Myoprotective Whole Foods, Muscle Health and Sarcopenia: A Systematic Review of Observational and Intervention Studies in Older Adults
Abstract
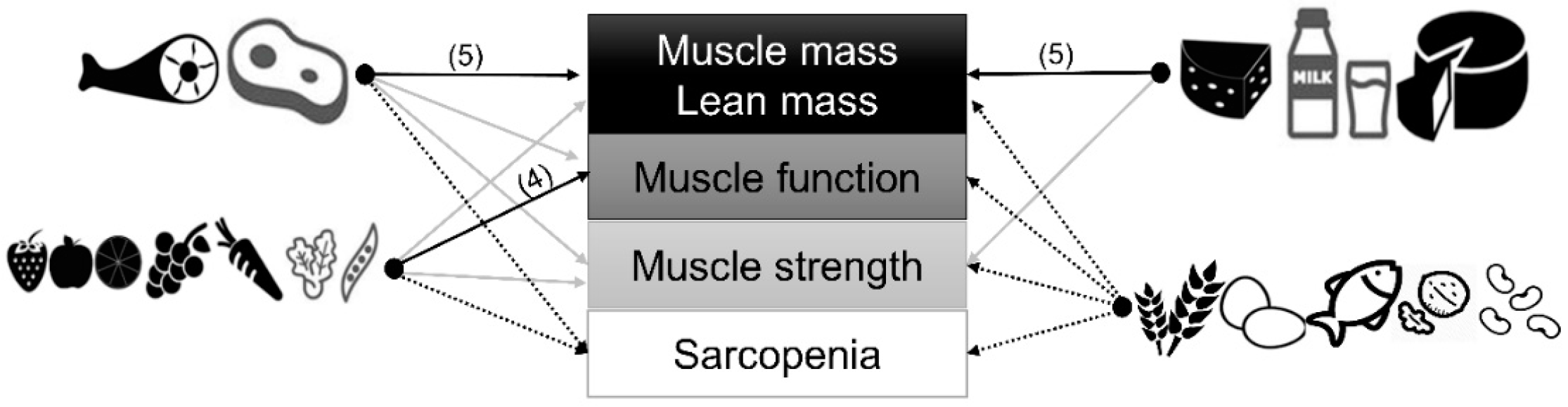
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Study Selection
2.6. Data Extraction and Data Items
2.7. Risk of Bias in Individual Studies
2.8. Summary Measures and Qualitative Synthesis of Results
3. Results
3.1. Study Selection
3.1.1. Observational Studies
3.1.2. Intervention Studies
3.2. Risk of Bias within the Studies
3.2.1. Risk of Bias in Observational Studies
3.2.2. Risk of Bias in Intervention Studies
3.3. Characteristics and Results of Individual Studies
3.3.1. Characteristics and Results of Observational Studies by Whole Foods
Meats
Fruit and Vegetables (FV)
Multiple Whole Foods
Dairy (including Semi-Solids and Cheese)
3.3.2. Characteristics and Results of Intervention Studies by Whole Foods
Meats
Fruit and Vegetables (FV)
Multiple Whole Foods
Dairy (including Semi-Solids and Cheese)
Eggs
3.4. Summary of Findings
3.4.1. Whole Foods and Muscle Health in Later Life: Summary of Observational Studies
Myoprotective Whole Foods for Muscle Health
Detrimental Whole Foods for Muscle Health
3.4.2. Whole Foods and Muscle Health in Later Life: Summary of Intervention Studies
Myoprotective Whole Foods for Muscle Health
3.5. Myoprotective Whole Foods in Later Life: Strongest Evidence
4. Discussion
4.1. Protein-Rich Whole Foods and Ageing Muscle: Meats and Non-Liquid Dairy
4.2. Fruit and Vegetables and Ageing Muscle
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Espinoza, B.; Salinas-Rodríguez, A.; Rosas-Carrasco, O.; Gutiérrez-Robledo, L.M.; Avila-Funes, J.A. Sarcopenia is associated with physical and mental components of health-related quality of life in older adults. J. Am. Med. Dir. Assoc. 2017, 18, 636.e1. [Google Scholar] [CrossRef]
- Tang, T.; Wu, L.; Yang, L.; Jiang, J.; Hao, Q.; Dong, B.; Yang, M. A sarcopenia screening test predicts mortality in hospitalized older adults. Sci. Rep. 2018, 8, 2923. [Google Scholar] [CrossRef]
- Bruyère, O.; Beaudart, C.; Ethgen, O.; Reginster, J.Y.; Locquet, M. The health economics burden of sarcopenia: A systematic review. Maturitas 2019, 119, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyère, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporosis Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J. Am. Med. Dir. Assoc. 2017, 18, 553.e1. [Google Scholar] [CrossRef]
- Liao, C.D.; Chen, H.C.; Huang, S.W.; Liou, T.H. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: A systematic review and meta-regression analysis of randomized trials. Nutrients 2019, 11, 1713. [Google Scholar] [CrossRef] [Green Version]
- ten Haaf, D.S.; Nuijten, M.A.; Maessen, M.F.; Horstman, A.M.; Eijsvogels, T.M.; Hopman, M.T. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 1043–1059. [Google Scholar] [CrossRef] [Green Version]
- Tieland, M.; Dirks, M.L.; van der Zwaluw, N.; Verdijk, L.B.; van de Rest, O.; de Groot, L.C.; van Loon, L.J. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef]
- Witard, O.C.; Combet, E.; Gray, S.R. Long-chain n-3 fatty acids as an essential link between musculoskeletal and cardio-metabolic health in older adults. Proc. Nutr. Soc. 2020, 79, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessier, A.J.; Chevalier, S. An update on protein, leucine, omega-3 fatty acids, and vitamin D in the prevention and treatment of sarcopenia and functional decline. Nutrients 2018, 10, 1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, S.M.; Martinson, W. Nutrient-rich, high-quality, protein-containing dairy foods in combination with exercise in aging persons to mitigate sarcopenia. Nutr Rev. 2019, 77, 216–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorissen, S.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef]
