Trimethylamine N-Oxide in Relation to Cardiometabolic Health—Cause or Effect?
Abstract
:1. Introduction
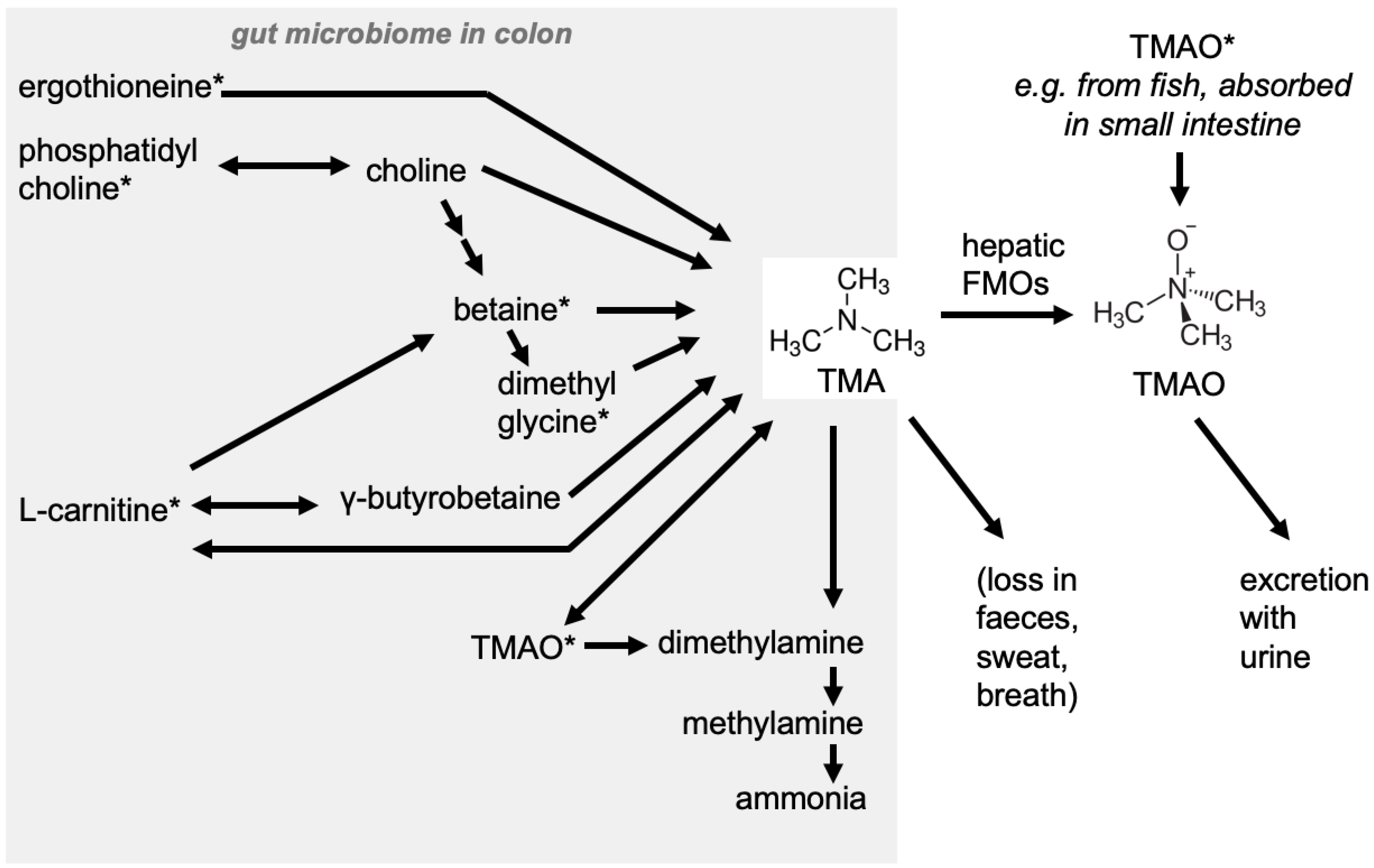
2. Where Does TMAO Come From?
3. Physiological Role/Effects of TMAO
3.1. TMAO Variability
3.1.1. TMAO Variability with Age and Sex
3.1.2. TMAO Variability through FMO
3.1.3. TMAO Variability by Diet
3.1.4. TMAO Variability by Microbiome Variability
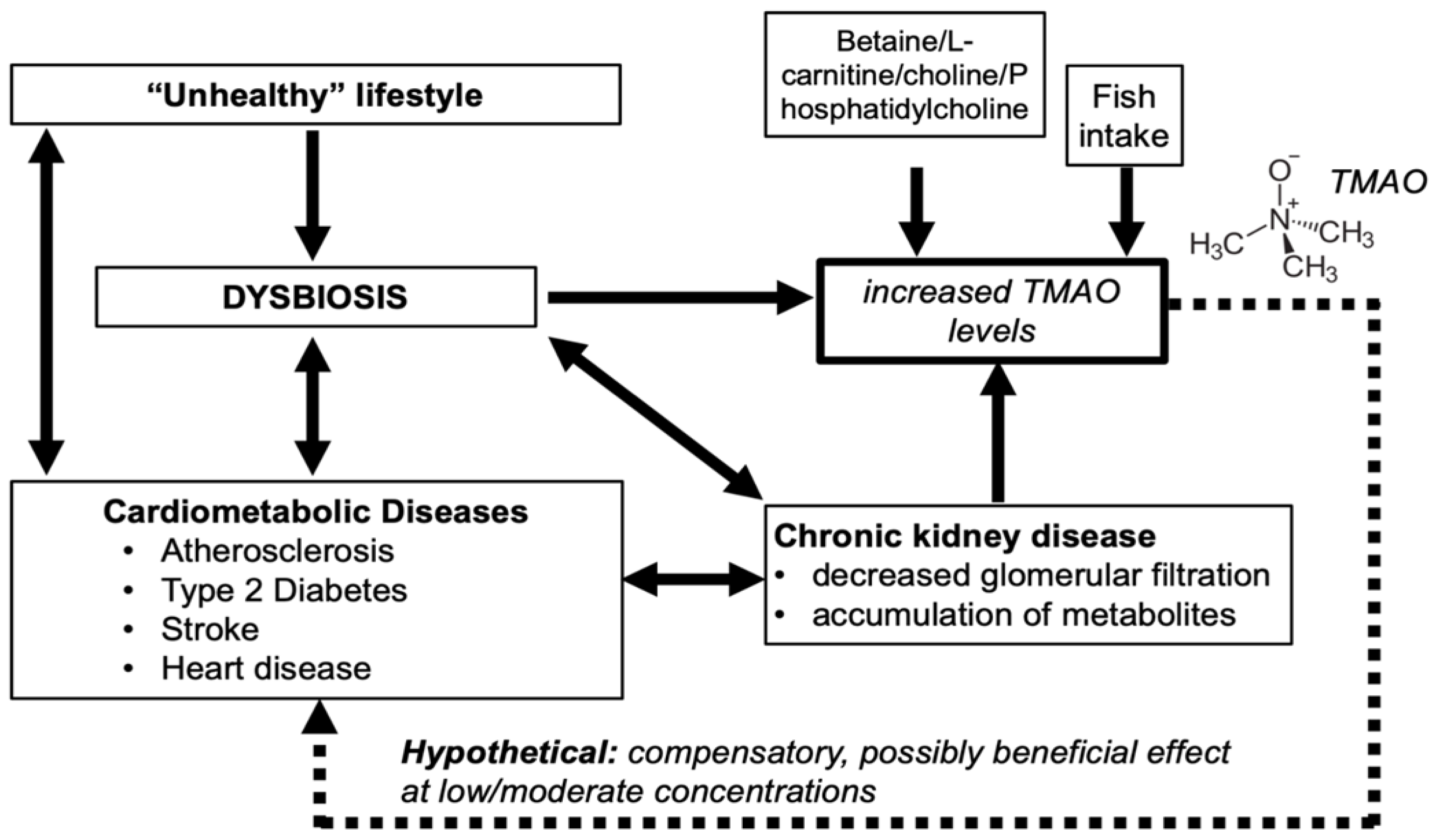
4. TMAO and Cardiometabolic Diseases
4.1. TMAO Levels in Humans Associated with CVD and T2D
4.2. TMAO in Patients with Compromised Renal Function
4.3. Cause to Effects
5. TMAO in Experimental Models
5.1. TMAO in Cell Culture
5.2. TMAO in Animal Studies
5.2.1. Unfavorable Effects of TMAO in Animals
5.2.2. Favorable or Neutral Effects of TMAO in Animals
5.2.3. Why TMAO Is a Marker but Not the Effector
6. Summary and Conclusions
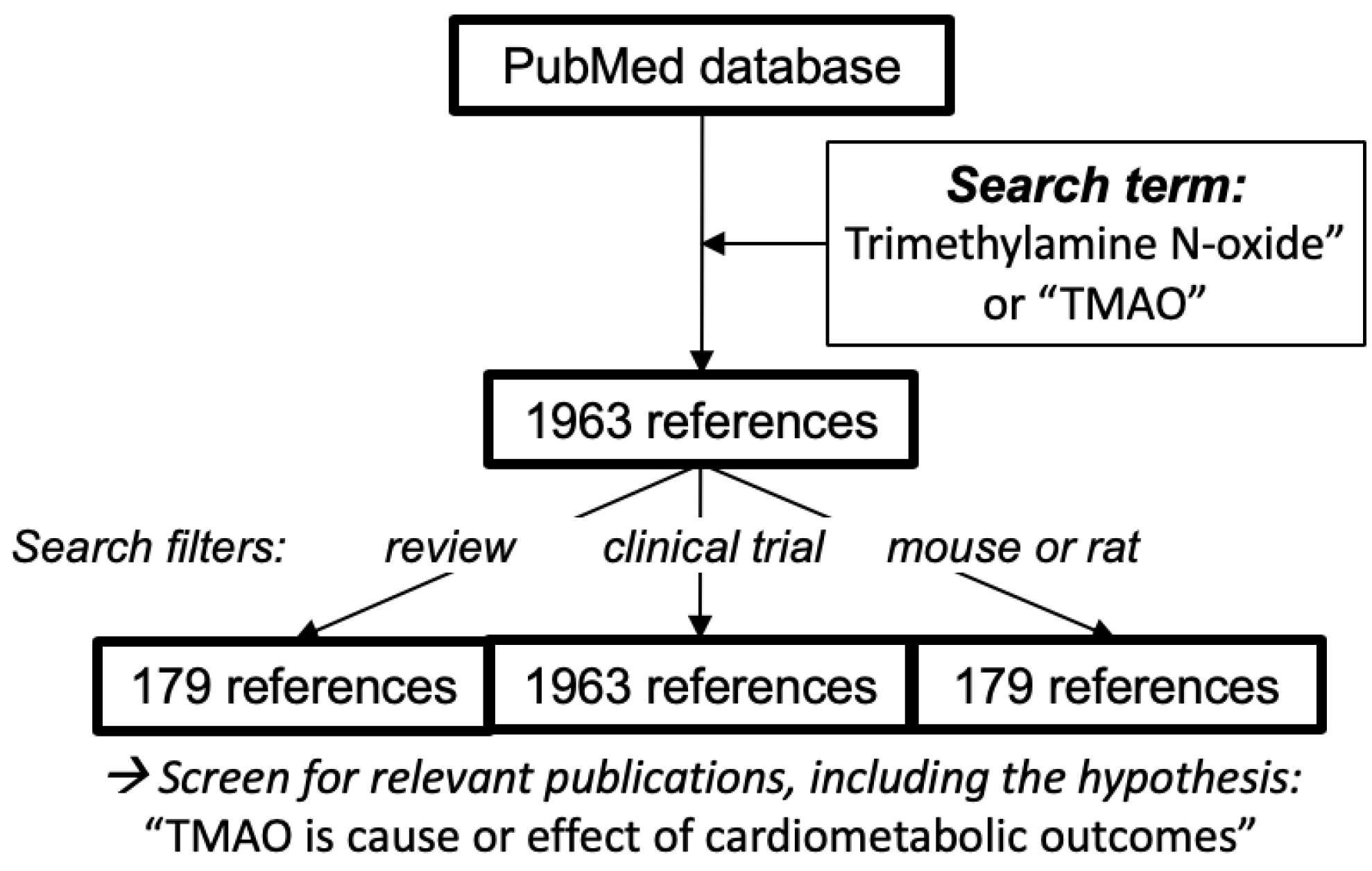
7. Literature Search Methods
Author Contributions
Funding
Conflicts of Interest
References
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Global Report on Diabetes. Online Publication. 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=BB2C89145E6EB31BD42C0A3A68D965DD?sequence=1 (accessed on 6 May 2020).
