The Effect of 4′-hydroxy-3,4,5-trimetoxystilbene, the Metabolite of Resveratrol Analogue DMU-212, on Growth, Cell Cycle and Apoptosis in DLD-1 and LOVO Colon Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and the Cytotoxicity of DMU-281 Analysis
2.3. Apoptosis and Necrosis Assays
2.4. Cell Cycle Analysis
2.5. Assessment of Caspase-3/7, -9 and Caspase-8 Activation
2.6. PCR Array
2.7. Protein Expression Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of the Metabolites of DMU-212 on Cells Viability and Cell Cycle
3.2. Apoptosis Induction by DMU-281
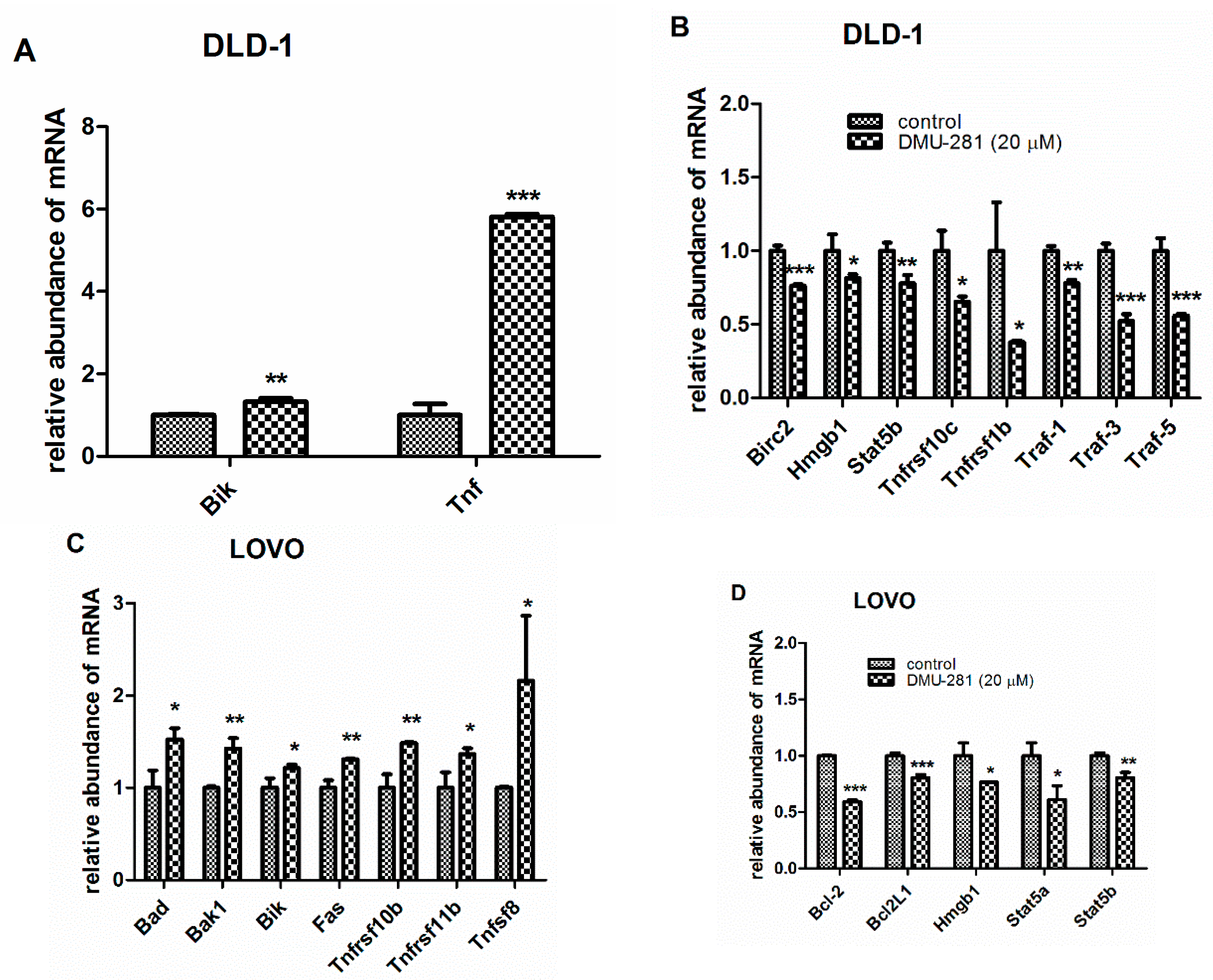
3.3. The Changes in the Expression of Apoptosis-Related Genes by DMU-281
3.4. The Changes in Pro- and Anti-Apoptotic Proteins Expression by DMU-281
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cancer (accessed on 10 September 2019).
