Effects of Dietary or Supplementary Micronutrients on Sex Hormones and IGF-1 in Middle and Older Age: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Methods
2.2. Eligibility Criteria
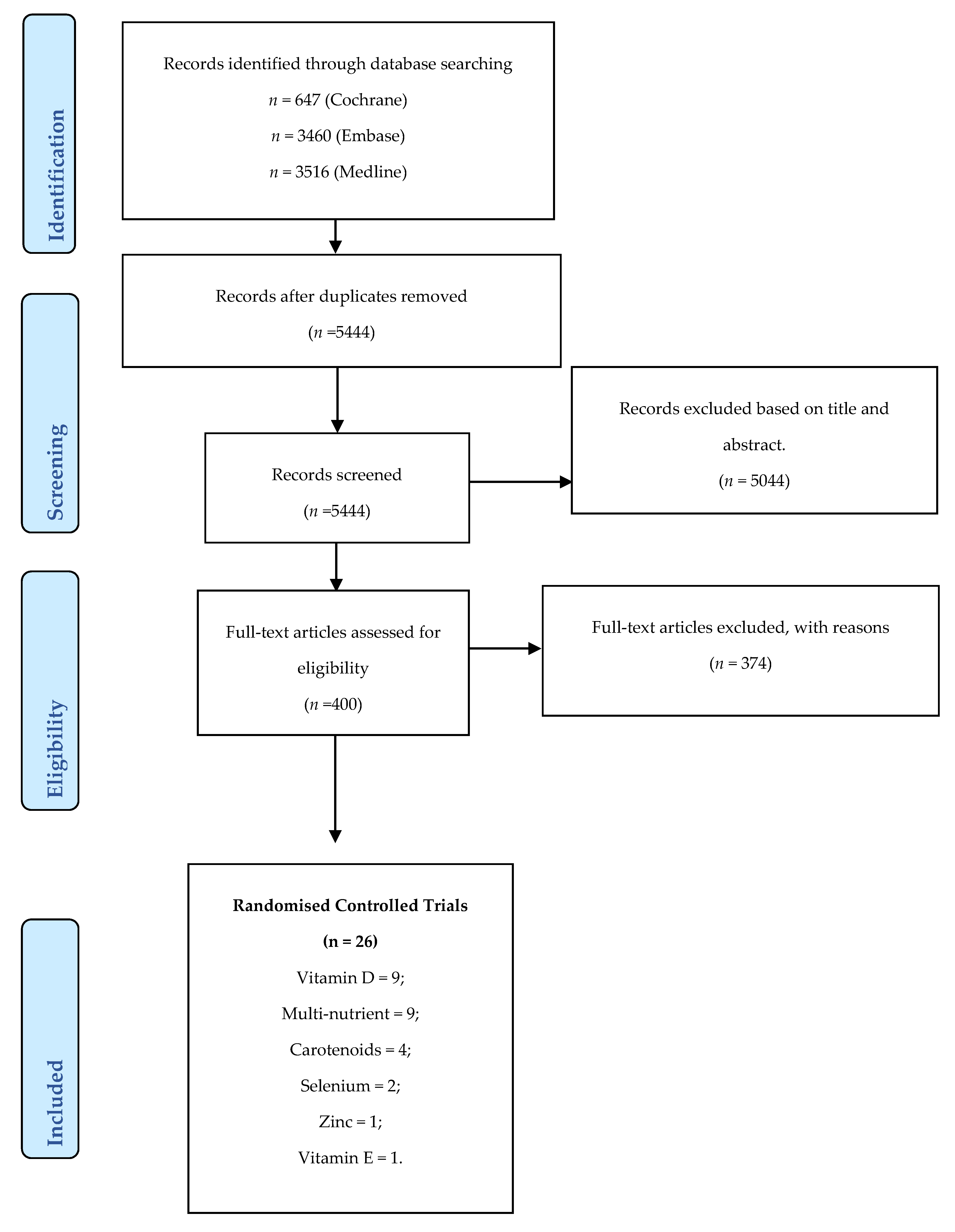
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias (Quality Assessment)
2.6. Data Synthesis and Statistical Analysis
3. Results
3.1. Risk of Bias of Included RCTs
3.2. Vitamin D
3.2.1. Effects of Vitamin D on IGF-1
3.2.2. Effects of Vitamin D on Testosterone
3.2.3. Effects of Vitamin D on Oestradiol
3.2.4. Effects of Vitamin D on SHBG
3.3. Multi-Nutrients
3.3.1. Effects of Multi-Nutrient Supplements on IGF-1
3.3.2. Effects of Multi-Nutrients on Testosterone
3.3.3. Effects of Multi-Nutrients on Dihydrotestosterone and SHBG
3.4. Carotenoids
Effects of Carotenoids on IGF-1
3.5. Selenium
Effects of Selenium on Testosterone
3.6. Vitamin E
Effects of Vitamin E on DHEAS
3.7. Zinc
Effects of Zinc on IGF-1
4. Discussion
Limitations of the Available Data
5. Recommendations for Future Studies
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19–21, 1988. Am. J. Clin. Nutr. 1989, 50 (Suppl. 5), 1121–1235.