- Miura, S.I.; Shiga, Y.; Ike, A.; Iwata, A. Atherosclerotic Coronary Artery Disease in Patients With Cardiometabolic Syndrome. Cardiol. Res. 2019, 10, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Martinez-Rodriguez, J.; Gonzalez-Lucan, M.; Fernandez-Fernandez, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. 2019, 13, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S. Inflammation in atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Bruck, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Volzke, H.; Arnlov, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. CKD Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W.; et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2018, 71, A7. [Google Scholar] [CrossRef]
- Lau, K.; Srivatsav, V.; Rizwan, A.; Nashed, A.; Liu, R.; Shen, R.; Akhtar, M. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrients 2017, 9, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.J. Risk factors for cardiovascular disease: A cautionary tale of diet-microbiome interactions. J. Am. Coll. Nutr. 2013, 32, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Fak, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Backhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, A.L.; Backhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Felizardo, R.J.F.; Watanabe, I.K.M.; Dardi, P.; Rossoni, L.V.; Camara, N.O.S. The interplay among gut microbiota, hypertension and kidney diseases: The role of short-chain fatty acids. Pharmacol. Res. 2019, 141, 366–377. [Google Scholar] [CrossRef]
- Ratajczak, W.; Ryl, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczynska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Santos-Marcos, J.A.; Perez-Jimenez, F.; Camargo, A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J. Nutr. Biochem. 2019, 70, 1–27. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Marko, L.; Hoges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Wang, J.; Wang, Y.; Fang, Y. Intestinal fatty acid-binding protein mediates atherosclerotic progress through increasing intestinal inflammation and permeability. J. Cell Mol. Med. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plata, C.; Cruz, C.; Cervantes, L.G.; Ramirez, V. The gut microbiota and its relationship with chronic kidney disease. Int. Urol. Nephrol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Rajani, C.; Jia, W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin. Sci. (London) 2018, 132, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Invest. 2014, 124, 4204–4211. [Google Scholar] [CrossRef]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef]
- Oellgaard, J.; Winther, S.A.; Hansen, T.S.; Rossing, P.; von Scholten, B.J. Trimethylamine N-oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [Green Version]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [Green Version]
- al-Waiz, M.; Mikov, M.; Mitchell, S.C.; Smith, R.L. The exogenous origin of trimethylamine in the mouse. Metabolism 1992, 41, 135–136. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Seim, H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev. Nutr. 1998, 18, 39–61. [Google Scholar] [CrossRef]
- Zeisel, S.H.; daCosta, K.A.; Youssef, M.; Hensey, S. Conversion of dietary choline to trimethylamine and dimethylamine in rats: Dose-response relationship. J. Nutr. 1989, 119, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Treacy, E.P.; Akerman, B.R.; Chow, L.M.; Youil, R.; Bibeau, C.; Lin, J.; Bruce, A.G.; Knight, M.; Danks, D.M.; Cashman, J.R.; et al. Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum. Mol. Genet. 1998, 7, 839–845. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Smith, R.L. Trimethylaminuria: The fish malodor syndrome. Drug Metab Dispos 2001, 29, 517–521. [Google Scholar] [PubMed]
- Hoyles, L.; Jimenez-Pranteda, M.L.; Chilloux, J.; Brial, F.; Myridakis, A.; Aranias, T.; Magnan, C.; Gibson, G.R.; Sanderson, J.D.; Nicholson, J.K.; et al. Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, M.A.; Faull, R.; Fornasini, G.; Milne, R.W.; Evans, A.M. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1300–1304. [Google Scholar] [CrossRef] [Green Version]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab Dispos 2016, 44, 1839–1850. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramirez, M.J.; Milagro, F.I.; Martinez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H.; Wishnok, J.S.; Blusztajn, J.K. Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Ther 1983, 225, 320–324. [Google Scholar]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Mitchell, S.C.; Smith, R.L. Dietary precursors of trimethylamine in man: A pilot study. Food Chem. Toxicol. 1999, 37, 515–520. [Google Scholar] [CrossRef]
- NIH. Carnitine: Fact Sheet for Health Professionals. 2017. Available online: https://ods.od.nih.gov/factsheets/Carnitine-HealthProfessional/#en6 (accessed on 14 October 2019).
- Feller, A.G.; Rudman, D. Role of carnitine in human nutrition. J. Nutr. 1988, 118, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J. Carnitine function and requirements during the life cycle. FASEB J. 1992, 6, 3379–3386. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, N.a.A.N. L-carnitine and contribution to normal lipid metabolism: Evaluation of a health claim pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2018, 16, 5137–5139. [Google Scholar] [CrossRef]
- Rebouche, C.J. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann. N. Y. Acad. Sci 2004, 1033, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Samulak, J.J.; Sawicka, A.K.; Hartmane, D.; Grinberga, S.; Pugovics, O.; Lysiak-Szydlowska, W.; Olek, R.A. L-Carnitine Supplementation Increases Trimethylamine-N-Oxide but not Markers of Atherosclerosis in Healthy Aged Women. Ann. Nutr. Metab. 2019, 74, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Yamagishi, S.; Sakai, K.; Kaida, Y.; Yokoro, M.; Ueda, S.; Wada, Y.; Takeuchi, M.; Shimizu, M.; Yamazaki, H.; et al. Oral L-carnitine supplementation increases trimethylamine-N-oxide but reduces markers of vascular injury in hemodialysis patients. J. Cardiovasc. Pharmacol. 2015, 65, 289–295. [Google Scholar] [CrossRef]
- Fritz, I.B.; Mc, E.B. Effects of carnitine on fatty-acid oxidation by muscle. Science 1959, 129, 334–335. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- El-Hattab, A.W. Systemic Primary Carnitine Deficiency. In GeneReviews (R); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; GeneRviews: Seattle, WA, USA, 1993. [Google Scholar]
- Wang, Z.Y.; Liu, Y.Y.; Liu, G.H.; Lu, H.B.; Mao, C.Y. l-Carnitine and heart disease. Life Sci. 2018, 194, 88–97. [Google Scholar] [CrossRef]
- Lee, B.J.; Lin, J.S.; Lin, Y.C.; Lin, P.T. Effects of L-carnitine supplementation on lipid profiles in patients with coronary artery disease. Lipids Health Dis. 2016, 15, 107. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Moghaddas, A.; Dashti-Khavidaki, S. L-Carnitine and Potential Protective Effects Against Ischemia-Reperfusion Injury in Noncardiac Organs: From Experimental Data to Potential Clinical Applications. J. Diet. Suppl. 2018, 15, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Dinicolantonio, J.J.; Niazi, A.K.; McCarty, M.F.; Lavie, C.J.; Liberopoulos, E.; O’Keefe, J.H. L-carnitine for the treatment of acute myocardial infarction. Rev. Cardiovasc. Med. 2014, 15, 52–62. [Google Scholar] [PubMed]
- Mingrone, G.; Greco, A.V.; Capristo, E.; Benedetti, G.; Giancaterini, A.; De Gaetano, A.; Gasbarrini, G. L-carnitine improves glucose disposal in type 2 diabetic patients. J. Am. Coll Nutr. 1999, 18, 77–82. [Google Scholar] [CrossRef]
- Papandreou, C.; Bullo, M.; Zheng, Y.; Ruiz-Canela, M.; Yu, E.; Guasch-Ferre, M.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the Prevencion con Dieta Mediterranea (PREDIMED) trial. Am. J. Clin. Nutr. 2018, 108, 163–173. [Google Scholar] [CrossRef]
- Spanish Agency for Food Safety and Nutrition. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on the Use Conditions for Certain Substances Other Than Vitamins, Minerals and Plants in Food Supplements. Available online: http://www.aecosan.msssi.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_cc_ingles/FOOD_SUPPLEMENTS.pdf (accessed on 6 May 2020).
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef]
- NIH. Choline: Fact Sheet for Health Professionals. 2019. Available online: https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/ (accessed on 14 October 2019).
