Pyropia yezoensis Extract Suppresses IFN-Gamma- and TNF-Alpha-Induced Proinflammatory Chemokine Production in HaCaT Cells via the Down-Regulation of NF-κB
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Pyropia Yezoensis Extracts (PYE), Astaxanthin (AS), and Xanthophyll (X)
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. RNA Extraction and cDNA Synthesis
2.5. Real-Time qPCR
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) for MDC and TARC
2.7. Preparation of Whole Cell Lysates and Nuclear Extracts
2.8. Western Blot
2.9. High Performance Liquid Chromatography (HPLC) Analysis
2.10. Statistical Analysis
3. Results
3.1. Effect of P. Yezoensis Extract (PYE) on the Viabillity of HaCaT Cells
3.2. PYE Suppressed IFN-γ and TNF-α induced mRNA Expression Level of TARC and MDC in HaCaT Cells
3.3. PYE Decreased the IFN-γ- and TNF-α-induced Production of TARC and MDC in HaCaT Cells
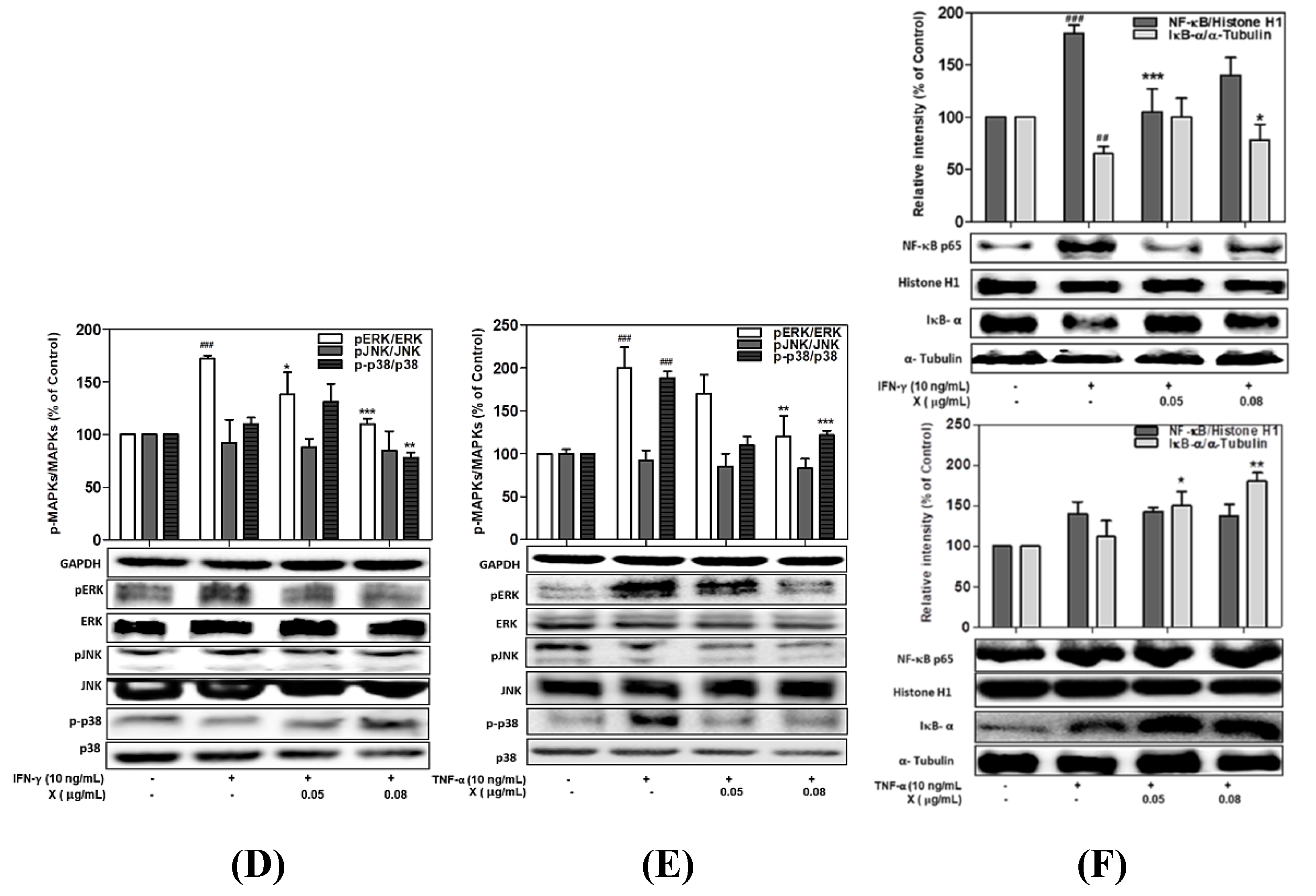
3.4. Effect of PYE on the Phosphorylation of MAPKs in IFN-γ- and TNF-α-induced HaCaT Cells
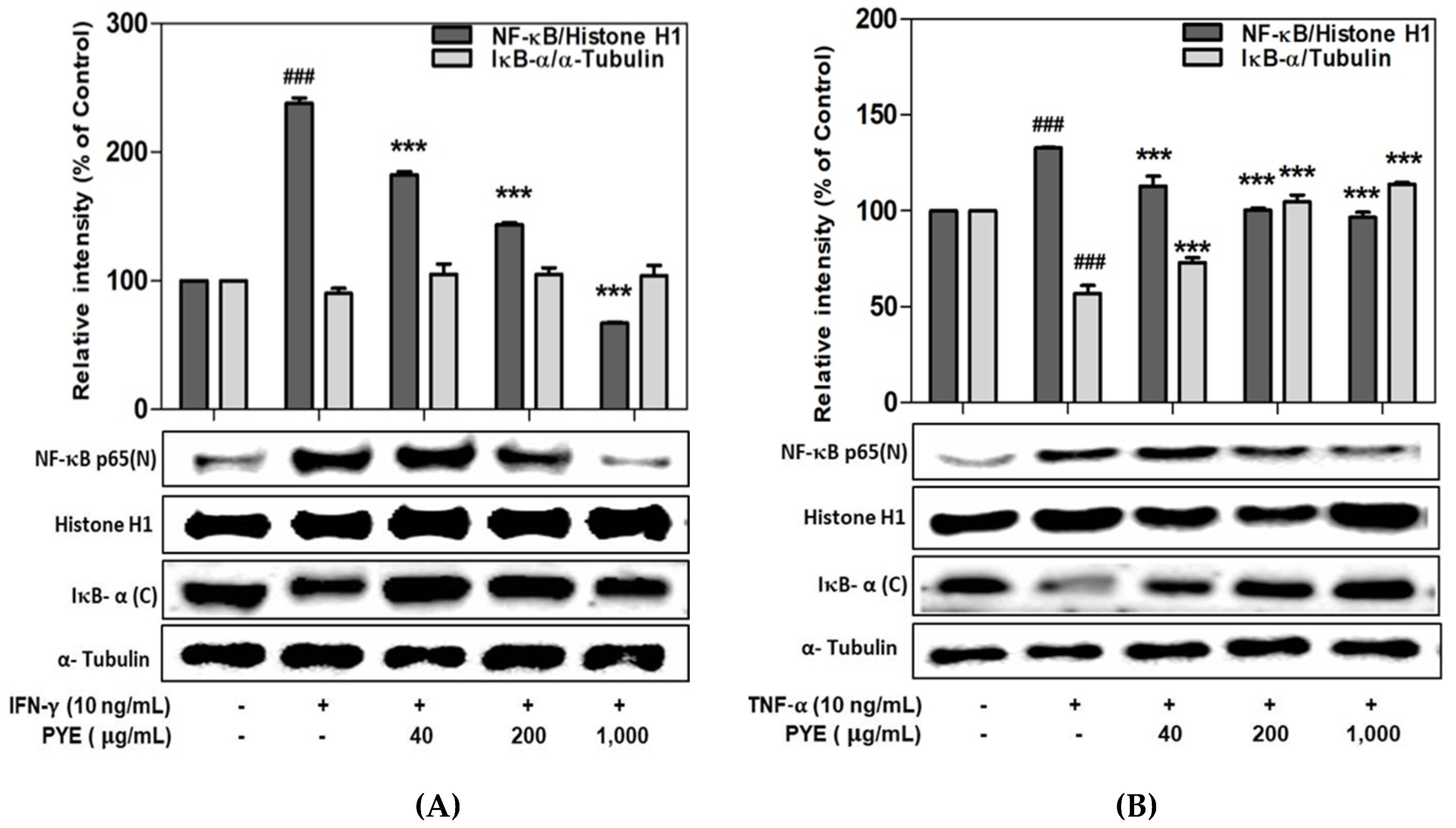
3.5. Effect of PYE on NF-κB Translocation in IFN-γ- and TNF-α-induced HaCaT Cells
3.6. Analysis of Candidate Carotenoid Substances in PYE by HPLC
3.7. Effect of Astaxanthin and Xanthophyll on the NF-kB/IkB-α and MAPK Signaling Pathway in IFN-γ- or TNF-α-Treated HaCaT Cells
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Leung, D.Y.M. Atopic dermatitis: New insights and opportunities for therapeutic intervention. J. Allergy Clin. Immunol. 2000, 105, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Liu, J.; Wang, J.; Luo, Q.; Zhang, H.; Liu, B.; Xu, F.; Pang, Q.; Liu, Y.; Dong, J. Icariin inhibits TNF-α/IFN-γ induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int. Immunopharmacol. 2015, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.B.; Chamian, F.; Masud, S.; Cardinale, I.; Abello, M.V.; Lowes, M.A.; Chen, F.; Magliocco, M.; Krueger, J.G. TNF Inhibition Rapidly Down-Regulates Multiple Proinflammatory Pathways in Psoriasis Plaques. J. Immunol. 2005, 175, 2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijs, J.; Krastev, T.; Weidinger, S.; Buckens, C.F.; de Bruin-Weller, M.; Bruijnzeel-Koomen, C.; Flohr, C.; Hijnen, D. Biomarkers for atopic dermatitis: A systematic review and meta-analysis. Curr. Opin. Allergy CL 2015, 15, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.; Kagami, S.; Saeki, H.; Sugaya, M.; Kakinuma, T.; Fujita, H.; Yano, S.; Mitsui, H.; Torii, H.; Komine, M.; et al. Both IL-4 and IL-13 inhibit the TNF-α and IFN-γ enhanced MDC production in a human keratinocyte cell line, HaCaT cells. J. Dermatol. Sci. 2003, 31, 111–117. [Google Scholar] [CrossRef]