- Boss, G.R.; Seegmiller, J.E. Age-Related Physiological Changes and Their Clinical Significance. West. J. Med. 1981, 135, 434–440. [Google Scholar] [PubMed]
- Marzetti, E.; on behalf of the SPRINTT Consortium; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Marty, E.; Liu, Y.; Samuel, A.; Or, O.; Lane, J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone 2017, 105, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, L.; Mellström, D.; A Laughlin, G.; Cawthon, P.M.; A Cauley, J.; Hoffman, A.R.; Karlsson, M.K.; Rosengren, B.E.; Ljunggren, Ö.; Nethander, M.; et al. Low Testosterone, but Not Estradiol, Is Associated With Incident Falls in Older Men: The International MrOS Study. J. Bone Min. Res. 2017, 32, 1174–1181. [Google Scholar] [CrossRef]
- Roddam, A.W.; Appleby, P.; Neale, R.E.; Dowsett, M.; Folkerd, E.; Tipper, S.; Allen, N.E.; Key, T.J. Association between endogenous plasma hormone concentrations and fracture risk in men and women: The EPIC-Oxford prospective cohort study. J. Bone Min. Metab. 2009, 27, 485–493. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Dowsett, M.; Folkerd, E.; Bingham, S.; Wareham, N.; Luben, R.N.; Welch, A.; Day, N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 2007, 116, 2694–2701. [Google Scholar] [CrossRef] [Green Version]
- Guadalupe-Grau, A.; Carnicero, J.A.; Losa-Reyna, J.; Tresguerres, J.; Gomez-Cabrera, M.C.; Castillo, C.; Alfaro-Acha, A.; Rosado-Artalejo, C.; Rodríguez-Mañas, L.; Garcia-Garcia, F.J. Endocrinology of Aging From a Muscle Function Point of View: Results from the Toledo Study for Healthy Aging. J. Am. Med. Dir. Assoc. 2017, 18, 234–239. [Google Scholar] [CrossRef]
- Clegg, D.; Hevener, A.L.; Moreau, K.L.; Morselli, E.; Criollo, A.; Van Pelt, R.E.; Vieira-Potter, V.J. Sex Hormones and Cardiometabolic Health: Role of Estrogen and Estrogen Receptors. Endocrinology 2017, 158, 1095–1105. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dattilo, M.; Macut, Đ.; Duntas, L.H.; Gonos, E.S.; Goulis, D.G.; Kanaka-Gantenbein, C.; Kapetanou, M.; Koukkou, E.G.; Lambrinoudaki, I.; et al. MECHANISMS IN ENDOCRINOLOGY: Aging and anti-aging: A Combo-Endocrinology overview. Eur. J. Endocrinol. 2017, 176, R283–R308. [Google Scholar] [CrossRef]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef] [Green Version]
- Yeap, B.B.; Alfonso, H.; Chubb, S.A.P.; Center, J.R.; Beilin, J.; Hankey, G.J.; Almeida, O.P.; Golledge, J.; Norman, P.; Flicker, L. U-Shaped Association of Plasma Testosterone, and no Association of Plasma Estradiol, with Incidence of Fractures in Men. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; Babcock, M.C.; Hildreth, K.L. Sex differences in vascular aging in response to testosterone. Biol. Sex Differ. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlamangla, A.S.; Burnett-Bowie, S.M.; Crandall, C.J. Bone Health during the Menopause Transition and Beyond. Obstet. Gynecol. Clin. N. Am. 2018, 45, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, X.; Shi, B.M. Sex hormone-binding globulin and risk of fracture in older adults: Systematic review and meta-analysis of observational studies. Osteoporos Int. 2018, 29, 2171–2180. [Google Scholar] [CrossRef]
- Brundle, C.; Heaven, A.; Brown, L.; Teale, E.; Young, J.; West, R.; Clegg, A. Convergent validity of the electronic frailty index. Age Ageing 2019, 48, 152–156. [Google Scholar] [CrossRef]
- Morley, J.E. Hormones and Sarcopenia. Curr. Pharm. Des. 2017, 23, 4484–4492. [Google Scholar] [CrossRef]
- Maggio, M.; Lauretani, F.; Ceda, G.P. Sex hormones and sarcopenia in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 3–13. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Waters, D.L.; Gallagher, D.; Morley, J.E.; Garry, P.J. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev. 1999, 107, 123–136. [Google Scholar] [CrossRef]
- Mouser, J.G.; Loprinzi, P.D.; Loenneke, J.P. The association between physiologic testosterone levels, lean mass, and fat mass in a nationally representative sample of men in the United States. Steroids 2016, 115, 62–66. [Google Scholar] [CrossRef]
- Ottenbacher, K.J.; Ottenbacher, M.E.; Ottenbacher, A.J.; Acha, A.A.; Ostir, G.V. Androgen Treatment and Muscle Strength in Elderly Males: A Meta-Analysis. J. Am. Geriatr. Soc. 2006, 54, 1666–1673. [Google Scholar] [CrossRef]
- Tiidus, P.M. Benefits of Estrogen Replacement for Skeletal Muscle Mass and Function in Post-Menopausal Females: Evidence from Human and Animal Studies. Eurasian J. Med. 2011, 43, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.M.; Meeuwsen, I.B.; Crowe, A.; Dessens, J.A.; Duursma, S.A.; Verhaar, H.J. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing 2000, 29, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.-M.; Keller, A.C.; Runchey, S.S.; Miller, B.F.; Kohrt, W.M.; Van Pelt, R.E.; Kang, C.; Jankowski, C.M.; Moreau, K.L. Acute estradiol treatment reduces skeletal muscle protein breakdown markers in early- but not late-postmenopausal women. Steroids 2019, 146, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Velders, M.; Diel, P. How sex hormones promote skeletal muscle regeneration. Sports Med. 2013, 43, 1089–1100. [Google Scholar] [CrossRef]
- Taekema, D.G.; Ling, C.H.; Blauw, G.J.; Meskers, C.G.; Westendorp, R.G.J.; De Craen, A.J.M.; Maier, A.B. Circulating levels of IGF1 are associated with muscle strength in middle-aged- and oldest-old women. Eur. J. Endocrinol. 2011, 164, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Warner, M.; Gustafsson, J.A. DHEA-a precursor of ERbeta ligands. J. Steroid Biochem. Mol. Biol. 2015, 145, 245–247. [Google Scholar] [CrossRef]