- EFSA Panel on Dietetic Products, N.a.A.N. Dietary Reference Values for choline. FEFSA J. 2016, 14, e04484. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.G.; Walk, A.M.; Cannavale, C.N.; Flemming, I.R.; Thompson, S.V.; Reeser, G.R.; Holscher, H.D.; Khan, N.A. Dietary choline is related to neural efficiency during a selective attention task among middle-aged adults with overweight and obesity. Nutr. Neurosci. 2019, 1–10. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to choline and contribution to normal lipid metabolism (ID 3186), maintenance of normal liver function (ID 1501), contribution to normal homocysteine metabolism (ID 3090), maintenance of normal neurological function (ID 1502), contribution to normal cognitive function (ID 1502), and brain and neurological development (ID 1503) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2056. [Google Scholar] [CrossRef]
- Schmitz, M.G.; Renooij, W. Phospholipids from rat, human, and canine gastric mucosa. Composition and metabolism of molecular classes of phosphatidylcholine. Gastroenterology 1990, 99, 1292–1296. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline deficiency. J. Nutr. Biochem. 1990, 1, 332–349. [Google Scholar] [CrossRef]
- Miller, C.A.; Corbin, K.D.; da Costa, K.A.; Zhang, S.; Zhao, X.; Galanko, J.A.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; Caudill, M.A.; Fernandez, M.L. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. J. Am. Coll. Nutr. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Berge, R.K.; Ramsvik, M.S.; Bohov, P.; Svardal, A.; Nordrehaug, J.E.; Rostrup, E.; Bruheim, I.; Bjorndal, B. Krill oil reduces plasma triacylglycerol level and improves related lipoprotein particle concentration, fatty acid composition and redox status in healthy young adults-a pilot study. Lipids Health Dis. 2015, 14, 163. [Google Scholar] [CrossRef] [Green Version]
- DiMarco, D.M.; Missimer, A.; Murillo, A.G.; Lemos, B.S.; Malysheva, O.V.; Caudill, M.A.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs/Day Increases HDL Cholesterol and Plasma Choline While Plasma Trimethylamine-N-oxide is Unchanged in a Healthy Population. Lipids 2017, 52, 255–263. [Google Scholar] [CrossRef]
- Konstantinova, S.V.; Tell, G.S.; Vollset, S.E.; Nygard, O.; Bleie, O.; Ueland, P.M. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J. Nutr. 2008, 138, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Lever, M.; George, P.M.; Elmslie, J.L.; Atkinson, W.; Slow, S.; Molyneux, S.L.; Troughton, R.W.; Richards, A.M.; Frampton, C.M.; Chambers, S.T. Betaine and secondary events in an acute coronary syndrome cohort. PLoS ONE 2012, 7, e37883. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Hu, F.B.; Ruiz-Canela, M.; Bullo, M.; Toledo, E.; Wang, D.D.; Corella, D.; Gomez-Gracia, E.; Fiol, M.; Estruch, R.; et al. Plasma Metabolites From Choline Pathway and Risk of Cardiovascular Disease in the PREDIMED (Prevention With Mediterranean Diet) Study. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.; Hua, X.; Gao, R.; Zeng, L.; Song, J.; Liu, J.; Zhang, J. Combinational Biomarkers for Atrial Fibrillation Derived from Atrial Appendage and Plasma Metabolomics Analysis. Sci. Rep. 2018, 8, 16930. [Google Scholar] [CrossRef] [Green Version]
- Zuo, H.; Svingen, G.F.T.; Tell, G.S.; Ueland, P.M.; Vollset, S.E.; Pedersen, E.R.; Ulvik, A.; Meyer, K.; Nordrehaug, J.E.; Nilsen, D.W.T.; et al. Plasma Concentrations and Dietary Intakes of Choline and Betaine in Association With Atrial Fibrillation Risk: Results From 3 Prospective Cohorts With Different Health Profiles. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vega, R.; Garrido, F.; Partearroyo, T.; Cediel, R.; Zeisel, S.H.; Martinez-Alvarez, C.; Varela-Moreiras, G.; Varela-Nieto, I.; Pajares, M.A. Folic acid deficiency induces premature hearing loss through mechanisms involving cochlear oxidative stress and impairment of homocysteine metabolism. FASEB J. 2015, 29, 418–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svingen, G.; Ueland, P.M.; Schartum-Hansen, H.; Pedersen, E.R.; Seifert, R.; Nygard, O.K. Metabolites in the choline oxidation pathway in relation to diabetes, glycemic control and insulin sensitivity among patients with angina pectoris. Atherosclerosis 2014, 235, e51. [Google Scholar] [CrossRef]
- Obeid, R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 2013, 5, 3481–3495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lever, M.; George, P.M.; Slow, S.; Bellamy, D.; Young, J.M.; Ho, M.; McEntyre, C.J.; Elmslie, J.L.; Atkinson, W.; Molyneux, S.L.; et al. Betaine and Trimethylamine-N-Oxide as Predictors of Cardiovascular Outcomes Show Different Patterns in Diabetes Mellitus: An Observational Study. PLoS ONE 2014, 9, e114969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejaz, A.; Martinez-Guino, L.; Goldfine, A.B.; Ribas-Aulinas, F.; De Nigris, V.; Ribo, S.; Gonzalez-Franquesa, A.; Garcia-Roves, P.M.; Li, E.; Dreyfuss, J.M.; et al. Dietary Betaine Supplementation Increases Fgf21 Levels to Improve Glucose Homeostasis and Reduce Hepatic Lipid Accumulation in Mice. Diabetes 2016, 65, 902–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Tang, W.H.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Binzak, B.; Vockley, J.; Jenkins, R.; Vockley, J. Structure and analysis of the human dimethylglycine dehydrogenase gene. Mol. Genet. Metab. 2000, 69, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z. Potential strategies to normalize the levels of homocysteine in chronic renal failure patients. Kidney Int. 2003, 63, S134–S136. [Google Scholar] [CrossRef] [Green Version]
- Svingen, G.; Ueland, P.; Pedersen, E.; Schartum-Hansen, H.; Seifert, R.; Ebbing, M.; Løland, K.; Tell, G.; Nygård, O. Plasma Dimethylglycine and Risk of Incident Acute Myocardial Infarction in Patients With Stable Angina Pectoris. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2041–2048. [Google Scholar] [CrossRef] [Green Version]
- Ey, J.; Schomig, E.; Taubert, D. Dietary sources and antioxidant effects of ergothioneine. J. Agric. Food Chem. 2007, 55, 6466–6474. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys Acta 2012, 1822, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.; Ottoson, F.; Hellstrand, S.; Ericson, U.; Orho-Melander, M.; Fernandez, C.; Melander, O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 2019, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Gibbons, H.; Rundle, M.; Frost, G.; McNulty, B.A.; Nugent, A.P.; Walton, J.; Flynn, A.; Brennan, L. The Relationship between Fish Intake and Urinary Trimethylamine-N-Oxide. Mol. Nutr. Food Res. 2020, 64, e1900799. [Google Scholar] [CrossRef] [PubMed]
- Al-Waiz, M.; Mitchell, S.C.; Idle, J.R.; Smith, R.L. The metabolism of 14C-labelled trimethylamine and its N-oxide in man. Xenobiotica 1987, 17, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Svensson, B.G.; Akesson, B.; Nilsson, A.; Paulsson, K. Urinary excretion of methylamines in men with varying intake of fish from the Baltic Sea. J. Toxicol. Environ. Health 1994, 41, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Corbin, K.D. Present Knowledge in Nutrition, 10th ed.; Erdman, J., Jr., MacDonald, I., Zeisel, S., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012; pp. 405–418. [Google Scholar]
- Rebouche, C. Carnitine. In Modern Nutrition in Health and Disease, 9th ed.; Shils, M., Olson, J., Shike, M., Ross, A., Eds.; Lippincott Williams and Wilkins: New York, NY, USA, 1999; pp. 505–512. [Google Scholar]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Park, J.E.; Miller, M.; Rhyne, J.; Wang, Z.; Hazen, S.L. Differential effect of short-term popular diets on TMAO and other cardio-metabolic risk markers. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 513–517. [Google Scholar] [CrossRef]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.H.; Siebenaller, J.F. Co-evolution of proteins and solutions: Protein adaptation versus cytoprotective micromolecules and their roles in marine organisms. J. Exp. Biol. 2015, 218, 1880–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treberg, J.R.; Driedzic, W.R. Elevated levels of trimethylamine oxide in deep-sea fish: Evidence for synthesis and intertissue physiological importance. J. Exp. Zool. 2002, 293, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pazos, I.M.; Gai, F. Microscopic insights into the protein-stabilizing effect of trimethylamine N-oxide (TMAO). Proc. Natl. Acad. Sci. USA 2014, 111, 8476–8481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennion, B.J.; Daggett, V. Counteraction of urea-induced protein denaturation by trimethylamine N-oxide: A chemical chaperone at atomic resolution. Proc. Natl. Acad. Sci. USA 2004, 101, 6433–6438. [Google Scholar] [CrossRef] [Green Version]
- Jackson-Atogi, R.; Sinha, P.K.; Rosgen, J. Distinctive solvation patterns make renal osmolytes diverse. Biophys J. 2013, 105, 2166–2174. [Google Scholar] [CrossRef] [Green Version]
- Hua, X.; Su, Z.; Deng, R.; Lin, J.; Li, D.Q.; Pflugfelder, S.C. Effects of L-carnitine, erythritol and betaine on pro-inflammatory markers in primary human corneal epithelial cells exposed to hyperosmotic stress. Curr. Eye Res. 2015, 40, 657–667. [Google Scholar] [CrossRef]
- Kuhn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; von Eckardstein, A.; Muller, D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017, 55, 261–268. [Google Scholar] [CrossRef] [Green Version]