- Giustizieri, M.L.; Mascia, F.; Frezzolini, A.; De Pità, O.; Chinni, L.M.; Giannetti, A.; Girolomoni, G.; Pastore, S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell–derived cytokines. J. Allergy Clin. Immunol. 2001, 107, 871–877. [Google Scholar] [CrossRef]
- Jeong, S.-I.; Choi, B.-M.; Jang, S.I. Sulforaphane suppresses TARC/CCL17 and MDC/CCL22 expression through heme oxygenase-1 and NF-κB in human keratinocytes. Arch. Pharmacal Res. 2010, 33, 1867–1876. [Google Scholar] [CrossRef]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J.H. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.L.; Lapadat, R. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and p38 Protein Kinases. Science 2002, 298, 1911. [Google Scholar] [CrossRef] [Green Version]
- Niwa, K. Genetic analysis of artificial green and red mutants of Porphyra yezoensis Ueda (Bangiales, Rhodophyta). Aquaculture 2010, 308, 6–12. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Biochem. 2011, 151, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, E.-S.; Hwang, H.-J.; Kim, I.-H.; Nam, T.-J. A glycoprotein from Porphyra yezoensis produces anti-inflammatory effects in liposaccharide-stimulated macrophages via the TLR4 signaling pathway. Int. J. Mol. Med. 2011, 28, 809–815. [Google Scholar] [PubMed] [Green Version]
- Toyosaki, T.; Iwabuchi, M. New antioxidant protein in seaweed (Porphyra yezoensis Ueda). Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 2), 46–56. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, Y.M.; Park, S.J.; Kim, I.H.; Nam, T.J. Protective effect of Porphyra yezoensis glycoprotein on D-galactosamine-induced cytotoxicity in Hepa 1c1c7 cells. Mol. Med. Rep. 2015, 11, 3914–3919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef]
- Eitsuka, T.; Nakagawa, K.; Igarashi, M.; Miyazawa, T. Telomerase inhibition by sulfoquinovosyldiacylglycerol from edible purple laver (Porphyra yezoensis). Cancer Lett. 2004, 212, 15–20. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kwon, M.J.; Kim, I.H.; Nam, T.J. Chemoprotective effects of a protein from the red algae Porphyra yezoensis on acetaminophen-induced liver injury in rats. Phytother. Res. 2008, 22, 1149–1153. [Google Scholar] [CrossRef]
- Guo, T.T.; Xu, H.L.; Zhang, L.X.; Zhang, J.P.; Guo, Y.F.; Gu, J.W.; He, P.M. In vivo protective effect of Porphyra yezoensis polysaccharide against carbon tetrachloride induced hepatotoxicity in mice. Regul Toxicol. Pharm. 2007, 49, 101–106. [Google Scholar] [CrossRef]