- Selby, C. Sex hormone binding globulin: Origin, function and clinical significance. Ann. Clin. Biochem. 1990, 27 Pt 6, 532–541. [Google Scholar] [CrossRef]
- Liu, P.Y.; Beilin, J.; Nguyen, T.V.; Center, J.R.; Meier, C.; Leedman, P.J.; Seibel, M.; A Eisman, J.; Handelsman, D.J. Age-Related Changes in Serum Testosterone and Sex Hormone Binding Globulin in Australian Men: Longitudinal Analyses of Two Geographically Separate Regional Cohorts. J. Clin. Endocrinol. Metab. 2007, 92, 3599–3603. [Google Scholar] [CrossRef] [Green Version]
- Longcope, C.; Goldfield, S.R.; Brambilla, D.J.; McKinlay, J. Androgens, estrogens, and sex hormone-binding globulin in middle-aged men. J. Clin. Endocrinol. Metab. 1990, 71, 1442–1446. [Google Scholar] [CrossRef]
- Pang, A.L.-Y.; Chan, W.-Y. Chapter 22-Molecular Basis of Diseases of the Endocrine System. In Essential Concepts in Molecular Pathology; Coleman, W.B., Tsongalis, G.J., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 289–307. [Google Scholar]
- Philippou, A.; Barton, E.R. Optimizing IGF-I for skeletal muscle therapeutics. Growth Horm. IGF Res. 2014, 24, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Secco, M.; Bueno, C.; Vieira, N.M.; Almeida, C.; Pelatti, M.; Zucconi, E.; Bartolini, P.; Vainzof, M.; Miyabara, E.; Okamoto, O.K.; et al. Systemic delivery of human mesenchymal stromal cells combined with IGF-1 enhances muscle functional recovery in LAMA2 dy/2j dystrophic mice. Stem Cell Rev. 2013, 9, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Rybalko, V.Y.; Pham, C.B.; Hsieh, P.-L.; Hammers, D.W.; Merscham-Banda, M.; Suggs, L.J.; Farrar, R.P. Controlled delivery of SDF-1alpha and IGF-1: CXCR4(+) cell recruitment and functional skeletal muscle recovery. Biomater. Sci. 2015, 3, 1475–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucci, L.; Yani, S.L.; Fabbri, C.; Bijlsma, A.Y.; Maier, A.B.; Meskers, C.G.; Narici, M.; Jones, D.A.; McPhee, J.S.; Seppet, E.; et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology 2013, 14, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, M.I.; Khater, M.S. Evaluation of insulin like growth factor-1 (IGF-1) level and its impact on muscle and bone mineral density in frail elderly male. Arch. Gerontol. Geriatr. 2015, 60, 124–127. [Google Scholar] [CrossRef]
- Harrison, P. Low Vitamin D Tied to Testosterone Dip in Healthy Men 2015 [Vitamin D and Testosterone]. Available online: https://www.medscape.com/viewarticle/845483 (accessed on 4 March 2020).
- Ceglia, L.; Harris, S.S. Vitamin D and its role in skeletal muscle. Calcif Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef]
- Azadi-Yazdi, M.; Nadjarzadeh, A.; Khosravi-Boroujeni, H.; Salehi-Abargouei, A. The Effect of Vitamin D Supplementation on the Androgenic Profile in Patients with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Clinical Trials. Horm. Metab. Res. 2017, 49, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Ouyang, P.; De Boer, I.H.; Lutsey, P.L.; Farag, Y.M.; Guallar, E.; Siscovick, D.S.; Post, W.S.; Kalyani, R.R.; Billups, K.L.; et al. Serum vitamin D and sex hormones levels in men and women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2017, 96, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, R.; Van Schoor, N.; Sohl, E.; Zillikens, M.; Oosterwerff, M.; Schaap, L.; Lips, P.; De Jongh, R. Associations of vitamin D status and vitamin D-related polymorphisms with sex hormones in older men. J. Steroid Biochem. Mol. Boil. 2016, 164 (Suppl. C), 11–17. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S.; Wan Ngah, W.Z. Vitamin D is significantly associated with total testosterone and sex hormone-binding globulin in Malaysian men. Aging Male 2015, 18, 175–179. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Pilz, S.; Trummer, C.; Rabe, T.; Schenk, M.; Heijboer, A.C.; Obermayer-Pietsch, B. Serum vitamin D levels and hypogonadism in men. Andrology 2014, 2, 748–754. [Google Scholar] [CrossRef]
- Anic, G.M.; Albanes, D.; Rohrmann, S.; Kanarek, N.; Nelson, W.G.; Bradwin, G.; Rifai, N.; McGlynn, K.A.; Platz, E.A.; Mondul, A.M. Association between serum 25-hydroxyvitamin D and serum sex steroid hormones among men in NHANES. Clin. Endocrinol. 2016, 85, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.M.; Kim, Y.S.; Won, H.J.; Yoon, T.K.; Lee, W.S. Association between Sex Steroids, Ovarian Reserve, and Vitamin D Levels in Healthy Nonobese Women. J. Clin. Endocrinol. Metab. 2014, 99, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Grimnes, G.; Hutchinson, M.S.; Kjaergaard, M.; Kamycheva, E.; Svartberg, J. Supplementation with vitamin D does not increase serum testosterone levels in healthy males. Horm. Metab. Res. 2013, 45, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, A.O.; Wayne Meikle, A.; Matthew Peterson, C.; Stanford, J.; Gibson, M.; Carrell, D.T. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J. Androl. 2012, 14, 855–859. [Google Scholar] [CrossRef] [Green Version]
- Mumford, S.L.; Browne, R.W.; Schliep, K.C.; Schmelzer, J.; Plowden, T.C.; A Michels, K.; Sjaarda, L.; Zarek, S.M.; Perkins, N.; Messer, L.; et al. Serum Antioxidants Are Associated with Serum Reproductive Hormones and Ovulation among Healthy Women. J. Nutr. 2016, 146, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Barella, L.; Rota, C.; Stocklin, E.; Rimbach, G. Alpha-tocopherol affects androgen metabolism in male rat. Ann. New York Acad. Sci. 2004, 1031, 334–336. [Google Scholar] [CrossRef]