- McEntyre, C.J.; Lever, M.; Chambers, S.T.; George, P.M.; Slow, S.; Elmslie, J.L.; Florkowski, C.M.; Lunt, H.; Krebs, J.D. Variation of betaine, N,N-dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann. Clin. Biochem. 2015, 52, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Ke, Y.; Li, D.; Zhao, M.; Liu, C.; Liu, J.; Zeng, A.; Shi, X.; Cheng, S.; Pan, B.; Zheng, L.; et al. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic. Biol. Med. 2018, 116, 88–100. [Google Scholar] [CrossRef]
- Razavi, A.C.; Potts, K.S.; Kelly, T.N.; Bazzano, L.A. Sex, gut microbiome, and cardiovascular disease risk. Biol Sex. Differ. 2019, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins (Basel) 2016, 8, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeravalli, S.; Karu, K.; Scott, F.; Fennema, D.; Phillips, I.R.; Shephard, E.A. Effect of Flavin-Containing Monooxygenase Genotype, Mouse Strain, and Gender on Trimethylamine N-oxide Production, Plasma Cholesterol Concentration, and an Index of Atherosclerosis. Drug Metab Dispos. 2018, 46, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkanoglu Ozcelik, A.; Can Demirdogen, B.; Demirkaya, S.; Adali, O. Flavin containing monooxygenase 3 genetic polymorphisms Glu158Lys and Glu308Gly and their relation to ischemic stroke. Gene 2013, 521, 116–121. [Google Scholar] [CrossRef]
- Zane, N.R.; Chen, Y.; Wang, M.Z.; Thakker, D.R. Cytochrome P450 and flavin-containing monooxygenase families: Age-dependent differences in expression and functional activity. Pediatr. Res. 2018, 83, 527–535. [Google Scholar] [CrossRef]
- Falls, J.G.; Blake, B.L.; Cao, Y.; Levi, P.E.; Hodgson, E. Gender differences in hepatic expression of flavin-containing monooxygenase isoforms (FMO1, FMO3, and FMO5) in mice. J. Biochem. Toxicol. 1995, 10, 171–177. [Google Scholar] [CrossRef]
- Mayr, M.; Chung, Y.L.; Mayr, U.; Yin, X.; Ly, L.; Troy, H.; Fredericks, S.; Hu, Y.; Griffiths, J.R.; Xu, Q. Proteomic and metabolomic analyses of atherosclerotic vessels from apolipoprotein E-deficient mice reveal alterations in inflammation, oxidative stress, and energy metabolism. Arter. Thromb. Vasc. Biol. 2005, 25, 2135–2142. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Warrier, M.; Shih, D.M.; Burrows, A.C.; Ferguson, D.; Gromovsky, A.D.; Brown, A.L.; Marshall, S.; McDaniel, A.; Schugar, R.C.; Wang, Z.; et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015, 10, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Ling, A.V.; Manthena, P.V.; Gearing, M.E.; Graham, M.J.; Crooke, R.M.; Croce, K.J.; Esquejo, R.M.; Clish, C.B.; Morbid Obesity Study, G.; et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 2015, 6, 6498. [Google Scholar] [CrossRef]
- Shih, D.M.; Wang, Z.; Lee, R.; Meng, Y.; Che, N.; Charugundla, S.; Qi, H.; Wu, J.; Pan, C.; Brown, J.M.; et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res. 2015, 56, 22–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.Y.; Clark, J.E.; Williams, D.E. Induction of flavin-containing monooxygenase (FMO B) in rabbit lung and kidney by sex steroids and glucocorticoids. Arch. Biochem. Biophys 1993, 302, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson-Cohen, C.; Newitt, R.; Shen, D.D.; Rettie, A.E.; Kestenbaum, B.R.; Himmelfarb, J.; Yeung, C.K. Association of FMO3 Variants and Trimethylamine N-Oxide Concentration, Disease Progression, and Mortality in CKD Patients. PLoS ONE 2016, 11, e0161074. [Google Scholar] [CrossRef]
- Prokopienko, A.J.; West, R.E., 3rd; Schrum, D.P.; Stubbs, J.R.; Leblond, F.A.; Pichette, V.; Nolin, T.D. Metabolic Activation of Flavin Monooxygenase-mediated Trimethylamine-N-Oxide Formation in Experimental Kidney Disease. Sci. Rep. 2019, 9, 15901. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.; Prokopienko, A.J.; West, R.E., 3rd; Nolin, T.D.; Stubbs, J.R. Decreased Kidney Function Is Associated with Enhanced Hepatic Flavin Monooxygenase Activity and Increased Circulating Trimethylamine N-Oxide Concentrations in Mice. Drug Metab. Dispos 2018, 46, 1304–1309. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.N.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Lu, P.C.; Tain, Y.L. Association of Trimethylamine, Trimethylamine N-oxide, and Dimethylamine with Cardiovascular Risk in Children with Chronic Kidney Disease. J. Clin. Med. 2020, 9, 336. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.N.; Lu, P.C.; Lo, M.H.; Lin, I.C.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Gut Microbiota-Dependent Trimethylamine N-Oxide Pathway Associated with Cardiovascular Risk in Children with Early-Stage Chronic Kidney Disease. Int. J. Mol. Sci 2018, 19, 3699. [Google Scholar] [CrossRef] [Green Version]
- Swidsinski, A.; Loening-Baucke, V.; Schulz, S.; Manowsky, J.; Verstraelen, H.; Swidsinski, S. Functional anatomy of the colonic bioreactor: Impact of antibiotics and Saccharomyces boulardii on bacterial composition in human fecal cylinders. Syst. Appl. Microbiol. 2016, 39, 67–75. [Google Scholar] [CrossRef]
- Rath, S.; Rud, T.; Pieper, D.H.; Vital, M. Potential TMA-Producing Bacteria Are Ubiquitously Found in Mammalia. Front. Microbiol. 2019, 10, 2966. [Google Scholar] [CrossRef]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergeron, N.; Williams, P.T.; Lamendella, R.; Faghihnia, N.; Grube, A.; Li, X.; Wang, Z.; Knight, R.; Jansson, J.K.; Hazen, S.L.; et al. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br. J. Nutr. 2016, 116, 2020–2029. [Google Scholar] [CrossRef] [Green Version]
- Genoni, A.; Christophersen, C.T.; Lo, J.; Coghlan, M.; Boyce, M.C.; Bird, A.R.; Lyons-Wall, P.; Devine, A. Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, L.E.; Djuric, Z.; Angiletta, C.J.; Mitchell, C.M.; Baugh, M.E.; Davy, K.P.; Neilson, A.P. A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food Funct. 2019, 10, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Muller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Invest. 2019, 129, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. (London) 2010, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Warmbrunn, M.V.; Herrema, H.; Aron-Wisnewsky, J.; Soeters, M.R.; Van Raalte, D.H.; Nieuwdorp, M. Gut microbiota: A promising target against cardiometabolic diseases. Expert Rev. Endocrinol. Metab. 2020, 15, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- More, M.I.; Swidsinski, A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis—A review. Clin. Exp. Gastroenterol. 2015, 8, 237–255. [Google Scholar] [CrossRef] [Green Version]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.K.; Chen, C.C.; Liu, P.Y.; Panyod, S.; Liao, B.Y.; Chen, P.C.; Kao, H.L.; Kuo, H.C.; Kuo, C.H.; Chiu, T.H.T.; et al. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut 2019, 68, 1439–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zhang, J.; Cai, S.; Dong, J.; Yang, J.Y.; Chen, Z. Metabonomics studies of intact hepatic and renal cortical tissues from diabetic db/db mice using high-resolution magic-angle spinning 1H NMR spectroscopy. Anal. Bioanal. Chem. 2009, 393, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Teft, W.A.; Morse, B.L.; Choi, Y.H.; Woolsey, S.; DeGorter, M.K.; Hegele, R.A.; Tirona, R.G.; Kim, R.B. Trimethylamine-N-oxide: A Novel Biomarker for the Identification of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 3620–3630. [Google Scholar] [CrossRef]
- Yin, J.; Liao, S.X.; He, Y.; Wang, S.; Xia, G.H.; Liu, F.T.; Zhu, J.J.; You, C.; Chen, Q.; Zhou, L.; et al. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.Y.; Xia, G.H.; Lu, J.Q.; Chen, M.X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.W.; Yin, J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7, 1445. [Google Scholar] [CrossRef]
- Sitkin, S.I.; Tkachenko, E.I.; Vakhitov, T.Y. Metabolic Dysbiosis of the Gut Microbiota and Its Biomarkers. Eksp Klin. Gastroenterol. 2016, 12, 6–29. [Google Scholar]
- Ahmadmehrabi, S.; Tang, W.H.W. Gut microbiome and its role in cardiovascular diseases. Curr. Opin. Cardiol. 2017, 32, 761–766. [Google Scholar] [CrossRef]
- Cigarran Guldris, S.; Gonzalez Parra, E.; Cases Amenos, A. Gut microbiota in chronic kidney disease. Nefrologia 2017, 37, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Jazani, N.H.; Savoj, J.; Lustgarten, M.; Lau, W.L.; Vaziri, N.D. Impact of Gut Dysbiosis on Neurohormonal Pathways in Chronic Kidney Disease. Diseases 2019, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Verhoog, S.; Taneri, P.E.; Roa Diaz, Z.M.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review. Nutrients 2019, 11, 1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhibber-Goel, J.; Singhal, V.; Parakh, N.; Bhargava, B.; Sharma, A. The Metabolite Trimethylamine-N-Oxide is an Emergent Biomarker of Human Health. Curr Med. Chem. 2017, 24, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Ulrich, C.M.; Neuhouser, M.L.; Malysheva, O.; Bailey, L.B.; Xiao, L.; Brown, E.C.; Cushing-Haugen, K.L.; Zheng, Y.; Cheng, T.Y.; et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014, 74, 7442–7452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.; Wang, Z.; Shrestha, K.; Borowski, A.G.; Wu, Y.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card Fail. 2015, 21, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Obeid, R.; Awwad, H.M.; Rabagny, Y.; Graeber, S.; Herrmann, W.; Geisel, J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am. J. Clin. Nutr. 2016, 103, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Ottiger, M.; Nickler, M.; Steuer, C.; Odermatt, J.; Huber, A.; Christ-Crain, M.; Henzen, C.; Hoess, C.; Thomann, R.; Zimmerli, W.; et al. Trimethylamine-N-oxide (TMAO) predicts fatal outcomes in community-acquired pneumonia patients without evident coronary artery disease. Eur. J. Intern. Med. 2016, 36, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Senthong, V.; Li, X.S.; Hudec, T.; Coughlin, J.; Wu, Y.; Levison, B.; Wang, Z.; Hazen, S.L.; Tang, W.H. Plasma Trimethylamine N-Oxide, a Gut Microbe-Generated Phosphatidylcholine Metabolite, Is Associated With Atherosclerotic Burden. J. Am. Coll. Cardiol. 2016, 67, 2620–2628. [Google Scholar] [CrossRef]
