44.21 | 16.85 | 29.75 |
| α-Linoleic acid (C18:3 n-3) | 0.61 | 63.98 | 0.11 | 0,35 | 0.57 | 0.11 |
| Eicosanoic acid (C20:0) | 0.45 | 0.15 | 0.28 | 0.54 | 0.49 | 0.81 |
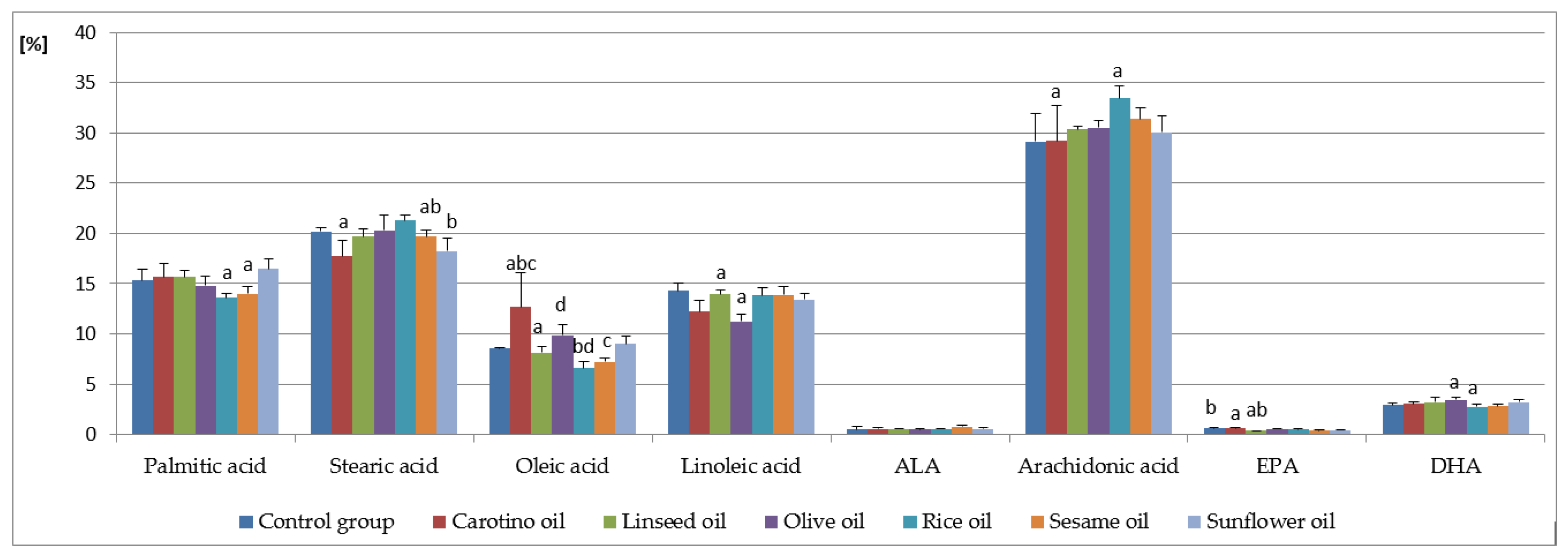
| Fatty Acids (%) | C | CAR | L | O | R | SES | SUN |
|---|---|---|---|---|---|---|---|
| SFA | |||||||
| Lauric acid (C12:0) | 0.07 ± 0.03 | 0.05 ± 0.01 | 0.10 ± 0.05 | 0.06 ± 0.03 | 0.02 ± 0.00 | 0.06 ± 0.00 | 0.11 ± 0.10 |
| Myristic acid (C14:0) | 0.37 ± 0.03 | 0.39 ± 0.04 | 0.40 ± 0.02 | 0.44 ± 0.16 | 0.40 ± 0.06 | 0.41 ± 0.00 | 0.45 ± 0.09 |
| Pentadecanoic acid (C15:0) | 0.31 ± 0.04 | 0.34 ± 0.02 | 0.32 ± 0.03 | 0.29 ± 0.02 | 0.34 ± 0.05 | 0.35 ± 0.02 | 0.36 ± 0.03 |
| Palmitic acid (C16:0) | 15.31 ± 1.10 | 15.66 ± 1.31 | 15.65 ± 0.69 | 14.78 ± 0.94 | 13.61 ± 0.40 a | 13.98 ± 0.69 | 16.47 ± 0.98 a |
| Heptadecanoic acid (C17:0) | 0.63 ± 0.03 a | 0.45 ± 0.05 ab | 0.56 ± 0.03 | 0.55 ± 0.06 | 0.57 ± 0.03 b | 0.50 ± 0.06 | 0.55 ± 0.07 |
| Stearic acid (C18:0) | 20.20 ± 0.32 | 17.72 ± 1.60 a | 19.70 ± 0.77 | 20.28 ± 1.54 | 21.27 ± 0.60 ab | 19.65 ± 0.72 | 18.25 ± 1.25 b |
| Arachidic acid (C20:0) | 0.05 ± 0.00 | 0.05 ± 0.02 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| Lignoceric acid (C24:0) | 0.13 ± 0.01 a | 0.15 ± 0.02 | 0.16 ± 0.02 | 0.15 ± 0.02 | 0.14 ± 0.02 | 0.14 ± 0.01 | 0.18 ± 0.02 a |
| Σ SFA | 37.06 ± 1.18 | 34.85 ± 0.97 a | 36.93 ± 0.78 a | 36.60 ± 0.57 | 36.46 ± 0.85 | 35.52 ± 1.01 | 36.50 ± 0.89 |
| MUFA | |||||||