- Hartman, T.J.; Dorgan, J.F.; Woodson, K.; Virtamo, J.; A Tangrea, J.; Heinonen, O.P.; Taylor, P.R.; Barrett, M.J.; Albanes, D. Effects of long-term alpha-tocopherol supplementation on serum hormones in older men. Prostate 2001, 46, 33–38. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Griswold, M.D. The key role of vitamin A in spermatogenesis. J. Clin. Investig. 2010, 120, 956–962. [Google Scholar] [CrossRef] [Green Version]
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 2001, 10, 861–868. [Google Scholar]
- Rotter, I.; Kosik-Bogacka, D.I.; Dolegowska, B.; Safranow, K.; Kuczynska, M.; Laszczynska, M. Analysis of the relationship between the blood concentration of several metals, macro- and micronutrients and endocrine disorders associated with male aging. Environ. Geochem. Health 2016, 38, 749–761. [Google Scholar] [CrossRef]
- Rotter, I.; Kosik-Bogacka, D.; Dolegowska, B.; Safranow, K.; Karakiewicz, B.; Laszczynska, M. Relationship between serum magnesium concentration and metabolic and hormonal disorders in middle-aged and older men. Magnes. Res. 2015, 28, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Ceda, G.; Lauretani, F.; Cattabiani, C.; Avantaggiato, E.; Morganti, S.; Ablondi, F.; Bandinelli, S.; Dominguez, L.-J.; Barbagallo, M.; et al. Magnesium and anabolic hormones in older men. Int. J. Androl. 2011, 34 Pt 2, e594–e600. [Google Scholar] [CrossRef]
- Cinar, V.; Polat, Y.; Baltaci, A.K.; Mogulkoc, R. Effects of magnesium supplementation on testosterone levels of athletes and sedentary subjects at rest and after exhaustion. Biol. Trace Elem. Res. 2011, 140, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Ceda, G.; Lauretani, F.; Bandinelli, S.; Dall’Aglio, E.; Guralnik, J.M.; Paolisso, G.; Semba, R.D.; Nouvenne, A.; Borghi, L.; et al. Association of plasma selenium concentrations with total IGF-1 among older community-dwelling adults: The InCHIANTI study. Clin. Nutr. 2010, 29, 674–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oluboyo, A.; Adijeh, R.U.; Onyenekwe, C.C.; O Oluboyo, B.; Mbaeri, T.C.; Odiegwu, C.N.; O Chukwuma, G.; Onwuasoanya, U.F. Relationship between serum levels of testosterone, zinc and selenium in infertile males attending fertility clinic in Nnewi, south east Nigeria. Afr. J. Med. Med. Sci. 2012, 41, 51–54. [Google Scholar] [PubMed]
- Hawkes, W.C.; Turek, P.J. Effects of dietary selenium on sperm motility in healthy men. J. Androl. 2001, 22, 764–772. [Google Scholar]
- Darago, A.; Klimczak, M.; Stragierowicz, J.; Stasikowska-Kanicka, O.; Kilanowicz, A. The Effect of Zinc, Selenium, and Their Combined Supplementation on Androgen Receptor Protein Expression in the Prostate Lobes and Serum Steroid Hormone Concentrations of Wistar Rats. Nutrients 2020, 12, 153. [Google Scholar] [CrossRef] [Green Version]
- Rodondi, A.; Ammann, P.; Ghilardi-Beuret, S.; Rizzoli, R. Zinc increases the effects of essential amino acids-whey protein supplements in frail elderly. J. Nutr. Health Aging 2009, 13, 491–497. [Google Scholar] [CrossRef]
- Blostein-Fujii, A.; DiSilvestro, R.A.; Frid, D.; Katz, C.; Malarkey, W. Short-term zinc supplementation in women with non-insulin-dependent diabetes mellitus: Effects on plasma 5’-nucleotidase activities, insulin-like growth factor I concentrations, and lipoprotein oxidation rates in vitro. Am. J. Clin. Nutr. 1997, 66, 639–642. [Google Scholar] [CrossRef] [Green Version]
- Vivoli, G.; Fantuzzi, G.; Bergomi, M.; Tonelli, E.; Gatto, M.; Zanetti, F.; Del Dot, M. Relationship between zinc in serum and hair and some hormones during sexual maturation in humans. Sci. Total Environ. 1990, 95, 29–40. [Google Scholar] [CrossRef]
- Shafiei Neek, L.; Gaeini, A.A.; Choobineh, S. Effect of zinc and selenium supplementation on serum testosterone and plasma lactate in cyclist after an exhaustive exercise bout. Biol. Trace Elem. Res. 2011, 144, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.; Baltaci, A.K.; Gunay, M.; Gokbel, H.; Okudan, N.; Cicioglu, I. The effect of exhaustion exercise on thyroid hormones and testosterone levels of elite athletes receiving oral zinc. Neuro Endocrinol. Lett. 2006, 27, 247–252. [Google Scholar] [PubMed]
- Ebisch, I.M.; Thomas, C.M.; Peters, W.H.; Braat, D.D.; Steegers-Theunissen, R.P. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum. Reprod. Update 2007, 13, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Ebisch, I.M.W.; Pierik, F.H.; De Jong, F.H.; Thomas, C.M.G.; Steegers-Theunissen, R.P.M. Does folic acid and zinc sulphate intervention affect endocrine parameters and sperm characteristics in men? Int. J. Androl. 2006, 29, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Vihtamaki, T.; Parantainen, J.; Koivisto, A.M.; Metsa-Ketela, T.; Tuimala, R. Oral ascorbic acid increases plasma oestradiol during postmenopausal hormone replacement therapy. Maturitas 2002, 42, 129–135. [Google Scholar] [CrossRef]
- Maggio, M.; De Vita, F.; Lauretani, F.; Bandinelli, S.; Semba, R.D.; Bartali, B.; Cherubini, A.; Cappola, A.R.; Ceda, G.; Ferrucci, L. Relationship between Carotenoids, Retinol, and Estradiol Levels in Older Women. Nutrients 2015, 7, 6506–6519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerchbaum, E. Vitamin D and menopause—A narrative review. Maturitas 2014, 79, 3–7. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Obermayer-Pietsch, B. Vitamin D and fertility: A systematic review. Eur. J. Endocrinol. 2012, 166, 765–778. [Google Scholar] [CrossRef]