- Vo, T.-S.; Ryu, B.; Kim, S.-K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Foods 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Qi, X.F.; Kim, D.H.; Yoon, Y.S.; Li, J.H.; Song, S.B.; Jin, D.; Huang, X.Z.; Teng, Y.C.; Lee, K.J. The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/CCL22 production through p38 MAPK and NF-kappaB in HaCaT keratinocytes. Mol. Immunol. 2009, 46, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Al saryi, N.; Hetta, H.F.; Abuderman, A.A. Marine Brevibacterium aureum Extract and Its Constituent’s Saphenic Acid a Derivative of 1-phenazinecarboxylic Acid (Tubermycin B), Initiate Apoptosis via Inhibition of NF-κB and MAPK Expression. Toxicol. Environ. Health Sci. 2018, 10, 321–329. [Google Scholar] [CrossRef]
- Kwon, M.J.; Nam, T.J. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006, 79, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhou, Y.; Ma, J.X. Hypolipidemic effect of the polysaccharides from Porphyra yezoensis. Int. J. Biol. Macromol. 2014, 68, 48–49. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.J.; Bae, Y.S.; Ju, S.M.; Goh, A.R.; Youn, G.S.; Choi, S.Y.; Park, J. Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-κB and STAT1 activation in HaCaT cells. Biochem. Biophys. Res. Commun. 2012, 417, 1254–1259. [Google Scholar] [CrossRef]
- Qi, X.F.; Kim, D.H.; Yoon, Y.S.; Song, S.B.; Teng, Y.C.; Cai, D.Q.; Lee, K.J. Bambusae caulis in Liquamen Suppresses the Expression of Thymus and Activation-Regulated Chemokine and Macrophage-Derived Chemokine in Human Keratinocytes due to Antioxidant Effect. Evid. Based Complement. Altern. Med. 2012, 2012, 617494. [Google Scholar] [CrossRef] [Green Version]
- Saeki, H.; Tamaki, K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J. Derm. Sci. 2006, 43, 75–84. [Google Scholar] [CrossRef]
- Vestergaard, C.; Yoneyama, H.; Murai, M.; Nakamura, K.; Tamaki, K.; Terashima, Y.; Imai, T.; Yoshie, O.; Irimura, T.; Mizutani, H.; et al. Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J. Clin. Investig. 1999, 104, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Nakamura, K.; Oyama, N.; Kaneko, F.; Tsunemi, Y.; Saeki, H.; Tamaki, K. Macrophage-derived chemokine (MDC)/CCL22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J. Derm. Sci. 2006, 44, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, Y.S.; Kim, H.K. Illicium verum extract inhibits TNF-α- and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J. Ethnopharmacol. 