- Granic, A.; Hurst, C.; Dismore, L.; Aspray, T.; Stevenson, E.; Witham, M.D.; Sayer, A.A.; Robinson, S. Milk for skeletal muscle health and sarcopenia in older adults: A narrative review. Clin. Interv. Aging 2020, 15, 695–714. [Google Scholar] [CrossRef]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary patterns, skeletal muscle health, and sarcopenia in older adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.; Granic, A.; Sayer, A.A. Nutrition and muscle strength, as the key component of sarcopenia: An overview of current evidence. Nutrients 2019, 11, 2942. [Google Scholar] [CrossRef] [Green Version]
- Peterson, M.D.; Sen, A.; Gordon, P.M. Influence of resistance exercise on lean body mass in aging adults: A meta-analysis. Med. Sci. Sports. Exerc. 2011, 43, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Asp, M.L.; Richardson, J.R.; Collene, A.L.; Droll, K.R.; Belury, M.A. Dietary protein and beef consumption predict for markers of muscle mass and nutrition status in older adults. J. Nutr. Health Aging 2012, 16, 784–790. [Google Scholar] [CrossRef]
- Morris, M.S.; Jacques, P.F. Total protein, animal protein and physical activity in relation to muscle mass in middle-aged and older Americans. Br. J. Nutr. 2013, 109, 1294–1303. [Google Scholar] [CrossRef] [Green Version]
- Struijk, E.A.; Banegas, J.R.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Consumption of meat in relation to physical functioning in the Seniors-ENRICA cohort. BMC Med. 2018, 16, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, K.M. Association of vegetables and fruits consumption with sarcopenia in older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2015, 44, 96–102. [Google Scholar] [CrossRef] [Green Version]
- García-Esquinas, E.; Rahi, B.; Peres, K.; Colpo, M.; Dartigues, J.F.; Bandinelli, S.; Feart, C.; Rodríguez-Artalejo, F. Consumption of fruit and vegetables and risk of frailty: A dose-response analysis of 3 prospective cohorts of community-dwelling older adults. Am. J. Clin. Nutr. 2010, 104, 132–142. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Morley, J.E.; Malmstrom, T.K.; Miller, D.K. Fruit and vegetable intake and physical activity as predictors of disability risk factors in African-American middle-aged individuals. J. Nutr. Health Aging 2016, 20, 891–896. [Google Scholar] [CrossRef]
- Sim, M.; Blekkenhorst, L.C.; Lewis, J.R.; Bondonno, C.P.; Devine, A.; Zhu, K.; Woodman, R.J.; Prince, R.L.; Hodgson, J.M. Vegetable and fruit intake and injurious falls risk in older women: A prospective cohort study. Br. J. Nutr. 2018, 120, 925–934. [Google Scholar] [CrossRef]
- Koyanagi, A.; Veronese, N.; Solmi, M.; Oh, H.; Shin, J.I.; Jacob, L.; Yang, L.; Haro, J.M.; Smith, L. Fruit and vegetable consumption and sarcopenia among older adults in low- and middle-income countries. Nutrients 2020, 12, 706. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.M.; Jameson, K.A.; Batelaan, S.F.; Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A. Hertfordshire Cohort Study Group. Diet and its relationship with grip strength in community-dwelling older men and women: The Hertfordshire cohort study. J. Am. Geriatr. Soc. 2008, 56, 84–90. [Google Scholar] [CrossRef]
- Martin, H.; Aihie Sayer, A.; Jameson, K.; Syddall, H.; Dennison, E.M.; Cooper, C.; Robinson, S. Does diet influence physical performance in community-dwelling older people? Findings from the Hertfordshire Cohort Study. Age Ageing 2011, 40, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, K.M. Association between healthy diet and exercise and greater muscle mass in older adults. J. Am. Geriatr. Soc. 2015, 63, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Kim, M.; Saito, K.; Yoshida, H.; Yoshida, Y.; Hirano, H.; Obuchi, S.; Shimada, H.; Suzuki, T.; Kim, H. Lifestyle-related factors contributing to decline in knee extension strength among elderly women: A cross-sectional and longitudinal cohort study. PLoS ONE 2015, 10, e0132523. [Google Scholar] [CrossRef] [Green Version]