- Karas, M.; Amir, H.; Fishman, D.; Danilenko, M.; Segal, S.; Nahum, A.; Koifmann, A.; Giat, Y.; Levy, J.; Sharoni, Y. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr. Cancer 2000, 36, 101–111. [Google Scholar] [CrossRef]
- Liu, C.; Lian, F.; Smith, D.E.; Russell, R.M.; Wang, X.D. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003, 63, 3138–3144. [Google Scholar]
- Hirsch, K.; Atzmon, A.; Danilenko, M.; Levy, J.; Sharoni, Y. Lycopene and other carotenoids inhibit estrogenic activity of 17beta-estradiol and genistein in cancer cells. Breast Cancer Res. Treat. 2007, 104, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, P.; Vijayababu, M.R.; Ravisankar, B.; Anbalagan, J.; Aruldhas, M.M.; Arunakaran, J. Effect of lycopene on insulin-like growth factor-I, IGF binding protein-3 and IGF type-I receptor in prostate cancer cells. J. Cancer Res. Clin. Oncol. 2007, 133, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A. Nutritional influences on age-related skeletal muscle loss. Proc. Nutr. Soc. 2014, 73, 16–33. [Google Scholar] [CrossRef] [Green Version]
- Van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, F.; Zhang, H.; Gao, Y.; Tan, A.; Zhang, S.; Xiao, Q.; Zhang, B.; Huang, L.; Ye, B.; et al. The association between the levels of serum ferritin and sex hormones in a large scale of Chinese male population. PLoS ONE 2013, 8, e75908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aihara, K.; Nishi, Y.; Hatano, S.; Kihara, M.; Ohta, M.; Sakoda, K.; Uozumi, T.; Usui, T. Zinc, copper, manganese, and selenium metabolism in patients with human growth hormone deficiency or acromegaly. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 610–615. [Google Scholar] [CrossRef]
- Walfisch, S.; Walfisch, Y.; Kirilov, E.; Linde, N.; Mnitentag, H.; Agbaria, R.; Sharoni, Y.; Levy, J. Tomato lycopene extract supplementation decreases insulin-like growth factor-I levels in colon cancer patients. Eur. J. Cancer Prev. 2007, 16, 298–303. [Google Scholar] [CrossRef]
- Vrieling, A.; Voskuil, D.W.; Bonfrer, J.M.; Korse, C.M.; Van Doorn, J.; Cats, A.; Depla, A.C.; Timmer, R.; Witteman, B.J.; E Van Leeuwen, F.; et al. Lycopene supplementation elevates circulating insulin-like growth factor binding protein-1 and -2 concentrations in persons at greater risk of colorectal cancer. Am. J. Clin. Nutr. 2007, 86, 1456–1462. [Google Scholar] [CrossRef] [Green Version]
- Gann, P.H.; Deaton, R.J.; Rueter, E.E.; Van Breemen, R.B.; Nonn, L.; Macias, V.; Han, M.; Ananthanarayanan, V. A Phase II Randomized Trial of Lycopene-Rich Tomato Extract Among Men with High-Grade Prostatic Intraepithelial Neoplasia. Nutr. Cancer 2015, 67, 1104–1112. [Google Scholar] [CrossRef] [Green Version]
- Darago, A.; Sapota, A.; Matych, J.; Nasiadek, M.; Skrzypinska-Gawrysiak, M.; Kilanowicz, A. The correlation between zinc and insulin-like growth factor 1 (IGF-1), its binding protein (IGFBP-3) and prostate-specific antigen (PSA) in prostate cancer. Clin. Chem. Lab. Med. 2011, 49, 1699–1705. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Trummer, C.; Schwetz, V.; Pachernegg, O.; Heijboer, A.C.; Pilz, S.; Obermayer-Pietsch, B. Vitamin D and Testosterone in Healthy Men: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2017, 102, 4292–4302. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Rabe, T. Vitamin D and female fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, J.; Norlin, M.; Wikvall, K. 1alpha,25-Dihydroxyvitamin D3 affects hormone production and expression of steroidogenic enzymes in human adrenocortical NCI-H295R cells. Biochim. Biophys. Acta 2010, 1801, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A. Sarcopenia. BMJ 2010, 341, 1. [Google Scholar] [CrossRef]
- Hayhoe, R.P.G.; Lentjes, M.A.H.; Mulligan, A.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Cross-sectional associations of dietary and circulating magnesium with skeletal muscle mass in the EPIC-Norfolk cohort. Clin. Nutr. 2019, 38, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.A.; Jennings, A.; Kelaiditi, E.; Skinner, J.; Steves, C.J. Cross-sectional associations between dietary antioxidant vitamins C,E and carotenoid intakes and sarcopenic indices in women aged 18–79 years. Calcif. Tissue Int. 2020, 106, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Cameron, D.; Welch, A.A.; Adelnia, F.; Bergeron, C.M.; Reiter, D.A.; Dominguez, L.J.; Ferrucci, L. Age and function are more closely associated with intracellular magnesium as assessed by 31P Magnetic Resonance Spectroscopy, than with serum mangesium. Front. Physiol. 2019, 10, 1454. [Google Scholar] [CrossRef]
- Landi, F.; Camprubi-Robles, M.; E Bear, D.; Cederholm, T.; Malafarina, V.; Welch, A.A.; Cruz-Jentoft, A.J.; Landi, F. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2019, 38, 2113–2120. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.A.; Skinner, J.; Hickson, M. Dietary Magnesium May Be Protective for Aging of Bone and Skeletal Muscle in Middle and Younger Older Age Men and Women: Cross-Sectional Findings from the UK Biobank Cohort. Nutrients 2017, 9, 1189. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.A.; Kelaiditi, E.; Jennings, A.; Steves, C.J.; Spector, T.D.; MacGregor, A. Dietary Magnesium Is Positively Associated With Skeletal Muscle Power and Indices of Muscle Mass and May Attenuate the Association Between Circulating C-Reactive Protein and Muscle Mass in Women. J. Bone Min. Res. 2016, 31, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Semba, R.D.; Lauretani, F.; Ferrucci, L. Carotenoids as protection against sarcopenia in older adults. Arch. Biochem. Biophys. 2007, 458, 141–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.; van Loon, L.J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickson, M. Nutritional interventions in sarcopenia: A critical review. Proc. Nutr. Soc. 2015, 74, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, E.; Mo, H.; Wang, S.; Zu, Y.; Elfakhani, M.; Rios, S.R.; Chyu, M.-C.; Yang, R.-S.; Shen, C.-L. Potential roles of vitamin E in age-related changes in skeletal muscle health. Nutr. Res. 2018, 49, 23–36. [Google Scholar] [CrossRef]