- Senthong, V.; Wang, Z.; Li, X.S.; Fan, Y.; Wu, Y.; Tang, W.H.; Hazen, S.L. Intestinal Microbiota-Generated Metabolite Trimethylamine-N-Oxide and 5-Year Mortality Risk in Stable Coronary Artery Disease: The Contributory Role of Intestinal Microbiota in a COURAGE-Like Patient Cohort. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Senthong, V.; Wang, Z.; Fan, Y.; Wu, Y.; Hazen, S.L.; Tang, W.H. Trimethylamine N-Oxide and Mortality Risk in Patients With Peripheral Artery Disease. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Suzuki, T.; Heaney, L.M.; Bhandari, S.S.; Jones, D.J.; Ng, L.L. Trimethylamine N-oxide and prognosis in acute heart failure. Heart 2016, 102, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Heaney, L.M.; Jones, D.J.; Ng, L.L. Trimethylamine N-oxide and Risk Stratification after Acute Myocardial Infarction. Clin. Chem. 2017, 63, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Li, X.S.; Fan, Y.; Li, D.S.; Wu, Y.; Hazen, S.L. Increased Trimethylamine N-Oxide Portends High Mortality Risk Independent of Glycemic Control in Patients with Type 2 Diabetes Mellitus. Clin. Chem. 2017, 63, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, J.; Xie, L.; Zhao, B.X.; Li, Y.; Qiu, B.; Zhu, F.; Li, G.F.; He, M.; Wang, Y.; Wang, B.; et al. Serum Trimethylamine N-Oxide Concentration Is Positively Associated With First Stroke in Hypertensive Patients. Stroke 2018, 49, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Hering, D.; Mosieniak, G.; Bielak-Zmijewska, A.; Pilz, M.; Konwerski, M.; Gasecka, A.; Kapłon-Cieślicka, A.; Filipiak, K.; Sikora, E.; et al. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins (Basel) 2019, 11, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiner, M.F.; Muller, D.; Gobbato, S.; Stalder, O.; Limacher, A.; Bonetti, N.R.; Pasterk, L.; Mean, M.; Rodondi, N.; Aujesky, D.; et al. Gut microbiota-dependent trimethylamine-N-oxide (TMAO) shows a U-shaped association with mortality but not with recurrent venous thromboembolism. Thromb Res. 2019, 174, 40–47. [Google Scholar] [CrossRef]
- Hai, X.; Landeras, V.; Dobre, M.A.; DeOreo, P.; Meyer, T.W.; Hostetter, T.H. Mechanism of Prominent Trimethylamine Oxide (TMAO) Accumulation in Hemodialysis Patients. PLoS ONE 2015, 10, e0143731. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Johansen, K.L.; Chertow, G.M.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Dwyer, T.; Chassy, A.W.; Fiehn, O. Associations of Trimethylamine N-Oxide With Nutritional and Inflammatory Biomarkers and Cardiovascular Outcomes in Patients New to Dialysis. J. Ren. Nutr. 2015, 25, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Missailidis, C.; Hallqvist, J.; Qureshi, A.R.; Barany, P.; Heimburger, O.; Lindholm, B.; Stenvinkel, P.; Bergman, P. Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PLoS ONE 2016, 11, e0141738. [Google Scholar] [CrossRef] [Green Version]
- Al-Obaide, M.A.I.; Singh, R.; Datta, P.; Rewers-Felkins, K.A.; Salguero, M.V.; Al-Obaidi, I.; Kottapalli, K.R.; Vasylyeva, T.L. Gut Microbiota-Dependent Trimethylamine-N-oxide and Serum Biomarkers in Patients with T2DM and Advanced CKD. J. Clin. Med. 2017, 6, 86. [Google Scholar] [CrossRef] [Green Version]
- Shafi, T.; Powe, N.R.; Meyer, T.W.; Hwang, S.; Hai, X.; Melamed, M.L.; Banerjee, T.; Coresh, J.; Hostetter, T.H. Trimethylamine N-Oxide and Cardiovascular Events in Hemodialysis Patients. J. Am. Soc. Nephrol. 2017, 28, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Ma, W.; DiDonato, J.A.; Sun, Q.; Rimm, E.B.; Hu, F.B.; Rexrode, K.M.; Manson, J.E.; Qi, L. Long-Term Changes in Gut Microbial Metabolite Trimethylamine N-Oxide and Coronary Heart Disease Risk. J. Am. Coll Cardiol. 2020, 75, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhong, C.; Li, S.; Sun, T.; Huang, H.; Chen, X.; Zhu, Y.; Hu, X.; Peng, X.; Zhang, X.; et al. Plasma concentration of trimethylamine-N-oxide and risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2018, 108, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Li, J.; Cao, Y.-F.; Li, S.-N.; Shao, P.; Leng, J.; Li, W.; Liu, J.; Yang, K.; Ma, R.; et al. Trimethylamine N-Oxide Metabolites in Early Pregnancy and Risk of Gestational Diabetes: A Nested Case-Control Study. J. Clin. Endocrinol. Metab. 2019, 104, 5529–5539. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Yuzefpolskaya, M.; Nandakumar, R.; Colombo, P.C.; Demmer, R.T. Plasma Trimethylamine-N-oxide and impaired glucose regulation: Results from The Oral Infections, Glucose Intolerance and Insulin Resistance Study (ORIGINS). PLoS ONE 2020, 15, e0227482. [Google Scholar] [CrossRef]
- Chou, R.H.; Chen, C.Y.; Chen, I.C.; Huang, H.L.; Lu, Y.W.; Kuo, C.S.; Chang, C.C.; Huang, P.H.; Chen, J.W.; Lin, S.J. Trimethylamine N-Oxide, Circulating Endothelial Progenitor Cells, and Endothelial Function in Patients with Stable Angina. Sci. Rep. 2019, 9, 4249. [Google Scholar] [CrossRef]
- Fu, B.C.; Hullar, M.A.J.; Randolph, T.W.; Franke, A.A.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Shepherd, J.A.; Madeleine, M.M.; Le Marchand, L.; et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Shepshelovich, J.; Goldstein-Magal, L.; Globerson, A.; Yen, P.M.; Rotman-Pikielny, P.; Hirschberg, K. Protein synthesis inhibitors and the chemical chaperone TMAO reverse endoplasmic reticulum perturbation induced by overexpression of the iodide transporter pendrin. J. Cell Sci. 2005, 118, 1577–1586. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Rodriguez, E.; Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Fernandez-Fernandez, B.; Kanbay, M.; Tejedor, A.; Lazaro, A.; Ruiz-Ortega, M.; et al. Impact of Altered Intestinal Microbiota on Chronic Kidney Disease Progression. Toxins (Basel) 2018, 10, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Gonzalez-Parra, E.; Sanz, A.B.; Ortiz, A.; Sanchez-Nino, M.D. Nutrients Turned into Toxins: Microbiota Modulation of Nutrient Properties in Chronic Kidney Disease. Nutrients 2017, 9, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, J.; Dou, P.; Gao, M.; Kong, X.; Li, C.; Liu, Z.; Huang, T. Assessment of Causal Direction Between Gut Microbiota-Dependent Metabolites and Cardiometabolic Health: Abi-Directional Mendelian Randomisation Analysis. Diabetes 2019, 68, 1747–1755. [Google Scholar] [CrossRef]
- Mair, R.D.; Sirich, T.L.; Meyer, T.W. Uremic Toxin Clearance and Cardiovascular Toxicities. Toxins (Basel) 2018, 10, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boini, K.M.; Hussain, T.; Li, P.L.; Koka, S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef]
- Collins, H.L.; Drazul-Schrader, D.; Sulpizio, A.C.; Koster, P.D.; Williamson, Y.; Adelman, S.J.; Owen, K.; Sanli, T.; Bellamine, A. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis 2016, 244, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Querio, G.; Antoniotti, S.; Levi, R.; Gallo, M.P. Trimethylamine N-Oxide Does Not Impact Viability, ROS Production, and Mitochondrial Membrane Potential of Adult Rat Cardiomyocytes. Int. J. Mol. Sci. 2019, 20, 3045. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151.e1145. [Google Scholar] [CrossRef]
- Ufnal, M.; Jazwiec, R.; Dadlez, M.; Drapala, A.; Sikora, M.; Skrzypecki, J. Trimethylamine-N-oxide: A carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can. J. Cardiol. 2014, 30, 1700–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Chen, Y.; Gua, C.; Li, X. Elevated Circulating Trimethylamine N-Oxide Levels Contribute to Endothelial Dysfunction in Aged Rats through Vascular Inflammation and Oxidative Stress. Front. Physiol. 2017, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Snell, K. Renal disease of the rat. In Pathology of Laboratory Rats and Mice; Cotchin, E., Roe, F.J., Eds.; Blackwell Scientific: Oxford, UK, 1967; pp. 105–147. [Google Scholar]
- Yang, W.; Zhang, S.; Zhu, J.; Jiang, H.; Jia, D.; Ou, T.; Qi, Z.; Zou, Y.; Qian, J.; Sun, A.; et al. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J. Mol. Cell Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Li, S.; Koh, Y.C.; Wu, J.C.; Yang, M.J.; Ho, C.T.; Pan, M.H. Oolong Tea Extract and Citrus Peel Polymethoxyflavones Reduce Transformation of l-Carnitine to Trimethylamine-N-Oxide and Decrease Vascular Inflammation in l-Carnitine Feeding Mice. J. Agric. Food Chem. 2019, 67, 7869–7879. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Zhang, X.; Wang, J. Reductions in gut microbiotaderived metabolite trimethylamine Noxide in the circulation may ameliorate myocardial infarctioninduced heart failure in rats, possibly by inhibiting interleukin8 secretion. Mol. Med. Rep. 2019, 20, 779–786. [Google Scholar] [CrossRef]
- Chen, Y.; Weng, Z.; Liu, Q.; Shao, W.; Guo, W.; Chen, C.; Jiao, L.; Wang, Q.; Lu, Q.; Sun, H.; et al. FMO3 and its metabolite TMAO contribute to the formation of gallstones. Biochim. Biophys Acta Mol. Basis Dis. 2019, 1865, 2576–2585. [Google Scholar] [CrossRef]
- John Hopkins University. Animal Care and Use Comittee.-The Mouse. Available online: http://web.jhu.edu/animalcare/procedures/mouse.html (accessed on 15 August 2019).
- The Jackson Laboratory. Body Weight Information fo B6.129P2-APOETM1UNC/J (002052). Available online: https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-002052 (accessed on 25 August 2019).