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Publ. Gr. 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Fadok, V. Corpse clearance defines the meaning of cell death. Nature 2000, 407, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, K.; Takahashi, M.; Watanabe, N.; Kobayashi, Y.; Alerts, E. Silent Cleanup of Very Early Apoptotic Cells by Macrophages. J. Immunol. 2003, 171, 4672–4679. [Google Scholar] [CrossRef] [Green Version]
- Hongmei, Z. Extrinsic and Intrinsic Apoptosis Signal Pathway Review. In Apoptosis and Medicine; Ntuli, T.M., Ed.; IntechOpen: London, UK, 2012; pp. 3–22. [Google Scholar] [CrossRef] [Green Version]
- de Gramont, A.; Figer, A.; Seymour, M.; Homerin, A.; Hmissi, J.; Cassidy, C.; Boni, H.; Cortes-Funes, A.; Cervantes, G.; Freyer, D.; et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2016, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Sale, S.; Tunstall, R.G.; Ruparelia, K.C.; Potter, G.A.; Steward, W.P.; Gescher, A.J. Comparison of the effects of the chemopreventive agent resveratrol and its development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int. J. Cancer 2005, 115, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef]
- Kang, S.; Lee, J.K.; Choi, O.; Kim, C.Y.; Jang, J.; Hwang, B.Y.; Hong, Y. Biosynthesis of methylated resveratrol analogs through the construction of an artificial biosynthetic pathway in E. coli. BMC Biotechnol. 2014, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Roberti, M.; Pizzirani, D.; Simoni, D.; Rondanin, R.; Baruchello, R.; Bonora, C.; Buscemi, F.; Grimaudo, S.; Tolomeo, M. Synthesis and Biological Evaluation of Resveratrol and Analogues as Apoptosis-Inducing Agents. J. Med. Chem. 2003, 46, 3546–3554. [Google Scholar] [CrossRef]
- Piotrowska, H.; Myszkowski, K.; Ziolkowska, A.; Kulcenty, K.; Wierzchowski, M.; Kaczmarek, M.; Murias, M.; Kwiatkowska-Borowczyk, E.; Jodynis-Liebert, J. Resveratrol analogue 3,4,4’,5-tetramethoxystilbene inhibits growth, arrests cell cycle and induces apoptosis in ovarian SKOV-3 and A-2780 cancer cells. Toxicol. Appl. Pharmacol. 2012, 263, 53–60. [Google Scholar] [CrossRef]
- Ma, Z.; Molavi, O.; Haddadi, A.; Lai, R.; Gossage, R.A.; Lavasanifar, A. Resveratrol analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) mediates anti-tumor effects via mechanism different from that of resveratrol. Cancer Chemother. Pharmacol. 2008, 63, 27–35. [Google Scholar] [CrossRef]
- Sale, S.; Verschoyle, R.D.; Boocock, D.; Jones, D.J.; Wilsher, N.; Ruparelia, K.C.; Potter, G.A.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Pharmacokinetics in mice and growth–inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4’- tetramethoxystilbene. Br. J. Cancer 2004, 90, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Ruparelia, K.C.; Papakyriakou, A.; Filippakis, H.; Tsatsakis, A.M.; Spandidos, D.A. Anticancer effects of the metabolic products of the resveratrol analogue, DMU-212: Structural requirements for potency. Eur. J. Med. Chem. 2011, 46, 2586–2595. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Scaffidi, C.; Fulda, S.; Srinivasan, A.; Friesen, C.; Li, F.; Tomaselli, K.J.; Debatin, K.M.; Krammer, P.H.; Peter, M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998, 17, 1675–1687. [Google Scholar] [CrossRef]