- Perälä, M.M.; von Bonsdorff, M.; Männistö, S.; Salonen, M.K.; Simonen, M.; Kanerva, N.; Pohjolainen, P.; Kajantie, E.; Rantanen, T.; Eriksson, J.G. A healthy Nordic diet and physical performance in old age: Findings from the longitudinal Helsinki Birth Cohort Study. Br. J. Nutr. 2016, 115, 878–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, S.; Wang, H.; Cao, L.; Liu, P.; Zhou, J.; Yang, Y.; Dong, B. Association between sarcopenia with lifestyle and family function among community-dwelling Chinese aged 60 years and older. BMC Geriatr. 2017, 17, 187. [Google Scholar] [CrossRef] [PubMed]
- Perälä, M.M.; von Bonsdorff, M.B.; Männistö, S.; Salonen, M.K.; Simonen, M.; Kanerva, N.; Rantanen, T.; Pohjolainen, P.; Eriksson, J.G. The healthy Nordic diet predicts muscle strength 10 years later in old women, but not old men. Age Ageing 2017, 46, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Bradlee, M.L.; Mustafa, J.; Singer, M.R.; Moore, L.L. High-protein foods and physical activity protect against age-related muscle loss and functional decline. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 88–94. [Google Scholar] [CrossRef]
- Radavelli-Bagatini, S.; Zhu, K.; Lewis, J.R.; Dhaliwal, S.S.; Prince, R.L. Association of dairy intake with body composition and physical function in older community-dwelling women. J. Acad. Nutr. Diet. 2013, 113, 1669–1674. [Google Scholar] [CrossRef]
- Radavelli-Bagatini, S.; Zhu, K.; Lewis, J.R.; Prince, R.L. Dairy food intake, peripheral bone structure, and muscle mass in elderly ambulatory women. J. Bone Miner. Res. 2014, 29, 1691–1700. [Google Scholar] [CrossRef]
- Lana, A.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. Dairy consumption and risk of frailty in older adults: A prospective cohort study. J. Am. Geriatr. Soc. 2015, 63, 1852–1860. [Google Scholar] [CrossRef]
- Daly, R.M.; O’Connell, S.L.; Mundell, N.L.; Grimes, C.A.; Dunstan, D.W.; Nowson, C.A. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: A cluster randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 899–910. [Google Scholar] [CrossRef]
- Charlton, K.; Walton, K.; Batterham, M.; Brock, E.; Langford, K.; McMahon, A.; Roodenrys, S.; Koh, F.; Host, A.; Crowe, R.; et al. Pork and chicken meals similarly impact on cognitive function and strength in community-living older adults: A pilot study. J. Nutr. Gerontol. Geriatr. 2016, 35, 124–145. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.J.; Robinson, S.; Orellana, L.; O’Connell, S.L.; Grimes, C.A.; Mundell, N.L.; Dunstan, D.W.; Nowson, C.A.; Daly, R.M. Effects of progressive resistance training combined with a protein-enriched lean red meat diet on health-related quality of life in elderly women: Secondary analysis of a 4-month cluster randomised controlled trial. Br. J. Nutr. 2017, 117, 1550–1559. [Google Scholar] [CrossRef]
- Neville, C.E.; Young, I.S.; Gilchrist, S.E.; McKinley, M.C.; Gibson, A.; Edgar, J.D.; Woodside, J.V. Effect of increased fruit and vegetable consumption on physical function and muscle strength in older adults. Age 2013, 35, 2409–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haub, M.D.; Wells, A.M.; Tarnopolsky, M.A.; Campbell, W.W. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am. J. Clin. Nutr. 2002, 76, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Haub, M.D.; Wells, A.M.; Campbell, W.W. Beef and soy-based food supplements differentially affect serum lipoprotein-lipid profiles because of changes in carbohydrate intake and novel nutrient intake ratios in older men who resistive-train. Metabolism 2005, 54, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Alemán-Mateo, H.; Macías, L.; Esparza-Romero, J.; Astiazaran-García, H.; Blancas, A.L. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: Evidence from a randomized clinical trial using a protein-rich food. Clin Interv. Aging 2012, 7, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Alemán-Mateo, H.; Carreón, V.R.; Macías, L.; Astiazaran-García, H.; Gallegos-Aguilar, A.C.; Enríquez, J.R. Nutrient-rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: A single-blind randomized clinical trial. Clin Interv. Aging 2014, 9, 1517–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, C.S.; Zhou, J.; Sayer, R.D.; Kim, J.E.; Campbell, W.W. Effects of a High-Protein Diet Including Whole Eggs on Muscle Composition and Indices of Cardiometabolic Health and Systemic Inflammation in Older Adults with Overweight or Obesity: A Randomized Controlled Trial. Nutrients 2018, 10, 946. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Beals, J.W.; Martinez, I.G.; Salvador, A.F.; Skinner, S.K. Food-First Approach to Enhance the Regulation of Post-exercise Skeletal Muscle Protein Synthesis and Remodeling. Sports Med. 2019, 49, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burd, N.A.; McKenna, C.F.; Salvador, A.F.; Paulussen, K.J.M.; Moore, D.R. Dietary protein quantity, quality, and exercise are key to healthy living: A muscle-centric perspective across the lifespan. Front. Nutr. 2019, 6, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, R.N.; Smeuninx, B.; Morgan, P.T.; Breen, L. Nutritional strategies to offset disuse-induced skeletal muscle atrophy and anabolic resistance in older adults: From whole-foods to isolated ingredients. Nutrients 2020, 12, 1533. [Google Scholar] [CrossRef]
- Hanach, N.I.; McCullough, F.; Avery, A. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: A systematic review and meta-analysis. Adv. Nutr. 2019, 10, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A. Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [Green Version]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Holwerda, A.M.; Paulussen, K.; Overkamp, M.; Goessens, J.; Kramer, I.F.; Wodzig, W.; Verdijk, L.B.; van Loon, L. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men. J. Nutr. 2019, 149, 221–230. [Google Scholar] [CrossRef]
- Maltais, M.L.; Ladouceur, J.P.; Dionne, I.J. The effect of resistance training and different sources of postexercise protein supplementation on muscle mass and physical capacity in sarcopenic elderly men. J. Strength Cond. Res. 2016, 30, 1680–1687. [Google Scholar] [CrossRef]
- Chernoff, R. Protein and older adults. J. Am. Coll. Nutr. 2004, 23, 627S–630S. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Burd, N.A.; Breen, L.; Rerecich, T.; Yang, Y.; Hector, A.J.; Baker, S.K.; Phillips, S.M. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl. Physiol. Nutr. Metab. 2013, 38, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Fulgoni, V.L., 3rd; Heaney, R.P.; Nicklas, T.A.; Slavin, J.L.; Weaver, C.M. Commonly consumed protein foods contribute to nutrient intake, diet quality, and nutrient adequacy. Am. J. Clin. Nutr. 2015, 101, 1346S–1352S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouvari, M.; Tyrovolas, S.; Panagiotakos, D.B. Red meat consumption and healthy ageing: A review. Maturitas 2016, 84, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Mata, F.; Morales, J.S.; Castillo-García, A.; Lucia, A. Does beef protein supplementation improve body composition and exercise performance? A systematic review and meta-analysis of randomized controlled trials. Nutrients 2019, 11, 1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the provide study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Brioche, T.; Lemoine-Morel, S. Oxidative stress, sarcopenia, antioxidant strategies and exercise: Molecular aspects. Curr. Pharm. Des. 2016, 22, 2664–2678. [Google Scholar] [CrossRef]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; Nunes Dos Santos, C.; Pinto, P.; Re, R.; Rémond, D.; et al. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food. Sci. Nutr. 2017, 57, 2497–2525. [Google Scholar] [CrossRef]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef]
- Banaszek, A.; Townsend, J.R.; Bender, D.; Vantrease, W.C.; Marshall, A.C.; Johnson, K.D. The effects of whey vs. pea protein on physical adaptations following 8-weeks of high-intensity functional training (HIFT): A pilot study. Sports 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Reinders, I.; Visser, M.; Wijnhoven, H. Two dietary advice strategies to increase protein intake among community-dwelling older adults: A feasibility study. Clin. Nutr. 2020, 37, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapuente, M.; Estruch, R.; Shahbaz, M.; Casas, R. Relation of fruits and vegetables with major cardiometabolic risk factors, markers of oxidation, and inflammation. Nutrients 2019, 11, 2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Ref. | Study Participants & SETTING | Study Design | Exposure | Outcome Measures | Summary of Main Findings |
|---|---|---|---|---|---|
| Asp et al. (2012) [21] | 142 adults aged 60–88; community-dwelling and ambulatory; OH, USA; | CS | Beef intake in the past 12 months assessed by the Diet History Questionnaire; | Muscle mass; GS; | Beef intake (g/day) was positively correlated to muscle mass measured by mid-arm muscle area (R = 0.128, p = 0.030). From multiple linear regression analysis, a 1 oz/day (~28 g/day) increase in beef consumption predicted a 2.3 cm2 increase in mid-arm muscle area. GS was not correlated with beef intake. |
| Morris & Jacques (2013) [22] | 2425 participants in the NHANES (2003–2006) aged ≥ 50 years; Boston, MA, USA; | CS | Beef intake estimated from 2 × 24-h recall; | Appendicular skeletal muscle (ASM) index assessed by DXA; | Each 100 g/week increase in beef intake was associated with a 0.10 (p = 0.04) and 0.13 (p = 0.006) point increase in AMS index in non-obese participants who engaged in vigorous aerobic and muscle-strengthening exercises, respectively. |
| Struijk et al. (2018) [23] | 2982 participants aged ≥ 60 years in the Seniors-ENRICA cohort; Spain; | PS | Meat intake assessed by a validated computer-assisted face-to-face diet history at baseline (2008–2010); | Functional tasks assessed by the Roscow–Breslau scale (agility and mobility); SPPB (a median follow-up 5.2 years); | Those in the highest tertile of processed meat had a higher risk of impaired agility (HR = 1.33; 95% CI 1.08–1.64, p = 0.01), and lower extremity function (SPPB) (HR = 1.32; 95% CI 1.02–1.68, p = 0.04) compared to those in the lowest tertile. A 100 g/day increase in processed meat consumption was associated with a 23% higher risk of poor agility. No associations were found with red meats (of any kind) and poultry. |
| Fruit and vegetables | |||||
| Kim et al. (2015) [24] | 823 men and 1089 women aged ≥ 65 years; the Fourth KNHANES (2008–2009); Korea; | CS | Fruit and vegetables from FFQ; | Sarcopenia defined as relative lean mass (height and fat mass-adjusted lean mass) assessed by DXA; | Vegetables, fruit and combined FV was significantly associated with reduced risk of sarcopenia in older men (p = 0.03, p = 0.01, p = 0.003, respectively). Men in the highest quintile of vegetables (OR = 0.48, 95% CI: 0.24–0.95), fruit (OR = 0.30; 95% CI: 0.13–0.70) and FV consumption (OR = 0.32; 95% CI: 0.16–0.67) had lower risk of sarcopenia compared to those in the lowest quintile. Women in the highest quintile of fruit intake had a lower risk of sarcopenia (OR = 0.39; 95% CI: 0.18–0.83) compared to those in the lowest quintile. |