- Zhao, Y.; Yang, N.; Gao, J.; Li, H.; Cai, W.; Zhang, X.; Ma, Y.; Niu, X.; Yang, G.; Zhou, X.; et al. The Effect of Different l-Carnitine Administration Routes on the Development of Atherosclerosis in ApoE Knockout Mice. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Koh, Y.C.; Li, S.; Chen, P.Y.; Wu, J.C.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Prevention of Vascular Inflammation by Pterostilbene via Trimethylamine-N-Oxide Reduction and Mechanism of Microbiota Regulation. Mol. Nutr. Food Res. 2019, e1900514. [Google Scholar] [CrossRef]
- Liu, M.; Han, Q.; Yang, J. Trimethylamine-N-oxide (TMAO) increased aquaporin-2 expression in spontaneously hypertensive rats. Clin. Exp. Hypertens 2019, 41, 312–322. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Invest. 2019, 99, 346–357. [Google Scholar] [CrossRef]
- Sun, G.; Yin, Z.; Liu, N.; Bian, X.; Yu, R.; Su, X.; Zhang, B.; Wang, Y. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochem. Biophys Res. Commun. 2017, 493, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Canlet, C.; Delplanque, B.; Agnani, G.; Lairon, D.; Gottardi, G.; Bencharif, K.; Gripois, D.; Thaminy, A.; Paris, A. 1H NMR metabonomics can differentiate the early atherogenic effect of dairy products in hyperlipidemic hamsters. Atherosclerosis 2009, 206, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Liu, X.; Xu, J.; Xue, C.; Xue, Y.; Wang, Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J. Biosci Bioeng 2014, 118, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Empl, M.T.; Kammeyer, P.; Ulrich, R.; Joseph, J.F.; Parr, M.K.; Willenberg, I.; Schebb, N.H.; Baumgartner, W.; Rohrdanz, E.; Steffen, C.; et al. The influence of chronic L-carnitine supplementation on the formation of preneoplastic and atherosclerotic lesions in the colon and aorta of male F344 rats. Arch. Toxicol. 2015, 89, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Weinert, C.H.; Empl, M.T.; Kruger, R.; Frommherz, L.; Egert, B.; Steinberg, P.; Kulling, S.E. The influence of a chronic L-carnitine administration on the plasma metabolome of male Fischer 344 rats. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Huc, T.; Drapala, A.; Gawrys, M.; Konop, M.; Bielinska, K.; Zaorska, E.; Samborowska, E.; Wyczalkowska-Tomasik, A.; Paczek, L.; Dadlez, M.; et al. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1805–H1820. [Google Scholar] [CrossRef]
- Lindskog Jonsson, A.; Caesar, R.; Akrami, R.; Reinhardt, C.; Fak Hallenius, F.; Boren, J.; Backhed, F. Impact of Gut Microbiota and Diet on the Development of Atherosclerosis in Apoe(-/-) Mice. Arter. Thromb Vasc. Biol. 2018, 38, 2318–2326. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Xin, F.Z.; Zhou, D.; Xue, Y.Q.; Liu, X.L.; Yang, R.X.; Pan, Q.; Fan, J.G. Trimethylamine N-oxide attenuates high-fat high-cholesterol diet-induced steatohepatitis by reducing hepatic cholesterol overload in rats. World J. Gastroenterol. 2019, 25, 2450–2462. [Google Scholar] [CrossRef]
- Aldana-Hernandez, P.; Leonard, K.A.; Zhao, Y.Y.; Curtis, J.M.; Field, C.J.; Jacobs, R.L. Dietary Choline or Trimethylamine N-oxide Supplementation Does Not Influence Atherosclerosis Development in Ldlr-/- and Apoe-/- Male Mice. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Jawien, J.; Nastalek, P.; Korbut, R. Mouse models of experimental atherosclerosis. J. Physiol. Pharmacol. 2004, 55, 503–517. [Google Scholar]
- Bennett, B.J.; Davis, R.C.; Civelek, M.; Orozco, L.; Wu, J.; Qi, H.; Pan, C.; Packard, R.R.; Eskin, E.; Yan, M.; et al. Genetic Architecture of Atherosclerosis in Mice: A Systems Genetics Analysis of Common Inbred Strains. PLoS Genet. 2015, 11, e1005711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, S.D.; Burton, C.; Hernandez, M.; Hassing, H.; Montenegro, J.; Mundt, S.; Patel, S.; Card, D.J.; Hermanowski-Vosatka, A.; Bergstrom, J.D.; et al. Infectious agents are not necessary for murine atherogenesis. J. Exp. Med. 2000, 191, 1437–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepankova, R.; Tonar, Z.; Bartova, J.; Nedorost, L.; Rossman, P.; Poledne, R.; Schwarzer, M.; Tlaskalova-Hogenova, H. Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J. Atheroscler Thromb. 2010, 17, 796–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Tain, Y.L. Targeting on Gut Microbial Metabolite Trimethylamine-N-Oxide and Short-Chain Fatty Acid to Prevent Maternal High-Fructose-Diet-Induced Developmental Programming of Hypertension in Adult Male Offspring. Mol. Nutr. Food Res. 2019, 63, e1900073. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Lavie, C.J.; Fares, H.; Menezes, A.R.; O’Keefe, J.H. L-carnitine in the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Mayo. Clin. Proc. 2013, 88, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Qu, H.; Yang, Z.; Rong, J.; Cai, W.; Zhou, H. Efficacy and Safety of L-Carnitine Treatment for Chronic Heart Failure: A Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2017, 2017, 6274854. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Jiang, W.; Chen, G.; Zhu, W.; Ding, W.; Ge, Z.; Tan, Y.; Ma, T.; Cui, G. L-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2017, 26, 333–338. [Google Scholar] [CrossRef]
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; Van Horn, L.; Dyer, A.R.; Greenland, P. Accumulated evidence on fish consumption and coronary heart disease mortality: A meta-analysis of cohort studies. Circulation 2004, 109, 2705–2711. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [Green Version]
- Takata, Y.; Zhang, X.; Li, H.; Gao, Y.T.; Yang, G.; Gao, J.; Cai, H.; Xiang, Y.B.; Zheng, W.; Shu, X.O. Fish intake and risks of total and cause-specific mortality in 2 population-based cohort studies of 134,296 men and women. Am. J. Epidemiol. 2013, 178, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aadland, E.K.; Lavigne, C.; Graff, I.E.; Eng, O.; Paquette, M.; Holthe, A.; Mellgren, G.; Jacques, H.; Liaset, B. Lean-seafood intake reduces cardiovascular lipid risk factors in healthy subjects: Results from a randomized controlled trial with a crossover design. Am. J. Clin. Nutr. 2015, 102, 582–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhassan, A.; Young, J.; Lean, M.E.J.; Lara, J. Consumption of fish and vascular risk factors: A systematic review and meta-analysis of intervention studies. Atherosclerosis 2017, 266, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
| First Author, Year and Citation | Number of Subjects | Study Population | Mean Age (Years) | TMAO Level Median (or Mean) (µM) | TMAO Interquartile Range (µM) | Increased Disease Severity with Higher TMAO Levels |
|---|---|---|---|---|---|---|
| Tang, 2013 [136] | 720 | Patients with stable heart failure undergoing cardiac evaluation | 63.0 | 5.0 | 3.0–8.5 | yes |
| Koeth 2013 [94] | 2595 | Patients undergoing elective cardiac evaluation, GeneBank study | 62 | 4.6 | -- | yes |
| Bae 2014 [150] | 835 | Postmenopausal women with colorectal cancer within the Women’s Health Initiative Observational Study | 66 | 4.0 | 2.9–6.0 | yes |
| 835 | Postmenopausal women healthy controls | 67 | 3.8 | 2.6–5.7 | NA | |
| Lever 2014 [80] | 79 | Coronary Disease Cohort Study (CDCS), participants with T2D | 74.0 | 7.5 | 4.4–12.1 | yes |
| 396 | CDCS participants without T2D | 68.0 | 4.8 | 3.0–9.1 | yes | |
| Wang 2014 [82] | 3903 | Patients undergoing elective diagnostic coronary angiography | 63 | 3.7 | 2.4–6.2 | yes |
| Tang 2015 [151] | 112 | Patients with chronic systolic HF | 57.0 | 5.8 | 3.6–12.1 | yes |
| Obeid 2016 [152] | 283 | Subjects participating in a diabetes case-control study or a vitamin-supplementation trial | 66.7 | 4.36 ** | Not indicated | yes |
| Ottiger 2016 [153] | 317 | Community-acquired pneumonia patients | 72.0 | 3.0 | 1.7–5.4 | Yes |
| Rohrmann 2016 [132] | 104 | Healthy men | 50 | (geometric mean 2.55 | (95% CI 2.17–2.99) | Only TNFα but not CRP and IL-6 |
| 167 | Healthy women | 44 | (geometric mean 2.52) | (95% CI 2.22–2.86) | ||
| Senthong 2016 [154] | 353 | Atherosclerotic CAD patients | 65.0 | 5.5 | 3.4–9.8 | yes |
| Senthong 2016 [155] | 2235 | Patients with stable CAD who underwent elective coronary angiography | 63.0 | 3.8 | 2.5–6.5 | yes |
| Senthong 2016 [156] | 821 | Patients with peripheral artery disease | 66 | 4.8 | 2.9–8 | yes |
| Suzuki 2016 [157] | 972 | Patients with acute HF | 78.0 | 5.6 | 3.4–10.5 | yes |
| Suzuki 2017 [158] | 1079 | Acute MI patients | 67 | 3.7 | 4.6–6.4 | yes |
| Schugar 2017 [120] | 102 | Patients with elective cardiac risk factor evaluation with T2D | 55.9 | 4.8 | 3.3–7.7 | yes |
| 333 | Patients with elective cardiac risk factor evaluation without T2D | 49.7 | 3.2 | 2.2–5.1 | NA | |
| Tang 2017 [159] | 1216 | Patients with T2D who underwent elective diagnostic coronary angiography | 64.4 | 4.4 | 2.8–7.7 | yes |
| 300 | Apparently healthy controls | 53.6 | 3.6 | 2.3–5.7 | yes | |
| Nie 2018 [160] | 622 | Hypertensive stroke patients | 62.2 | 2.5 | 1.6–4.0 | yes |
| 622 | Matched controls | 62.2 | 2.3 | 1.4–3.7 | NA | |
| Jaworska 2019 [161] | 19 | Cardiovascular patients qualified for aortic valve replacement | 74.5 | 5.5 ± 0.6 | -- | No |
| 9 | Healthy control | 38.9 | 3.6 ± 0.4 | -- | NA | |
| Reiner 2019 [162] | 859 | Venous thromboembolism patients | 75.0 | -- | 2.28–6.57 | U-shaped optimum level at 4 µM |
| Hai 2015 [163] | 7 | Hemodialysis patients | Not indicated | (mean 77 ± 26) | Not indicated | Not indicated |
| 6 | Control subjects | Not indicated | (mean 2 ± 1) | Not indicated | NA | |
| Kaysen 2015 [164] | 235 | Patients new to hemodialysis | 61.8 | 43 | 27.5–66.6 | no |
| NA | Two commercially available pooled control samples | Not indicated | (mean 1.41 ± 0.49) | NA | NA | |
| Tang 2015 [165] | 521 | CKD patients | 70 | 7.9 | 5.2–12.4 | yes |
| 3166 | Control subjects | 62 | 3.4 | 2.3–5.3 | NA | |
| Missailidis 2016 [166] | 56 | CDK 3‒4 patients | 42 | 14.6 * | 5.6–71.2 | yes |
| 55 | CDK 5 patients on hemodialysis starting renal replacement | 74 | 73.5 * | 26.4–191.0 | ||
| 80 | Controls | 62 | 5.8 * | 3.1–13.3 | NA | |
| Al-Obaide 2017 [167] | 20 | Diabetic CKD patients | 64.4 | 12.5 (mean 20.2) | 9.9–22.9 | yes |