- Takisawa, S.; Funakoshi, T.; Yatsu, T.; Nagata, K.; Aigaki, T.; Machida, S.; Ishigami, A. Vitamin C deficiency causes muscle atrophy and a deterioration in physical performance. Sci. Rep. 2019, 9, 4702. [Google Scholar] [CrossRef] [Green Version]
- Demirbag, R.; Yilmaz, R.; Erel, O. The association of total antioxidant capacity with sex hormones. Scand Cardiovasc. J. 2005, 39, 172–176. [Google Scholar] [CrossRef]
- Van Poppel, G.; Goldbohm, R.A. Epidemiologic evidence for beta-carotene and cancer prevention. Am. J. Clin. Nutr. 1995, 62 (Suppl. 6), 1393s–1402s. [Google Scholar] [CrossRef]
- Sharoni, Y.; Danilenko, M.; Dubi, N.; Ben-Dor, A.; Levy, J. Carotenoids and transcription. Arch. Biochem. Biophys. 2004, 430, 89–96. [Google Scholar] [CrossRef]
- Davison, G.W.; Ashton, T.; George, L.; Young, I.S.; McEneny, J.; Davies, B.; Jackson, S.K.; Peters, J.R.; Bailey, D.M. Molecular detection of exercise-induced free radicals following ascorbate prophylaxis in type 1 diabetes mellitus: A randomised controlled trial. Diabetologia 2008, 51, 2049. [Google Scholar] [CrossRef] [Green Version]
- Alessio, H.M.; Goldfarb, A.H.; Cao, G. Exercise-induced oxidative stress before and after vitamin C supplementation. Int. J. Sport Nutr. 1997, 7, 1–9. [Google Scholar] [CrossRef]
- Rokitzki, L.; Logemann, E.; Huber, G.; Keck, E.; Keul, J. alpha-Tocopherol supplementation in racing cyclists during extreme endurance training. Int. J. Sport Nutr. 1994, 4, 253–264. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Morrow, J.D.; Shooter, L.A.; Holmes, S.; Heward, C.; Henson, D.A. Effect of alpha-tocopherol supplementation on plasma homocysteine and oxidative stress in highly trained athletes before and after exhaustive exercise. J. Nutr. Biochem. 2005, 16, 530–537. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Kde, J.; Donangelo, C.M.; de Oliveira, A.V., Jr.; da Silveira, C.L.; Koury, J.C. Effect of zinc supplementation on the antioxidant, copper, and iron status of physically active adolescents. Cell Biochem. Funct. 2009, 27, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Fusco, D.; Sisto, A.; Ortolani, E.; Savera, G.; Salini, S.; Marzetti, E. Age-Related Variations of Muscle Mass, Strength, and Physical Performance in Community-Dwellers: Results From the Milan EXPO Survey. J. Am. Med. Dir. Assoc. 2017, 18, 88 e17–88 e24. [Google Scholar] [CrossRef]
- Reviews, C.S. Cochrane Handbook for Systematic Reviews of Interventions. Available online: http://community.cochrane.org/handbook (accessed on 4 March 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred reporting items for sytematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e100009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PRISMA. The PRISMA Checklist: Prisma-statement.org. 2009. Available online: http://prisma-statement.org/documents/PRISMA%202009%20checklist.pdf (accessed on 4 March 2020).
- Network SIG. SIGN 50: A Guideline Developers Handbook 2019. Available online: https://www.sign.ac.uk/sign-50 (accessed on 4 March 2020).
- Schmidt, A.; Luger, A.; Hörl, W.H. Sexual hormone abnormalities in male patients with renal failure. Nephrol. Dial. Transplant. 2002, 17, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Moller, S.; Becker, U. Insulin-like growth factor 1 and growth hormone in chronic liver disease. Dig. Dis. 1992, 10, 239–248. [Google Scholar] [CrossRef]
- Oh, Y. The insulin-like growth factor system in chronic kidney disease: Pathophysiology and therapeutic opportunities. Kidney Res. Clin. Pract. 2012, 31, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Cochrane. Cochrane Handbook 2017. Available online: http://handbook-5-1.cochrane.org/ (accessed on 4 March 2020).
- Handelsman, D.J.; Wartofsky, L. Requirement for Mass Spectrometry Sex Steroid Assays in the Journal of Clinical Endocrinology and Metabolism. J. Clin. Endocrinol. Metab. 2013, 98, 3971–3973. [Google Scholar] [CrossRef] [Green Version]
- Stanczyk, F.Z.; Cho, M.M.; Endres, D.B.; Morrison, J.L.; Patel, S.; Paulson, R.J. Limitations of direct estradiol and testosterone immunoassay kits. Steroids 2003, 68, 1173–1178. [Google Scholar] [CrossRef]
- Vieira, J.G.H.; Nakamura, O.H.; Ferrer, C.M.; Tachibana, T.T.; Endo, M.H.K.; Carvalho, V.M. The importance of methodology in serum testosterone measurement: Comparison between a direct immunoassay and a method based on high performance liquid chromatography and tandem mass spectrometry (HPLC/MS-MS). Arq. Bras. Endocrinol. Metabol. 2008, 52, 1050–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochran. Review Manager 5 (ReVMan5) [Computer Program]. Version 5.3: Copenhagen: The Nordic Cochrane Centre, The Cochran Collaboration. 2014. Available online: http://Community.cochrane.org/ (accessed on 4 March 2020).