| Garcia-Esquinas et al. (2016) [25] | Three cohorts: 1872 men and women aged 68.7 ± 6.4 years in the Seniors-ENRICA cohort, Spain; 581 participants aged 81.8 ± 4.1 years in Three-City (3C) Bordeaux, France; 473 participants aged 74.5 ± 5.8 years in the Integrated Multidisciplinary Approach Cohort (AMI), rural France; | PS | Fruit and vegetables assessed by either a validated computerised diet history (the Seniors-ENRICA) or FFQ (3C Bordeaux, AMI); | Gait speed (3-m walking speed test); | Decreased risk of slow walking speed with increasing portions of fruit consumed per day. An inverse dose-response relation was found between the baseline consumption of fruit and risk of slow walking speed. |
| Ribeiro et al. (2016) [26] | 432 African Americans aged 59.2 ± 4.4 years at in the African American Health Study. Study reports findings from multiple waves obtained (up to 10 year later); St. Louis, MO, USA; | PS | Fruit and vegetables assessed by the Behavioral Risk Factor Surveillance System at wave 8 (2008); | Gait speed, GS, SPPB, LBFL (wave 4 (2004) and wave 10 (2010)); | Longitudinally, higher vegetables intake different from carrots, potatoes or salad was independently associated with better outcomes for GS, while fruit juice was associated with worse changes over time for GS. |
| Sim et al. (2018) [27] | 1429 women aged ≥ 70 years in the Perth Longitudinal Study of Aging in Women; Perth, Australia; | PS | Fruit and vegetables from FFQ (validated by the Cancer Council of Victoria); | GS, TUG; | Vegetables consumption resulted in superior muscular strength (GS) and physical function (TUG). A 13% (p = 0.01) decreased risk of low GS (<22 kg) for every 75 g of vegetable serving. A 12% lower odds of slow TUG for every 75 g/day increase in vegetable intake. High vegetables intake (≥3 servings/day) was associated with 31% lower odds of low GS and 31% lower odds of slow TUG (both p = 0.02) compared with low vegetables intake (<2 servings/day). |
| Koyanagi et al. (2020) [28] | 14,585 older adults aged ≥ 65 years from low- and middle-income countries in the Study on Global Ageing and Adults Health (China, Ghana, India, Mexico, Russia, South Africa); | CS | Fruit and vegetables assessed by a question ‘How many servings of fruit and vegetables do you eat on a typical day?’ and calculated in quintiles (Q1–Q5); | Sarcopenia; | In unadjusted analysis, increased fruit consumption was associated with lower prevalence of sarcopenia in women (21% in Q1 (0 servings) versus 7.9% in Q5 (≥4 servings)). In adjusted analysis, Q5 was associated with lower odds of sarcopenia (OR = 0.60, 95% CI 0.42–0.84, p < 0.01) compared to Q1 in all participants, and in women (OR = 0.42, 95% CI 0.24–0.73, p < 0.01) and not in men. No associations were found for vegetables intake. |
| Multiple whole foods | |||||
| Robinson et al. (2008) [29] | 1569 men and 1414 women aged 59 to 73 from The Hertfordshire Cohort Study; Hertfordshire, UK; | CS; RS | White fish and shellfish, fatty fish, breakfast cereals, fruit and vegetables, nuts, eggs, offal, and other meats) assessed by FFQ based on the EPIC study questionnaire; | GS; | In multivariate analysis adjusted for height, age, and birth weight, each additional portion of fatty fish consumed per week was associated with a gain in GS of 0.43 kg (p = 0.005) in men and 0.48 kg (p < 0.001) in women. |