| Myristoleic acid (C14:1) | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.00 |
| Palmitoleic acid (C16:1) | 1.03 ± 0.01 | 1.53 ± 0.75 a | 1.04 ± 0.11 | 1.12 ± 0.15 | 0.78 ± 0.09 ab | 1.55 ± 0.36 b | 1.18 ± 0.27 |
| Heptadecenoic acid (C17:1) | 0.10 ± 0.02 | 0.13 ± 0.03 | 1.12 ± 0.05 | 0.11 ± 0.01 | 0.08 ± 0.01 a | 0.10 ± 0.02 | 0.15 ± 0.02 a |
| Oleic acid (C18:1 n-9) | 8.56 ± 0.07 | 12.70 ± 3.35 abc | 8.15 ± 0.55 a | 9.89 ± 1.03 d | 6.60 ± 0.60 bd | 7.21 ± 0.37 c | 9.00 ± 0.72 |
| Eicosenoic acid (C20:1) | 0.11 ± 0.02 | 0.12 ± 0.05 | 0.08 ± 0.03 | 0.13 ± 0.06 | 0.09 ± 0.01 | 0.12 ± 0.03 | 0.09 ± 0.02 |
| Nervonic acid (C24:1 n-9) | 0.41 ± 0.00 a | 0.33 ± 0.03 | 0.27 ± 0.03 ab | 0.31 ± 0.03 | 0.32 ± 0.03 | 0.30 ± 0.05 | 0.35 ± 0.03 b |
| Σ MUFA | 11.03 ± 1.37 | 13.42 ± 5.67 a | 9.67 ± 0.64 | 11.56 ± 1.18 | 7.92 ± 0.67 a | 9.42 ± 0.74 | 10.93 ± 0.98 |
| PUFA | |||||||
| Linolelaidic acid (C18:2 n-6 trans) | 1.59 ± 0.22 | 1.67 ± 0.33 | 1.44 ± 0.07 | 1.51 ± 0.10 | 1.24 ± 0.36 | 1.55 ± 0.42 | 1.49 ± 0.14 |
| Linoleic acid (C18:2 n-6) | 14.30 ± 0.81 | 12.22 ± 1.09 | 13.94 ± 0.44 a | 11.26 ± 0.70 a | 13.80 ± 0.85 | 13.86 ± 0.83 | 13.40 ± 0.57 |
| γ-Linolenic acid (C18:3 n-6) | 0.38 ± 0.08 b | 0.43 ± 0.04 a | 0.55 ± 0.03 ab | 0.44 ± 0.03 | 0.47 ± 0.07 | 0.49 ± 0.07 | 0.48 ± 0.03 |
| α-Linolenic acid (C18:3 n-3) | 0.51 ± 0.37 | 0.57 ± 0.13 | 0.55 ± 0.09 | 0.49 ± 0.13 | 0.54 ± 0.07 | 0.77 ± 0.21 | 0.56 ± 0.10 |
| Dihomo-γ-linolenic acid (DGLA, C20:3 n-6) | 0.35 ± 0.04 | 0.36 ± 0.05 | 0.31 ± 0.07 | 0.30 ± 0.00 | 0.34 ± 0.05 | 0.35 ± 0.12 | 0.38 ± 0.06 |