- Koehler, B.C.; Scherr, A.L.; Lorenz, S.; Urbanik, T.; Kautz, N.; Elssner, C.; Welte, S.; Bermejo, J.L.; Jäger, D.; Schulze-Bergkamen, H. Beyond cell death—Antiapoptotic Bcl-2 proteins regulate migration and invasion of colorectal cancer cells In Vitro. PLoS ONE 2013, 8, 76446. [Google Scholar] [CrossRef]
- Placzek, W.J.; Wei, J.; Kitada, S.; Zhai, D.; Reed, J.C.; Pellecchia, M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010, 1, 40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gu, J.; Lin, T.; Huang, X.; Roth, J.A.; Fang, B. Mechanisms involved in development of resistance to adenovirus-mediated proapoptotic gene therapy in DLD1 human colon cancer cell line. Gene Ther. 2002, 9, 1262–1270. [Google Scholar] [CrossRef] [Green Version]
- Miserocchi, G.; Mercatali, L.; Liverani, C.; De Vita, A.; Spadazzi, C.; Pieri, F.; Bongiovanni, A.; Recine, F.; Amadori, D.; Ibrahim, T. Management and potentialities of primary cancer cultures in preclinical and translational studies. J. Transl. Med. 2017, 15, 229. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.Y.; Lee, J.E.; Kim, H.; Sim, M.H.; Kim, K.K.; Lee, G.; Kim, H.I.; An, J.Y.; Hyung, W.J.; Kim, C.B.; et al. Establishment and characterisation of patient-derived xenografts as paraclinical models for gastric cancer. Sci. Rep. 2016, 6, 22172. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, H.; Myszkowski, K.; Amarowicz, R.; Murias, M.; Kulcenty, K.; Wierzchowski, M.; Jodynis-Liebert, J. Different susceptibility of colon cancer DLD-1 and LOVO cell lines to apoptosis induced by DMU-212, a synthetic resveratrol analogue. Toxicol. In Vitro 2013, 27, 2127–2134. [Google Scholar] [CrossRef]
- Chung, S.S.; Dutta, P.; Austin, D.; Wang, P.; Awad, A.; Vadgama, J.V. Combination of resveratrol and 5-flurouracil enhanced anti-telomerase activity and apoptosis by inhibiting STAT3 and Akt signaling pathways in human colorectal cancer cells. Oncotarget 2018, 9, 32943–32957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Martins-Dinis, M.M.D.D.C.; Ferretti, G.D.D.S.; Ferreira, V.F.; Silva, J.L. Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues. Molecules 2020, 25, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosslau, A.; Chen, H.; Ho, C.T.; Chen, K.Y. A methoxy derivative of resveratrol analogue selectively induced activation of the mitochondrial apoptotic pathway in transformed fibroblasts. Br. J. Cancer 2005, 92, 513–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska-Kempisty, H.; Ruciński, M.; Borys, S.; Kucińska, M.; Kaczmarek, M.; Zawierucha, P.; Wierzchowski, M.; Łażewski, D.; Murias, M.; Jodynis-Liebert, J. 3′-hydroxy-3,4,5,4′-tetramethoxystilbene, the metabolite of resveratrol analogue DMU-212, inhibits ovarian cancer cell growth in vitro and in a mice xenograft model. Sci. Rep. 2016, 6, 32627. [Google Scholar] [CrossRef] [Green Version]
- Ceballos-Cancino, G.; Espinosa, M.; Maldonado, V.; Melendez-Zajgla, J. Regulation of mitochondrial Smac/DIABLO-selective release by survivin. Oncogene 2007, 26, 7569–7575. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Ruiz, G.; Maldonado, V.; Ceballos-cancino, G.; Grajeda, J.P.R.; Melendez-zajgla, J. Role of Smac/ DIABLO in cancer progression. J. Exp. Clin. Cancer Res. 2008, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kashkar, H.; Seeger, J.M.; Hombach, A.; Deggerich, A.; Yazdanpanah, B.; Utermöhlen, O.; Heimlich, G.; Abken, H.; Krönke, M. XIAP targeting sensitizes Hodgkin lymphoma cells for cytolytic T-cell attack. Blood 2006, 108, 3434–3440. [Google Scholar] [CrossRef]