| 20 | Healthy controls | 54.3 | 1.2 (mean 2.4) | 0.68–4.5 | NA | |
| Shafi 2017 [168] | 1232 | Hemodialysis patients, 35% Caucasians and 65% African American | 58 | White 87 Black 88 (total mean 101.9 ± 63.9) | 63–120 62–125 | Yes in Caucasians No in African Americans |
| Literature Source | Test System | Treatment | Outcome | Model and Supplementation | Daily Dosage of TMAO or Precursors * | Plasma TMAO Level * (or Control Level) |
|---|---|---|---|---|---|---|
| Wang 2011 [115] | Atherosclerosis-prone male and female mice (apoE−/−) | Control or diet (around 0.08% choline) or diet with 0.5% or 1.0% additional choline or 0.12% TMAO; from weaning–20 weeks of age (16 weeks treatment) | Choline supplementation groups resulted in increased TMAO levels, with correlation between plasma TMAO and atherosclerotic plaque size. Female mice had higher FMO activity and higher TMAO levels upon supplementation, however plaques were observed similarly in both genders, introducing a confounding factor. Parallel examination of plasma cholesterol, triglycerides, lipoproteins, glucose levels, and hepatic triglyceride content in the mice failed to show significant increases that could account for the enhanced atherosclerosis. | apoE−/− mice; high choline/TMAO supplementation | 800 or 1600 mg choline/kg ** or 192 mg TMAO/kg ** | Males/females w/0.5% choline: 13 µM/140 µM; w/1% choline: 24 µM/215 µM w/0.12% TMAO: 23 µM/70 µM (5 µM/10 µM controls) |
| Atherosclerosis-prone mice (apoE−/−) Gender not clearly indicated (Figure S7b) | Control or diet or diet with 1.0% additional choline or 0.12% or TMAO or 1% betaine; for at least 3 weeks half of them +antibiotics, for 3 weeks | Mice supplemented with either choline, TMAO, or betaine showed enhanced levels of scavenger receptors CD36 and SR-A1 (which bind to lipoproteins), markers for activated macrophages. Increase in scavenger receptors was inhibited by antibiotics. Mice on the control diet showed modest foam cell formation; 1% choline supplemented diet resulted in enhanced lipid-laden macrophages (foam cells). Foam cell formation was inhibited by antibiotics. | High choline or betaine supplementation | 1600 mg choline/kg ** or 192 mg TMAO/kg ** or 1600 mg betaine/kg ** | 50 µM (4 h after challenge w/d9(trimethyl)-choline (baseline 0 µM) TMAO conc. after other supplementation not indicated | |
| Koeth 2013 [94] | Atherosclerosis-prone female mice (apoE−/−) | 1.3% L-carnitine in drinking water (or control water) +/− antibiotics; standard chow, for at least 4 weeks | Significant increase in plasma TMA and TMAO in the L-carnitine group without antibiotics; negligible concentrations in the other groups. L-carnitine did not increase other pro-atherogenic biomarkers (plasma lipids, lipoproteins, glucose, or insulin levels) in the plasma. 1.8-fold increase of aortic root lesion size in the L-carnitine group without antibiotics compared to the control, other groups similar to control. The difference between supplemented group and control was only 3 mice. | apoE−/− mice; high L-carnitine supplementation | 2080 mg L-carnitine/kg ** | 130 µM (10 µM) |
| Atherosclerosis-prone female mice (apoE−/−) | 1.3% L-carnitine diet or 1.3% choline in diet; +/− antibiotics; standard chow, for 8 weeks | TMA/TMAO production abolished with antibiotics. TMAO was shown to inhibit reverse cholesterol transport in aortic cells. | apoE−/− mice; high L-carnitine or choline supplementation | 2080 mg L-carnitine/kg ** 2080 mg choline/kg ** | 190 µM-w/carnitine 110 µM-w/choline (10 µM) | |
| Atherosclerosis-prone female mice (apoE−/−) | Supplementation of diet with 0.12% TMAO for 4 weeks | TMAO was shown to inhibit reverse cholesterol transport and reduce the expression of Cyp7a1 (enzyme involved in bile acid synthesis and cholesterol metabolism). | apoE−/−; TMAO supplementation | 192 mg TMAO/kg ** | 35 µM (9 µM) | |
| Ufnal 2014 [188] | Rats gender not relevant | Osmotic pump infusion with saline, TMAO, low-dose Angiotensin II, or both | TMAO did not affect blood pressure in normotensive animals. However, it prolonged the hypertensive effect of Angiotensin II. | High TMAO/angiotensin | Osmotic pump infusion with TMAO | TMAO: 58 µM (0.57 µM) |
| Seldin 2016 [182] | Female LDLR(-/-) mice | “Chow with 1.3% choline provided ad libitum in drinking water” or control | Aortas of LDLR(-/-) mice fed a choline diet showed elevated inflammatory gene expression compared with controls. | LDLr(-/-); high choline supplementation | 2080 mg choline/kg ** | 55 µM (9 µM) |
| Tang 2015 [165] | Male mice | Diet with 1.0% choline or 0.12% TMAO, or control for 6 weeks or 16 weeks | Elevated TMAO levels were associated with increases in tubulointerstitial fibrosis, collagen deposition and phosphorylation of Smad3 (regulator of the pro-fibrotic TGF-β/Smad3 signaling pathway). TMAO-fed and choline-fed mice experienced increased kidney injury marker-1. After 16 weeks increased serum cystatin C levels compared to chow-fed mice were observed. Altogether, dose-dependent relationships were noted between plasma TMAO levels and monitored indices of renal histopathological and functional impairment. | High choline or TMAO diet | 1600 mg choline/kg ** or 192 mg TMAO/kg ** | 100 µM-w/choline 40 µM-w/TMAO (5 µM control) |
| Zhu 2016 [183] | Female mice | Diet supplemented with either 0.12% TMAO or 1% choline or control; antibiotic control for 6 weeks | Choline or TMAO supplementation led to increased TMAO levels. Ex vivo platelet aggregation (induced by ADP stimulation) was significantly increased in these groups; antibiotics suppressed this effect for the choline supplemented mice (not the TMAO supplemented mice). | High choline supplementation | 192 mg TMAO/kg ** 1600 mg choline/kg | Not indicated |
| Boini 2017 [184] | Male mice with partially ligated carotid artery | Osmotic pump infusion with TMAO (dosage not apparent) or control for 2 weeks post ligation | Mice with partially ligated carotid artery and infused with TMAO for 2 weeks had increased inflammasome formation. | Partially ligated carotid artery; TMAO infusion | Osmotic pump infusion with TMAO (dosage not apparent) | Not indicated |
| Li 2017 [189] | Male Fischer 344 rats (Most Fischer 344 rats older than 2 years exhibit small local areas of nephritis; less than 25% show severe nephritis [190]) | Young or old (22 months) rats treated with 1% DMB in the drinking water for 8 weeks; controls | Compared with the young control group, the old control group had higher plasma TMAO levels. In both age groups DMB reduced TMAO levels. Old aortae exhibited increased expression of proinflammatory cytokines and superoxide production and decreased expression of endothelial nitric-oxide synthase (eNOS), all of which were restored by DMB treatment. | Old age: causes renal problems with these type of rats [190] | DMB treatment to inhibit TMAO formation | Old rats: 14.3 µM (reduced by DMB to 6.0 µM) (young rats 6.4 µM; reduced by DMB to 3.9 µM) |
| Yang 2019 [191] | Coronary ligation to induce myocardial infarction (MI), or sham operation in male mice | Mice were fed a control diet, high choline diet (1.2%) or/and DMB (choline analogue and inhibitor) diet or a TMAO diet (0.12%) starting 3 weeks before MI; treatment for one more week after MI | Cardiac fibrosis increased with 0.12% TMAO or 0.24% TMAO, but not with 0.06% TMAO. In the MI model, TMAO or choline supplementation led to decreased cardiac function and cardiac fibrosis. DMB inhibited the TMAO-effect. Choline or TAMO treated MI animals transformed fibroblasts into myofibroblasts and activated the TGF-βRI/Smad2 pathway, indicating cardiac fibrosis. | MI, choline or high TMAO or choline supplementation | 1920 mg choline/kg or 192 mg TMAO/kg ** | Before MI: 42.4 µM w/TMAO (8.8 µM control) before MI: 91.6 µM w/choline (6.1 µM control; 27.8 µM w/choline + DMB) |
| Chen 2019 [192] | Female mice | Mice treated with 1.3% L-carnitine in drinking water (drinking volume and body mass provided), flavonoids (or oolong tea) | Mice treated with L-carnitine significantly increased plasma TMAO levels compared to control. L-carnitine also increased inflammation markers. TMAO was remarkedly reduced by flavonoids. Antibiotics strongly reduced TMAO production. Inflammation markers (TNF-α, E-selectin, and VCAM-1) were reduced by flavonoids or oolong tea or antibiotics. | High L-carnitine → high TMAO | 2941 mg L-carnitine in L-carnitine group; 2657 mg L-carnitine in flavonoid group; 2424 mg L-carnitine in antibiotics group | 400 µM w/L-carnitine (26 µM with no diet; 322 µM with flavonoids; 13 µM with antibiotics) |
| Li 2019 [193] | Male rats with coronary ligation to induce MI or sham operation | MI and sham rats treated with either vehicle (tap water) or 1.0% DMB in tap water, for 8 weeks | Plasma TMAO levels were elevated in vehicle-treated MI rats compared with vehicle-treated sham rats; plasma TMAO levels were reduced in DMB-treated MI rats. Manifestations of MI-induced heart failure were improved in DMB-treated MI rats. Elevated plasma TMAO levels went along with increased proinflammatory IL-8 plasma level in MI groups. In sham rats, DMB treatment reduced plasma TMAO but did not alter other parameters. | Coronary ligation → myocardial infarction | DMB treatment to inhibit TMAO formation | 30 µM w/o DMB (Sham: 12 µM w/o DMB) (MI & DMB: 10 µM) (Sham & DMB: 3 µM) |
| Chen 2019 [194] | Male mice | 1% cholesterol diet with/without 1% choline | Plasma TMAO levels increased about 4-fold with diet + choline; increased mRNA of cholesterol uptake and secretion genes (Abcg5 and g8, Ldlr); no difference in bile acid composition. | High cholesterol diet, high choline | 1600 mg choline/kg ** | 7.7 µM (1 µM) |
| Male mice | 1% cholesterol diet and supplemented with low dose (0.12%) or high dose (0.3%) TMAO | No pathological differences in liver tissue; cholesterol concentration in gallbladder bile increased with TMAO, more apparent at high dose; increased mRNA of cholesterol uptake and secretion genes (Abcg5 and g8, Ldlr, Srb1). | High cholesterol diet, TMAO supplementation | 192 mg TMAO/kg or 480 mg TMAO/kg | Not indicated | |