| Arachidonic acid (C20:4 n-6) | 29.12 ± 2.79 | 29.21 ± 3.51 a | 30.32 ± 0.37 | 30.50 ± 0.75 | 33.42 ± 1.25 a | 31.40 ± 1.06 | 30.05 ± 1.62 |
| Eicosapentaenoic acid (EPA, C20:5 n-3) | 0.64 ± 0.03 b | 0.63 ± 0.09 a | 0.37 ± 0.04 ab | 0.55 ± 0.09 | 0.49 ± 0.06 | 0.39 ± 0.09 | 0.48 ± 0.05 |
| Docosahexaenoic acid (DHA, C22:6 n-3) | 2.93 ± 0.18 | 3.03 ± 0.24 | 3.20 ± 0.48 | 3.44 ± 0.26 a | 2.74 ± 0.29 a | 2.82 ± 0.22 | 3.21 ± 0.27 |
| Σ PUFA | 49.81 ± 1.69 | 49.41 ± 1.27 a | 50.77 ± 1.36 | 48.23 ± 1.45 b | 53.27 ± 1.11 ab | 51.45 ± 2.56 | 50.06 ± 1.26 |
| n-3 | 4.08 ± 0.25 | 4.38 ± 0.09 | 3.84 ± 0.80 | 4.47 ± 0.30 | 3.77 ± 0.34 | 3.98 ± 0.24 | 4.26 ± 0.23 |
| n-6 | 45.73 ± 1.88 | 45.07 ± 1.29 a | 46.94 ± 0.88 | 43.76 ± 1.29 b | 49.82 ± 0.53 ab | 47.47 ± 2.32 | 45.80 ± 1.13 |
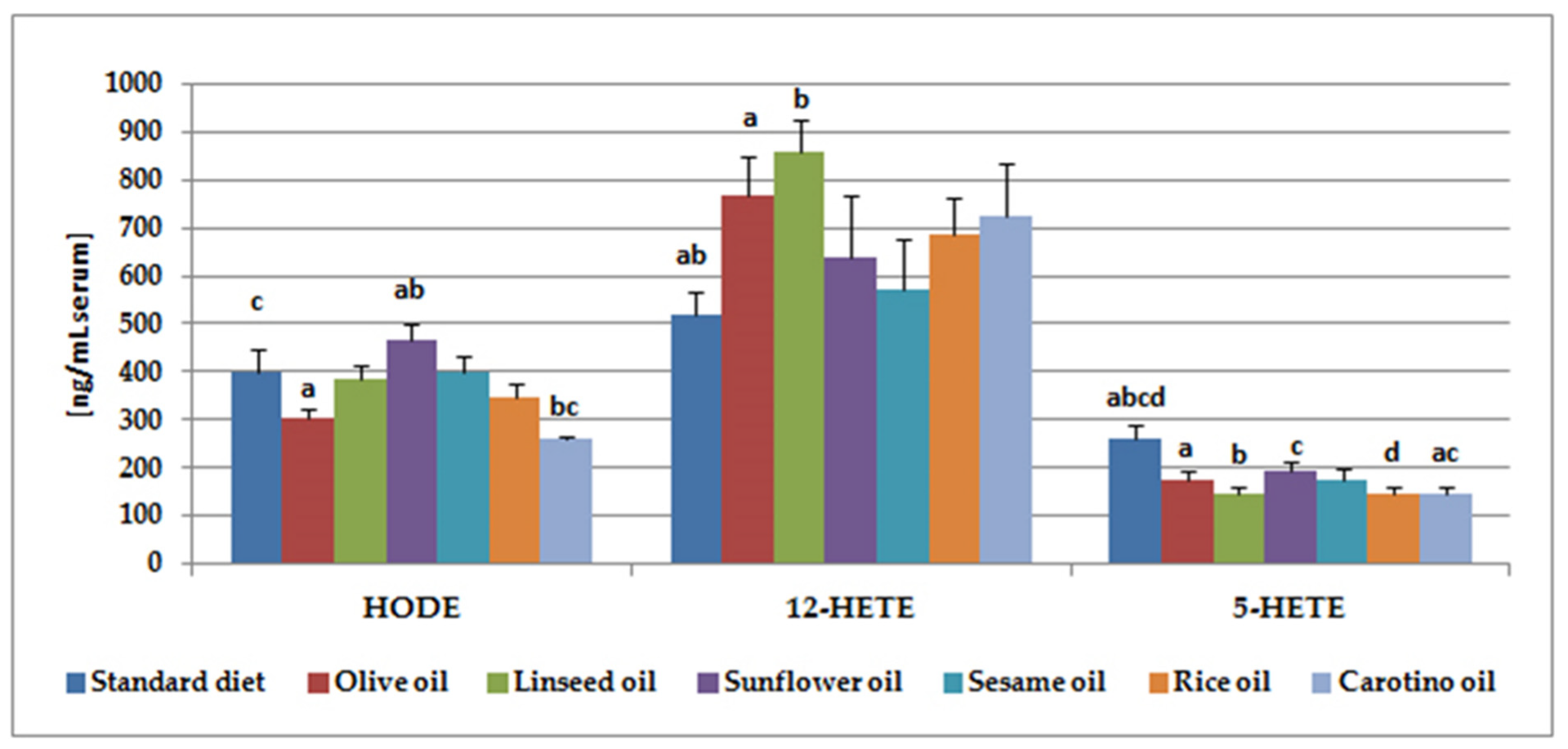
| Hydroxyfatty Acid | Standard Diet | Olive Oil | Linseed Oil | Sunflower Oil | Sesame Oil | Rice Oil | Carotino Oil |
|---|---|---|---|---|---|---|---|
| 12-HEPE | 35.5 ± 4.8 ab | 37.5 ± 4.7 | 30.4 ± 3.2 | 22.1 ± 3.8 a | 22.9 ± 3.4 b | 26.4 ± 2.4 | 34.7 ± 5.9 |
| HODE | 400.9 ± 46.0 c | 302.9 ± 21.6 a | 383.3 ± 31.5 | 467.8 ± 31.3 ab | 398.3 ± 36.4 | 348.5 ± 29.2 | 259.9 ± 6.9 bc |
| 15-HETE | 32.0 ± 6.1 | 26.3 ± 3.8 | 28.6 ± 2.1 | 39.+6 ± 4.0 a | 46.8 ± 8.0 b | 31.0 ± 3.5 | 19.1 ± 2.6 ab |
| 12-HETE | 516.7 ± 51.2 ab | 765.2 ± 85.4 a | 857.3 ± 66.2 b | 638.0 ± 127.7 | 571.6 ± 104.2 | 683.9 ± 77.3 | 723.0 ± 110.7 |