2012, 144, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Lee, B.; Ma, J.Y. Anti-Inflammatory Effects of Melandrii Herba Ethanol Extract via Inhibition of NF-κB and MAPK Signaling Pathways and Induction of HO-1 in RAW 264.7 Cells and Mouse Primary Macrophages. Molecules 2016, 21, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, G.; Shen, Z.; Chen, H.; Liu, J.; Jiang, K.; Fan, L.; Jia, L.; Shao, J. Metapristone suppresses non-small cell lung cancer proliferation and metastasis via modulating RAS/RAF/MEK/MAPK signaling pathway. Biomed. Pharmacother. 2017, 90, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Sun, Y.; Li, X.; Wang, J.; Yan, L.; Zhang, Z.; Wang, D.; Dai, J.; He, J.; Wang, S. Scutellarin inhibits RANKL-mediated osteoclastogenesis and titanium particle-induced osteolysis via suppression of NF-κB and MAPK signaling pathway. Int. Immunopharmacol. 2016, 40, 458–465. [Google Scholar] [CrossRef]
- Park, J.W.; Kwon, O.K.; Yuniato, P.; Marwoto, B.; Lee, J.; Oh, S.R.; Kim, J.H.; Ahn, K.S. Amelioration of an LPS-induced inflammatory response using a methanolic extract of Lagerstroemia ovalifolia to suppress the activation of NF-κB in RAW264.7 macrophages. Int. J. Mol. Med. 2016, 38, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Takada, Y.; Khuri, F.R.; Aggarwal, B.B. Protein farnesyltransferase inhibitor (SCH 66336) abolishes NF-kappaB activation induced by various carcinogens and inflammatory stimuli leading to suppression of NF-kappaB-regulated gene expression and up-regulation of apoptosis. J. Biol. Chem. 2004, 279, 26287–26299. [Google Scholar] [CrossRef] [Green Version]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef] [Green Version]
- Widmann, C.; Gibson, S.; Jarpe, M.B.; Johnson, G.L. Mitogen-Activated Protein Kinase: Conservation of a Three-Kinase Module from Yeast to Human. Physiol. Rev. 1999, 79, 143–180. [Google Scholar] [CrossRef]
- Lee, J.C.; Laydon, J.T.; McDonnell, P.C.; Gallagher, T.F.; Kumar, S.; Green, D.; McNulty, D.; Blumenthal, M.J.; Keys, J.R.; Land vatter, S.W.; et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994, 372, 739–746. [Google Scholar] [CrossRef]
- Begum, R.; Kim, C.-S.; Fadriquela, A.; Bajgai, J.; Jing, X.; Kim, D.-H.; Kim, S.-K.; Lee, K.-J. Molecular hydrogen protects against oxidative stress-induced RAW 264.7 macrophage cells through the activation of Nrf2 and inhibition of MAPK signaling pathway. Mol. Cell. Toxicol. 2020, 16, 103–118. [Google Scholar] [CrossRef]