| Gallstone-susceptible AKR/J male mice (biliary cholesterol hypersecretion) | Lithogenic diet supplemented with 0.3% TMAO or not supplemented (control). | With TMAO, the incidence of gallstones rose to 70%, compared no gallstones in the control mice. TMAO also induced increased hepatic Abcg5 and g8 expression. | Lithogenic diet; gallstone-susceptible AKR/J mice (cholesterol hypersecretion); high TMAO supplementation | 480 mg TMAO/kg | 23,3 µM (1 µM lithogenic diet) |
| Literature Source | Test System | Treatment | Outcome | Distress Factors | Daily Dosage of TMAO or Precursors * | Plasma TMAO Level * (or Control Level) |
|---|---|---|---|---|---|---|
| Mayr 2005 [114] | Male and female apoE−/− and apoE+/+mice on normal chow diet | Proteomics and metabolomics to identify protein and metabolite changes in vessels | No significant difference in TMAO concentration in the aortas of 18-month-old apoE−/− and ApoE+/+mice. Lesion formation in apoE−/− mice due to increase in oxidative stress, not to increased TMAO. | apoE−/− mice | none | Females 0.06 Males 0.25 (units not stated, controls not stated) |
| Martin 2009 [202] | Male hamsters | Hyperlipidemic diet (normal diet plus 100 g/kg fat for 5 weeks + 200 g/kg for 12 weeks; fat as anhydrous butter or cheese) or controls; (1)H NMR-based metabonomics | VLDL lipids, cholesterol, and N-acetylglycoproteins had the best correlation to onset of atherosclerosis. TMAO was found to be negatively associated with atherogenesis. | High-fat diet | none | Absolute concentrations not determined (only relative ones; personal communication) |
| Gao 2014 [203] | Male mice | Control, diet with 25% fat +/− 0.2% TMAO for 4 weeks | Dietary TMAO increased fasting insulin levels and insulin resistance and exacerbated impaired glucose tolerance and MCP-1 mRNA (pro-inflammatory cytokine) in HFD-fed mice. IL-10 mRNA (anti-inflammatory cytokine) in adipose tissue was (more than already by the high-fat diet) decreased by TMAO. However, the increase in atherosclerosis associated with a high-fat diet was prevented by TMAO, suggesting a protective effect of TMAO with regard to atherosclerosis. | High-fat diet; TMAO supplementation | 320 mg TMAO/kg ** | 17.5 µM (normal chow 11.9 µM; high-fat chow 12 µM) |
| Shih 2015 [118] | Male mice, transgenic FMO3 overexpression | Transgenic compared to control mice, supplemented with water containing 1.3% choline chloride for 6 weeks | FMO3 overexpression caused a 75% increase in plasma TMAO levels and increased hepatic and plasma lipids. | FMO3 overexpression; high choline supplementation | FMO3 overexpression; 2080 mg choline/kg ** | 16 µM (9 µM for control transgene) |
| Shih 2015 [29,118] | Male hyperlipidemic mouse “E3L Tg” with transgenic FMO3 overexpression | Transgenic compared to control mice; low-fat or high-fat/1% cholesterol chow for 16 weeks | Increased plasma TG, VLDL/IDL/LDL, and unesterified cholesterol with both diets, increased glucose and insulin levels, increased levels of TG, TC, and phosphatidylcholine in the VLDL plasma fractions with high-fat diet. Small increase (20%) in atherosclerotic lesion size compared to knockdown mouse (see above); it is implausible to explain the effect with the slight change in TMAO levels. | Hyperlipidemic mouse with transgenic FMO3 overexpression; high-fat/high-cholesterol chow | High-fat diet | w/high-fat/cholesterol: 2.6 µM (2.2 µM for control transgene) (difference = trend) |
| Collins 2016 [185] | Male apoE−/− mice expressing human cholesteryl ester transfer protein (hCETEP) | 12 week treatment with L-carnitine (87 mg/kg and 352 mg/kg; equivalent to 500 and 2000 mg/day in humans); (and/or methimazole =FMO inhibitor) | High doses of L-carnitine resulted in a significant increase in plasma L-carnitine and TMAO levels. Plasma lipid and lipoprotein levels did not change. Aortic root analysis showed significant decrease in lesion size with L-carnitine treatment and high TMAO compared to the control. Significantly lower levels of lesion were found with elevated plasma TMAO (>0.05 ppm) compared to the low plasma TMAO (<0.05 ppm). TMAO may be protective against atherosclerosis development. | apoE−/− mice (plus hCETEP) | 352 mg L-carnitine/kg | 0.2 ppm = 2.7 µM (0.08 ppm = 1.07 µM) |
| Empl 2015 [204] | Male Fischer 344 rats | Daily 0, 0.1, 0.2 or 0.5 g/L L-carnitine (0; 70; 141; 352 mg/kg) via drinking water for one year | L-carnitine did not cause any preneoplastic, atherosclerotic, or other lesions. | None | 352 mg L-carnitine/kg (highest dosage) | See below |
| Weinert 2017 [205] | Male Fischer 344 rats from study [204] | Daily 0, 0.1, 0.2 or 0.5 g/L L-carnitine (0; 70; 141; 352 mg/kg) via drinking water for one year | High dose L-carnitine resulted in tenfold higher plasma TMAO concentration compared to the control (25.0 μM). Supplementation did not cause changes in the plasma metabolome. | None | 352 mg L-carnitine/kg (highest dosage) | 25.0 μM (2.5 µM for control) |
| Huc 2018 [206] | Male spontaneously hypertensive rats (SHR) with pressure-overloaded hearts, 2 age groups | TMAO 333 mg/L with drinking water, water controls and normotensive rat controls for 9 weeks; metabolic cage for 2 days at end of study | Chronic, low-dose trimethylamine oxide (TMAO) treatment increased plasma TMAO by 4 to 5-fold and reduced plasma NH2-terminal pro-B-type natriuretic peptide and vasopressin, left ventricular end-diastolic pressure, and cardiac fibrosis. TMAO may be beneficial for reduction of hypertension. | Hypertension | 37 (16 weeks)/32 mg (56 weeks) TMAO/kg (personal communication) | 16 weeks: 37.3 µM (SHR control: 8.8 µM) (normal control: 6.3 µM) 56 weeks: 40.9 µM (SHR control: 8.1 µM) (normal control: 5.2 µM) |
| Lindskog Jonsson 2018 [207] | Male germ-free or conventionally raised apoE−/− mice | Western diet alone or supplemented with 1.2% choline for 12 weeks | Conventionally raised mice had smaller aortic lesions and lower plasma cholesterol levels on a chow diet compared to a Western diet; choline supplementation increased plasma TMAO levels in conventionally raised mice but not in germ-free mice. However, choline supplementation did not affect the size of aortic lesions or plasma cholesterol levels; increased plasma TMAO levels observed in conventionally raised apoE−/− mice fed a choline-supplemented diet did not correlate with aortic lesion size. The microbiota was required for TMAO production from dietary choline, but this process could not be linked to increased atherosclerosis. Choline supplementation reduced body weight and epididymal fat weight in conventionally but not germ-free mice when fed Western diet. | Western diet/germ-free existence; apoE−/− mice | 1920 mg choline/kg ** | Conventionally raised: Western diet +choline: 8 µM Chow +choline: 21 µM (Western diet: 0.5 µM Chow: 1 µM) No TMAO in germ-free mice |
| Zhao 2019 [208] | Male rats with steatohepatitis induced by high-fat high-cholesterol diet | 16-wk high-fat high-cholesterol (HFHC) diet feeding; daily TMAO (120 mg/kg/day) by oral gavage for 8 weeks | Hepatic and serum levels of cholesterol were both decreased by TMAO treatment in rats on HFHC diet. TMAO treatment also downregulated cholesterol influx-related Niemann-Pick C1-like 1 (intestinal cholesterol absorption transporter) and upregulated cholesterol efflux-related ABCG5/8 in the small intestine; thus, TMAO inhibits intestinal cholesterol absorption. Gut microbiota analysis showed that TMAO could alter the gut microbial profile and restore the diversity of gut flora. TMAO also ameliorated hepatic ER stress and cell death under cholesterol overload, thereby attenuating HFHC diet-induced steatohepatitis in rats. | Steatohepatitis induced by high-fat high-cholesterol diet | 120 mg TMAO/kg | Not determined (personal communication) |
| Aldana-Hernandez 2019 [209] | Male Ldlr-/- mice and male apoE−/− mice | 40% high-fat diet for atherosclerosis induction; control (0.1% choline) or supplemented with 1% choline or 0.9% betaine or 0.2% TMAO; for 8 or 16 weeks control diet (0.1% choline) or diet for ≤28 weeks supplemented with 1% choline or 0.9% betaine or 0.12% TMAO for 12 or 28 weeks | In LDLr-/- mice, dietary supplementation for 8 wk with choline or TMAO increased plasma TMAO concentrations by 1.6-and 4-fold, respectively. After 16 wk, there was a 2-fold increase in plasma TMAO after dietary TMAO supplementation. In apoe-/- mice, dietary supplementation with choline, betaine, or TMAO for 12 wk did not increase plasma TMAO concentrations. However, choline and TMAO supplementation for 28 wk significantly increased plasma TMAO concentrations by 1.8-and 1.5-fold, respectively. Atherosclerotic lesion size was not altered by any of the dietary interventions, irrespective of mouse model. | Ldlr-/- mice (40% high-fat diet) apoE−/− mice | 1600 mg choline/kg (1%) ** 1440 mg betaine/kg (0.9%) ** 320 mg TMAO/kg (high TMAO dosage of 0.2%) ** 192 mg TMAO/kg (low TMAO dosage of 0.12%) ** | LDLr-/- mice (8 weeks/16 weeks): w/choline: 1.3 µM/1.8 µM w/betaine: 0.9 µM/1.1 µM w/TMAO: 2.5 µM/1.1 µM (0.5-1.1 µM for controls) apoE−/− mice (12 weeks/28 weeks): w/choline: 1.3µM/1.6 µM w/betaine: 0.8 µM/0.8 µM w/TMAO: 0.9 µM/1.3 µM (1.0 µM/0.8 µM for controls) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papandreou, C.; Moré, M.; Bellamine, A. Trimethylamine N-Oxide in Relation to Cardiometabolic Health—Cause or Effect? Nutrients 2020, 12, 1330. https://doi.org/10.3390/nu12051330
Papandreou C, Moré M, Bellamine A. Trimethylamine N-Oxide in Relation to Cardiometabolic Health—Cause or Effect? Nutrients. 2020; 12(5):1330. https://doi.org/10.3390/nu12051330
Chicago/Turabian StylePapandreou, Christopher, Margret Moré, and Aouatef Bellamine. 2020. "Trimethylamine N-Oxide in Relation to Cardiometabolic Health—Cause or Effect?" Nutrients 12, no. 5: 1330. https://doi.org/10.3390/nu12051330
APA StylePapandreou, C., Moré, M., & Bellamine, A. (2020). Trimethylamine N-Oxide in Relation to Cardiometabolic Health—Cause or Effect? Nutrients, 12(5), 1330. https://doi.org/10.3390/nu12051330