| 5-HETE | 262.4 ± 28.6 abcd | 175.8 ± 17.5 a | 147.2 ± 13.2 b | 195.2 ± 17.1c | 176.3 ± 19.8 | 145.0 ± 13.0d | 145.0 ± 13.0 ac |
| Correlation | Spearman’s Correlation Coefficient (r) | p-Value |
|---|---|---|
| Linseed oil | ||
| 12-HEPE vs. Linoleic acid | −0.980 | 0.0033 |
| 12-HEPE vs. Arachidonic acid | −0.850 | 0.0684 *) |
| 12-HEPE vs. EPA | 0.882 | 0.0480 |
| 15-HETE vs. Linoleic acid | −0.881 | 0.0481 |
| 5-HETE vs. Linoleic acid | −0.930 | 0.0221 |
| Sunflower oil | ||
| 15-HETE vs. Arachidonic acid | 0.817 | 0.0249 |
| 12-HETE vs. Arachidonic acid | 0.748 | 0.0532*) |
| 5-HETE vs. Arachidonic acid | 0.905 | 0.0051 |
| Rice oil | ||
| HODE vs. Linoleic acid | −0.966 | 0.0074 |
| HODE vs. Arachidonic acid | −0.945 | 0.0151 |
| 15-HETE vs. Linoleic acid | −0.993 | 0.0006 |
| 15-HETE vs. Arachidonic acid | −0.888 | 0.0439 |
| Control Group | Carotino Oil | Linseed Oil | Olive Oil | Rice Oil | Sesame Oil | Sunflower Oil | |
|---|---|---|---|---|---|---|---|
| Mass–start (g) | 108.2 ± 11.0 ab | 97.0 ± 10.8 | 101.9 ± 16.9 | 95.9 ± 20.0 | 80.1 ± 12.9 a | 81.7 ± 10.7 b | 93.2 ± 7.5 |
| Mass–end (g) | 193.0 ± 14.8 | 180.9 ± 10.1 | 181.2 ± 19.5 | 172.1 ± 16.1 | 175.2 ± 13.1 | 180.3 ± 17.5 | 182.1 ± 20.4 |
| Mass–increase (g) | 84.8 ± 9.5 | 83.9 ± 10.8 | 79.3 ± 9.6 ab | 76.2 ± 8.9 cd | 95.1 ± 7.1 ac | 98.7 ± 8.9 bd | 85.5 ± 16.2 |
| Liver (g) | 5.8 ± 0.7 | 5.6 ± 0.5 | 5.5 ± 0.6 | 5.6 ± 0.3 | 5.8 ± 0.4 | 5.3 ± 0.5 | 5.8 ± 0.8 |
| Liver (%) | 3.0 ± 0.2 | 3.1 ± 0.1 | 3.0 ± 0.1 | 3.2 ± 0.3 | 3.3 ± 0.2 | 2.9 ± 0.3 | 3.2 ± 0.2 |