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions Version 60; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Olmedilla-Alonso, B.; Granado-Lorencio, F.; Blanco-Navarro, I. Carotenoids, retinol and tocopherols in blood: Comparability between serum and plasma (Li-heparin) values. Clin. Biochem. 2005, 38, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Kamycheva, E.; Berg, V.; Jorde, R. Insulin-like growth factor I, growth hormone, and insulin sensitivity: The effects of a one-year cholecalciferol supplementation in middle-aged overweight and obese subjects. Endocrine 2013, 43, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Gee, J. Phase II Open Label, Multi-Center Clinical Trial of Modulation of Intermediate Endpoint Biomarkers by 1α-Hydroxyvitamin D2 in Patients With Clinically Localized Prostate Cancer and High Grade Pin. Prostate 2013, 73, 970–978. [Google Scholar] [CrossRef] [Green Version]
- Sinha-Hikim, I.; Duran, P.; Shen, R.; Lee, M.; Friedman, T.C.; Davidson, M.B. Effect of long term vitamin D supplementation on biomarkers of inflammation in Latino and African-American subjects with pre-diabetes and hypovitaminosis D. Horm. Metab. Res. 2015, 47, 280–283. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.; Tapsoba, J.D.D.; Duggan, C.; Imayama, I.; Wang, C.-Y.; Korde, L.A.; Stanczyk, F.; McTiernan, A. Effects of vitamin D supplementation during weight loss on sex hormones in postmenopausal women. Menopause 2016, 23, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.H.; Chen, K.J.; Lu, D.X.; Zhu, X.F.; Ma, X.C. A clinical study of Yigu capsule in treating postmenopausal osteoporosis. Chin. J. Integr. Med. 2005, 11, 97–103. [Google Scholar]
- Heijboer, A.C.; Oosterwerff, M.; Schroten, N.F.; Eekhoff, E.M.; Chel, V.G.; De Boer, R.A.; Blankenstein, M.; Lips, P. Vitamin D supplementation and testosterone concentrations in male human subjects. Clin. Endocrinol. 2015, 83, 105–110. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Trummer, C.; Theiler-Schwetz, V.; Kollmann, M.; Wölfler, M.; Heijboer, A.C.; Pilz, S.; Obermayer-Pietsch, B. Effects of vitamin D supplementation on androgens in men with low testosterone levels: A randomized controlled trial. Eur. J. Nutr. 2018, 58, 3135–3146. [Google Scholar] [CrossRef] [Green Version]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Berthold, H.; Gouni-Berthold, I.; Gummert, J.F.; et al. Vitamin D supplementation does not prevent the testosterone decline in males with advanced heart failure: The EVITA trial. Eur. J. Nutr. 2019, 58, 673–680. [Google Scholar] [CrossRef]
- Bonjour, J.P.; Benoit, V.; Pourchaire, O.; Rousseau, B.; Souberbielle, J.C. Nutritional approach for inhibiting bone resorption in institutionalized elderly women with vitamin D insufficiency and high prevalence of fracture. J. Nutr. Health Aging 2011, 15, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Trummer, C.; Theiler-Schwetz, V.; Pandis, M.; Grübler, M.R.; Verheyen, N.; Gaksch, M.; Zittermann, A.; März, W.; Aberer, F.; Lang, A.; et al. Effects of Vitamin D Supplementation on IGF-1 and Calcitriol: A Randomized-Controlled Trial. Nutrients 2017, 9, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, M.; Hytter-Landahl, A.; Brismar, K.; Cederholm, T. Nutritional supplementation and dietary advice in geriatric patients at risk of malnutrition. Clin. Nutr. 2007, 26, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Lamb, J.; Lerman, R.H.; Konda, V.R.; Darland, G.; Minich, D.M.; Desai, A.; Chen, T.; Austin, M.; Kornberg, J.; et al. Hop rho iso-alpha acids, berberine, vitamin D3 and vitamin K1 favorably impact biomarkers of bone turnover in postmenopausal women in a 14-week trial. J. Bone Miner. Metab. 2010, 28, 342–350. [Google Scholar] [CrossRef]
- Lamb, J.; Holick, M.F.; Lerman, R.H.; Konda, V.R.; Minich, D.M.; Desai, A.; Chen, T.; Austin, M.; Kornberg, J.; Chang, J.-L.; et al. Nutritional supplementation of hop rho iso-alpha acids, berberine, vitamin D(3), and vitamin K(1) produces a favorable bone biomarker profile supporting healthy bone metabolism in postmenopausal women with metabolic syndrome. Nutr. Res. 2011, 31, 347–355. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Aaseth, J.; Alexander, J.; Brismar, K. Increase in insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 1 after supplementation with selenium and coenzyme Q10. A prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. PLoS ONE 2017, 12, e0178614. [Google Scholar] [CrossRef]
- Torbergsen, A.C.; Watne, L.O.; Frihagen, F.; Wyller, T.B.; Mowe, M. Effects of nutritional intervention upon bone turnover in elderly hip fracture patients. Randomized controlled trial. Clin. Nutr. ESPEN 2019, 29, 52–58. [Google Scholar] [CrossRef]
- Jensen, C.; Holloway, L.; Block, G.; Spiller, G.; Gildengorin, G.; Gunderson, E.; Butterfield, G.; Marcus, R. Long-term effects of nutrient intervention on markers of bone remodeling and calciotropic hormones in late-postmenopausal women. Am. J. Clin. Nutr. 2002, 75, 1114–1120. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.S.; Aggarwal, R. Common pitfalls in statistical analysis: Intention-to-treat versus per-protocol analysis. Perspect. Clin. Res. 2016, 7, 144–146. [Google Scholar] [CrossRef]