- Ju, S.M.; Song, H.Y.; Lee, S.J.; Seo, W.Y.; Sin, D.H.; Goh, A.R.; Kang, Y.H.; Kang, I.J.; Won, M.H.; Yi, J.S.; et al. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose via blockade of NF-kappaB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 2009, 387, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kim, S.H. Inhibitory effect of galangin on atopic dermatitis-like skin lesions. Food Chem. Toxicol. 2014, 68, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Trzyna, W.C.; McClintock, D.S.; Schumacker, P.T. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J. Immunol. 2000, 165, 1013–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, G.J.; Dang, H.T.; Han, S.C.; Kang, N.J.; Koo, D.H.; Koh, Y.S.; Hyun, J.W.; Kang, H.K.; Jung, J.H.; Yoo, E.S. Methyl 5-chloro-4,5-didehydrojasmonate (J7) inhibits macrophage-derived chemokine production via down-regulation of the signal transducers and activators of transcription 1 pathway in HaCaT human keratinocytes. Chem. Pharm. Bull. 2013, 61, 1002–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eom, S.-H.; Lee, E.-H.; Park, K.; Kwon, J.-Y.; Kim, P.-H.; Jung, W.-K.; Kim, Y.-M. Eckol from Eisenia bicyclis Inhibits Inflammation Through the Akt/NF-κB Signaling in Propionibacterium acnes-Induced Human Keratinocyte Hacat Cells. J. Food Biochem. 2017, 41, e12312. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, H.S.; Lee, W.; Han, E.J.; Kim, S.Y.; Fernando, I.P.S.; Ahn, G.; Kim, K.N. Eckol from Ecklonia cava ameliorates TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells. Int. Immunopharmacol. 2020, 82, 106146. [Google Scholar] [CrossRef]
- Kang, N.J.; Koo, D.H.; Kang, G.J.; Han, S.C.; Lee, B.W.; Koh, Y.S.; Hyun, J.W.; Lee, N.H.; Ko, M.H.; Kang, H.K.; et al. Dieckol, a Component of Ecklonia cava, Suppresses the Production of MDC/CCL22 via Down-Regulating STAT1 Pathway in Interferon-γ Stimulated HaCaT Human Keratinocytes. Biomol. Ther. 2015, 23, 238–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, N.J.; Kang, G.J.; Han, S.C.; Hyun, E.A.; Koo, D.H.; Koh, Y.S.; Ko, M.H.; Hyun, J.W.; Kang, H.K.; Yoo, E.S. Ceramium boydenii, a red alga, inhibits MDC/CCL22 production via suppression of STAT1 activation in HaCaT keratinocyte. Korean J. Pharmacogn. 2013, 44, 154–160. [Google Scholar]
- Koizumi, J.; Takatani, N.; Kobayashi, N.; Mikami, K.; Miyashita, K.; Yamano, Y.; Wada, A.; Maoka, T.; Hosokawa, M. Carotenoid Profiling of a Red Seaweed Pyropia yezoensis: Insights into Biosynthetic Pathways in the Order Bangiales. Mar. Drugs 2018, 16, 426. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Lee, Y.S.; Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B.; Hong, J.T. Astaxanthin Ameliorates Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Dysfunction through Inactivation of the Signal Transducer and Activator of Transcription 3 Pathway. Mar. Drugs 2019, 17, 123. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Singh, S.V. Inhibition of NF-κB signaling pathway by astaxanthin supplementation for prevention of heat stress-induced inflammatory changes and apoptosis in Karan Fries heifers. Trop Anim. Health Prod. 2019, 51, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Feng, J.; Xie, X.; Gao, S.; Wang, G. Involvement of cyclic electron flow in irradiance stress responding and its potential regulation of the mechanisms in Pyropia yezoensis. Chin. J. Oceanol. Limnol. 2016, 34, 730–739. [Google Scholar] [CrossRef]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol. Behav. 2019, 211, 112650. [Google Scholar] [CrossRef]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.-L.; Wang, G.; Chen, L.; et al. Fucoxanthin, a Marine Xanthophyll Isolated from Conticribra weissflogii ND-8: Preventive Anti-Inflammatory Effect in a Mouse Model of Sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef] [Green Version]







| Genes | Sequences (5′→3′) |
|---|---|
| GAPDH (Forward) | CGT CTC CTC TGA CTT CAA CA |
| GAPDH (Reverse) | AGC CAA ATT CGT TGT CAT AC |
| MDC (Forward) | CAG CAC GAG GGA CCA ATG TG |
| MDC (Reverse) | CTT GGG GTC CGA ACA GAT GG |
| TARC (Forward) | ACT GCA CTC CTG GTT GTC CT |
| TARC (Reverse) | AAG GTT AGC AAC ACC ACG CC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, Y.; Lee, W.-H.; Jeong, J.; Park, M.; Ko, J.-Y.; Kwon, O.W.; Lee, J.; Kim, Y.-J. Pyropia yezoensis Extract Suppresses IFN-Gamma- and TNF-Alpha-Induced Proinflammatory Chemokine Production in HaCaT Cells via the Down-Regulation of NF-κB. Nutrients 2020, 12, 1238. https://doi.org/10.3390/nu12051238
Ha Y, Lee W-H, Jeong J, Park M, Ko J-Y, Kwon OW, Lee J, Kim Y-J. Pyropia yezoensis Extract Suppresses IFN-Gamma- and TNF-Alpha-Induced Proinflammatory Chemokine Production in HaCaT Cells via the Down-Regulation of NF-κB. Nutrients. 2020; 12(5):1238. https://doi.org/10.3390/nu12051238
Chicago/Turabian StyleHa, Yuna, Won-Hwi Lee, JaeWoo Jeong, Mira Park, Ju-Young Ko, Oh Wook Kwon, Jongsung Lee, and Youn-Jung Kim. 2020. "Pyropia yezoensis Extract Suppresses IFN-Gamma- and TNF-Alpha-Induced Proinflammatory Chemokine Production in HaCaT Cells via the Down-Regulation of NF-κB" Nutrients 12, no. 5: 1238. https://doi.org/10.3390/nu12051238
APA StyleHa, Y., Lee, W.-H., Jeong, J., Park, M., Ko, J.-Y., Kwon, O. W., Lee, J., & Kim, Y.-J. (2020). Pyropia yezoensis Extract Suppresses IFN-Gamma- and TNF-Alpha-Induced Proinflammatory Chemokine Production in HaCaT Cells via the Down-Regulation of NF-κB. Nutrients, 12(5), 1238. https://doi.org/10.3390/nu12051238