- Kashkar, H.; Haefs, C.; Shin, H.; Hamilton-Dutoit, S.J.; Salvesen, G.S.; Krönke, M.; Jürgensmeier, J.M. XIAP-mediated caspase inhibition in Hodgkin’s lymphoma-derived B cells. J. Exp. Med. 2003, 198, 341–347. [Google Scholar] [CrossRef]
- Adrain, C.; Creagh, E.M.; Martin, S.J. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001, 20, 6627–6636. [Google Scholar] [CrossRef] [Green Version]
- Würstle, M.L.; Laussmann, M.A.; Rehm, M. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp. Cell Res. 2012, 318, 1213–1220. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Green, D.R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008, 4, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; McFadden, G. Modulation of tumor necrosis factor by microbial pathogens. PLoS Pathog. 2006, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.S.; Choi, S.; Shin, B.; Yu, J.; Yu, Y.; Hwang, J.-M.; Hyeongseok, Y.; Chung, Y.-H.; Choi, J.-S.; Choi, Y.; et al. Tumor necrosis factor (TNF) receptor-associated factor (TRAF)-interacting protein (TRIP) negatively regulates the TRAF2 ubiquitin-dependent pathway by suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction. J. Biol. Chem. 2015, 290, 9660–9673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 1, 76–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickens, L.S.; Boyd, R.S.; Jukes-Jones, R.; Hughes, M.A.; Robinson, G.L.; Fairall, L.; Schwabe, J.W.R.; Cain, K.; Macfarlane, M. A death effector domain chain DISC model reveals a crucial role for Caspase-8 chain assembly in mediating apoptotic cell death. Mol. Cell 2012, 47, 291–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruey, J.M.; Ducasse, C.; Bonniaud, P.; Ravagnan, L.; Susin, S.A.; Diaz-Latoud, C.; Gurbuxani, S.; Arrigo, A.P.; Kroemer, G.; Solary, E.; et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2000, 2, 645–652. [Google Scholar] [CrossRef]
- Paul, C.; Manero, F.; Gonin, S.; Kretz-Remy, C.; Virot, S.; Arrigo, A.P. Hsp27 as a negative regulator of cytochrome C release. Mol. Cell. Biol. 2002, 22, 816–834. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef] [Green Version]
- Takayama, S.; Reed, J.C.; Homma, S. Heat-shock proteins as regulators of apoptosis. Oncogene 2003, 22, 9041–9047. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Su, W.; Liang, Q.; Zhang, Z.; Chen, H.; Du, W.; Chen, Y.-X.; Fang, J.-Y. Inhibition of STAT5 induces G1 cell cycle arrest and reduces tumor cell invasion in human colorectal cancer cells. Lab. Investig. 2009, 89, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Wang, Y.-C.; Hong, J.; Su, W.-Y.; Lin, Y.-W.; Lu, R.; Xiong, H.; Fang, J.-Y. STAT5 Isoforms regulate colorectal cancer cell apoptosis via reduction of mitochondrial membrane potential and generation of reactive oxygen species. J. Cell. Physiol. 2011, 227, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Zhu, X.; Lu, R.; Messer, J.S.; Xia, Y.; Chang, E.B.; Sun, J. Intestinal epithelial HMGB1 inhibits bacterial infection via STAT3 regulation of autophagy. Autophagy 2019, 15, 1935–1953. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Cheh, C.-W.; Livesey, K.M.; Liang, X.; Schapiro, N.E.; Benschop, R.; Sparvero, L.J.; Amoscato, A.A.; Tracey, K.J.; et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 2010, 29, 5299–5310. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Metabolites of DMU-212 | Time Exposure | Cell Viability [%] | ||