- Hoenjet, K.M.; Dagnelie, P.C.; Delaere, K.P.; Wijckmans, N.E.; Zambon, J.V.; Oosterhof, G.O. Effect of a nutritional supplement containing vitamin E, selenium, vitamin c and coenzyme Q10 on serum PSA in patients with hormonally untreated carcinoma of the prostate: A randomised placebo-controlled study. Eur. Urol. 2005, 47, 433–439. [Google Scholar] [CrossRef]
- Kranse, R.; Dagnelie, P.C.; Van Kemenade, M.C.; De Jong, F.H.; Blom, J.H.; Tijburg, L.B.; Weststrate, J.A.; Schröder, F.H. Dietary intervention in prostate cancer patients: PSA response in a randomized double-blind placebo-controlled study. Int. J. Cancer 2005, 113, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Vidlar, A.; Vostálová, J.; Ulrichová, J.; Student, V.; Krajicek, M.; Vrbkova, J.; Simanek, V. The safety and efficacy of a silymarin and selenium combination in men after radical prostatectomy-a six month placebo-controlled double-blind clinical trial. Biomed. Pap. 2010, 154, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vostalova, J.; Vidlar, A.; Ulrichova, J.; Vrbkova, J.; Simanek, V.; Student, V. Use of selenium-silymarin mix reduces lower urinary tract symptoms and prostate specific antigen in men. Phytomedicine 2013, 21, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Van Amsterdam, J.; van der Horst-Graat, J.; Bischoff, E.; Steerenberg, P.; Opperhuizen, A.; Schouten, E. The effect of vitamin E supplementation on serum DHEA and neopterin levels in elderly subjects. Int. J. Vitam. Nutr. Res. 2005, 75, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Meng, R.; A Kerr, D.; Devine, A.; Solah, V.; Binns, C.W.; Prince, R.L. The effects of a two-year randomized, controlled trial of whey protein supplementation on bone structure, IGF-1, and urinary calcium excretion in older postmenopausal women. J. Bone Miner. Res. 2011, 26, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Hanna, M.; Chang, S.L.; Jacob, S.; Têtu, B.; Diorio, C. Evaluation of Antioxidant Intakes in Relation to Inflammatory Markers Expression within the Normal Breast Tissue of Breast Cancer Patients. Integr. Cancer Ther. 2017, 16, 485–495. [Google Scholar] [CrossRef]
- Watts, E.L.; Appleby, P.N.; Albanes, D.; Black, A.; Chan, J.M.; Chen, C.; Cirillo, P.M.; Cohn, B.A.; Cook, M.B.; Donovan, J.L.; et al. Circulating sex hormones in relation to anthropometric, sociodemographic and behavioural factors in an international dataset of 12,300 men. PLoS ONE 2017, 12, e0187741. [Google Scholar] [CrossRef] [Green Version]
- Watts, E.L.; Perez-Cornago, A.; Appleby, P.N.; Albanes, D.; Ardanaz, E.; Black, A.; Bueno-De-Mesquita, H.B.; Chan, J.M.; Chen, C.; Chubb, S.P.; et al. The associations of anthropometric, behavioural and sociodemographic factors with circulating concentrations of IGF-I, IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3 in a pooled analysis of 16,024 men from 22 studies. Int. J. Cancer 2019, 145, 3244–3256. [Google Scholar] [CrossRef]
- Bingham, S.A.; Cassidy, A.; Cole, T.J.; Welch, A.; Runswick, S.A.; Black, A.E.; Thurnham, D.; Bates, C.; Khaw, K.T.; Key, T.J.A.; et al. Validation of weighed records and other methods of dietary assessment using the 24 h urine nitrogen technique and other biological markers. Br. J. Nutr. 1995, 73, 531–550. [Google Scholar] [CrossRef] [Green Version]
- A Bingham, S.; Luben, R.N.; Welch, A.; Low, Y.L.; Khaw, K.T.; Wareham, N.; Day, N. Associations between dietary methods and biomarkers, and between fruits and vegetables and risk of ischaemic heart disease, in the EPIC Norfolk Cohort Study. Int. J. Epidemiol. 2008, 37, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Hayhoe, R.P.G.; Lentjes, M.A.H.; Mulligan, A.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Br. J. Nutr. 2017, 117, 1439–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| P | Humans, adults only, aged >45 years. |
| I | Micronutrients |
| C | - |
| O | Sex hormones and IGF-1 |
| S | Randomised controlled trials (RCTs) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janjuha, R.; Bunn, D.; Hayhoe, R.; Hooper, L.; Abdelhamid, A.; Mahmood, S.; Hayden-Case, J.; Appleyard, W.; Morris, S.; Welch, A. Effects of Dietary or Supplementary Micronutrients on Sex Hormones and IGF-1 in Middle and Older Age: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1457. https://doi.org/10.3390/nu12051457
Janjuha R, Bunn D, Hayhoe R, Hooper L, Abdelhamid A, Mahmood S, Hayden-Case J, Appleyard W, Morris S, Welch A. Effects of Dietary or Supplementary Micronutrients on Sex Hormones and IGF-1 in Middle and Older Age: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(5):1457. https://doi.org/10.3390/nu12051457
Chicago/Turabian StyleJanjuha, Ryan, Diane Bunn, Richard Hayhoe, Lee Hooper, Asmaa Abdelhamid, Shaan Mahmood, Joseph Hayden-Case, Will Appleyard, Sophie Morris, and Ailsa Welch. 2020. "Effects of Dietary or Supplementary Micronutrients on Sex Hormones and IGF-1 in Middle and Older Age: A Systematic Review and Meta-Analysis" Nutrients 12, no. 5: 1457. https://doi.org/10.3390/nu12051457
APA StyleJanjuha, R., Bunn, D., Hayhoe, R., Hooper, L., Abdelhamid, A., Mahmood, S., Hayden-Case, J., Appleyard, W., Morris, S., & Welch, A. (2020). Effects of Dietary or Supplementary Micronutrients on Sex Hormones and IGF-1 in Middle and Older Age: A Systematic Review and Meta-Analysis. Nutrients, 12(5), 1457. https://doi.org/10.3390/nu12051457