| Spleen (g) | 0.5 ± 0.1 abcd | 0.5 ± 0.0 efhi | 0.4 ± 0.1 ae | 0.4 ± 0.0 bfg | 0.4 ± 0.0 ci | 0.5 ± 0.0 g | 0.4 ± 0.0 dh |
| Spleen (%) | 0.3 ± 0.0 | 0.3 ± 0.0 ab | 0.2 ± 0.0 a | 0.2 ± 0.0 b | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 |
| Kidneys (g) | 1.7 ± 0.1 a | 1.6 ± 0.1 | 1.6 ± 0.2 | 1.4 ± 0.2 a | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 |
| Kidneys (%) | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.1 | 0.8 ± 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawarska, A.; Jelińska, M.; Czaja, J.; Pacześniak, E.; Bobrowska-Korczak, B. Oils’ Impact on Comprehensive Fatty Acid Analysis and Their Metabolites in Rats. Nutrients 2020, 12, 1232. https://doi.org/10.3390/nu12051232
Stawarska A, Jelińska M, Czaja J, Pacześniak E, Bobrowska-Korczak B. Oils’ Impact on Comprehensive Fatty Acid Analysis and Their Metabolites in Rats. Nutrients. 2020; 12(5):1232. https://doi.org/10.3390/nu12051232
Chicago/Turabian StyleStawarska, Agnieszka, Małgorzata Jelińska, Julia Czaja, Ewelina Pacześniak, and Barbara Bobrowska-Korczak. 2020. "Oils’ Impact on Comprehensive Fatty Acid Analysis and Their Metabolites in Rats" Nutrients 12, no. 5: 1232. https://doi.org/10.3390/nu12051232
APA StyleStawarska, A., Jelińska, M., Czaja, J., Pacześniak, E., & Bobrowska-Korczak, B. (2020). Oils’ Impact on Comprehensive Fatty Acid Analysis and Their Metabolites in Rats. Nutrients, 12(5), 1232. https://doi.org/10.3390/nu12051232