|---|---|---|---|---|
| DLD-1 | LOVO | CaCo-2 | ||
| DMU-214 | 24 h | 66.97 ± 7.14 ** | 82.16 ± 5.91 * | 104.90 ± 2.91 |
| 48 h | 59.52 ± 12.78 ** | 53.60 ± 5.54 *** | 80.60 ± 26.47 | |
| 72 h | 56.55 ± 19.04 ** | 54.88 ± 12.18 *** | 68.28 ± 15.84 ** | |
| DMU-281 | 24 h | 89.50 ± 7.40 | 78.83 ± 7.93 * | 90.09 ± 8.40 |
| 48 h | 62.02 ± 5.78 *** | 56.37 ± 3.20 *** | 90.73 ± 11.49 | |
| 72 h | 44.87 ± 7.57 ** | 50.71 ± 14.94 *** | 84.87 ± 11.56 * | |
| DMU-291 | 24 h | 102.99 ± 9.05 | 94.30 ± 6.94 | 107.47 ± 5.90 |
| 48 h | 94.90 ± 19.43 | 78.15 ± 12.44 ** | 97.53 ± 17.38 | |
| 72 h | 75.90 ± 20.29 | 88.07 ± 9.09 * | 85.37 ± 21.46 | |
| DMU-807 | 24 h | 92.53 ± 8.60 | 79.92 ± 2.58 *** | 89.63 ± 2.01 * |
| 48 h | 95.32 ± 8.62 | 69.41 ± 16.59 * | 77.96 ± 4.68 ** | |
| 72 h | 101.18 ± 9.99 | 76.71 ± 25.15 | 80.59 ± 8.21 ** | |
| Cell Line | Genes Expression Profile | Proteins Expression Profile |
|---|---|---|
| DLD-1 | ↑ Bik, Tnf | ↑ Casp-3, Smac/Diablo |
| ↓ Birc2, Hmgb1, Stat5b, Tnfrsf10c, Tnfrsf1b, Traf-1, Traf-3, Traf-5 | ↓ Bcl-2, Bcl-xL, Procasp-3, Hsp27 | |
| LOVO | ↑ Bad, Bak1, Bik, Fas, Tnfrsf10b, Tnfrsf11b, Tnfsf 8 | ↑ Fadd, Hsp60 |
| ↓ Bcl-2, Bcl-2L1, Hmgb1, Stat5a, Stat5b | ↓ Hsp27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jozkowiak, M.; Skupin-Mrugalska, P.; Nowicki, A.; Borys-Wojcik, S.; Wierzchowski, M.; Kaczmarek, M.; Ramlau, P.; Jodynis-Liebert, J.; Piotrowska-Kempisty, H. The Effect of 4′-hydroxy-3,4,5-trimetoxystilbene, the Metabolite of Resveratrol Analogue DMU-212, on Growth, Cell Cycle and Apoptosis in DLD-1 and LOVO Colon Cancer Cell Lines. Nutrients 2020, 12, 1327. https://doi.org/10.3390/nu12051327
Jozkowiak M, Skupin-Mrugalska P, Nowicki A, Borys-Wojcik S, Wierzchowski M, Kaczmarek M, Ramlau P, Jodynis-Liebert J, Piotrowska-Kempisty H. The Effect of 4′-hydroxy-3,4,5-trimetoxystilbene, the Metabolite of Resveratrol Analogue DMU-212, on Growth, Cell Cycle and Apoptosis in DLD-1 and LOVO Colon Cancer Cell Lines. Nutrients. 2020; 12(5):1327. https://doi.org/10.3390/nu12051327
Chicago/Turabian StyleJozkowiak, Malgorzata, Paulina Skupin-Mrugalska, Andrzej Nowicki, Sylwia Borys-Wojcik, Marcin Wierzchowski, Mariusz Kaczmarek, Piotr Ramlau, Jadwiga Jodynis-Liebert, and Hanna Piotrowska-Kempisty. 2020. "The Effect of 4′-hydroxy-3,4,5-trimetoxystilbene, the Metabolite of Resveratrol Analogue DMU-212, on Growth, Cell Cycle and Apoptosis in DLD-1 and LOVO Colon Cancer Cell Lines" Nutrients 12, no. 5: 1327. https://doi.org/10.3390/nu12051327
APA StyleJozkowiak, M., Skupin-Mrugalska, P., Nowicki, A., Borys-Wojcik, S., Wierzchowski, M., Kaczmarek, M., Ramlau, P., Jodynis-Liebert, J., & Piotrowska-Kempisty, H. (2020). The Effect of 4′-hydroxy-3,4,5-trimetoxystilbene, the Metabolite of Resveratrol Analogue DMU-212, on Growth, Cell Cycle and Apoptosis in DLD-1 and LOVO Colon Cancer Cell Lines. Nutrients, 12(5), 1327. https://doi.org/10.3390/nu12051327