| Martin et al. (2011) [30] | 628 participants aged 63–73; Hertfordshire, UK; | CS | Fruit and vegetables, nuts, meat and meat dishes, white and shellfish and oily fish assessed by FFQ; | SPPB (3-m walk time, chair-rise test, one-legged balance test); | An inverse association between vegetables (p = 0.02), white and shell fish (p = 0.04), and oily fish (p = 0.007) and 3-m gait speed in women, which was not robust to adjustments. Higher nuts (p = 0.01) and vegetables intake (p = 0.02) was associated with shorter char-rise time in women. However, after adjustments only the association with vegetables remained robust. |
| Kim et al. (2015) [31] | 1486 men and 1799 women aged ≥ 65 years in the Fourth and Fifth KHANES (2008–2011); Korea; | CS | Meat, fish, eggs and legumes, fruit and vegetables assessed by FFQ; | ASM adjusted for weight and assessed by DXA; | In women, consuming recommended levels of vegetables (≥5/day from a list of 12 vegetables (Chinese cabbage, radish, dried radish leaves, bean sprouts, spinach, cucumber, hot peppers, carrots, pumpkin, cabbage, tomatoes, mushrooms) was associated with 48% lower odds of lower ASM (OR = 0.52, 95% CI 0.30–0.89). No associations with any of the food groups were observed in men. |
| Kojima et al. (2015) [32] | 575 community-dwelling women aged between 75–85 years; Itabashi Ward of Tokyo, Japan; | CS (2008); PS (2012) | Green and yellow vegetables, potatoes, fruit, soy products, seaweeds, seafood, meat, egg, and milk intake frequencies assessed by close-ended lifestyle questionnaire; | Isometric KES; | Cross sectional: No significant relationship between KES and frequency of food intake of the studied food groups. Prospective: Daily intake of soy products and green or yellow vegetables was protective of KES decline over 4 years. The decrease of KES (17.87 N) in participants who ate soy products almost every day was approximately 69% of that in those who ate soy products once in 2 days or less (26.06 N, p = 0.03). The decrease of KES (18.82 N) in participants who ate green or yellow vegetables almost daily was approximately 60% of that in those who ate these vegetables once in 2 days or less (31.46 N; p = 0.02). Those who ate seafood almost daily had a 1.5 times greater decrease in KES (24.68 N) than those who ate seafood once in 2 days or less (16.88 N, p = 0.02). |
| Perala et al. (2016) [33] | 1,072 men and women (mean age 71 years) in the Helsinki Birth Cohort Study (born 1934–1944); Helsinki, Finland; | PS | Elements of the Nordic Diet Score: Nordic fruit and berries (apples, pears and berries), Nordic vegetables (tomatoes, cucumber, leafy vegetables, roots, cabbages and peas), Nordic cereals (rye, oats and barley), Nordic fish (salmon and freshwater fishes), red and processed meat assessed with a validate FFQ at mean age of 61 (2001–2004); | SFT (assessed at mean age of 71 years (2011–2013)); | In women, high consumption of Nordic fruit and berries (p = 0.01), and Nordic cereals (p = 0.03) were positively related to the overall SFT score, whilst higher consumption of red and processed meat were inversely associated with the SFT score (p = 0.001). In men, high consumption of Nordic cereals was associated with better overall SFT score (p = 0.04). |
| Hai et al. (2017) [34] | 834 community-dwelling older adults aged between 60–92 years from Chengdu, Sichuan province, China | CS | Grains/cereals, fruit and vegetables, eggs, fish/shrimp, nuts, meat (pork, beef, mutton, poultry), milk/milk products, legumes assessed by a validated FFQ and based on the Chinese Food Guide Pagoda; | Sarcopenia defined according to the AWGS criteria; | Women with sarcopenia had lower frequency per week of nut consumption than those without sarcopenia (mean (SD): 0.05 (0.22) versus 0.81 (2.11), p = 0.02). Higher frequency per week of nut consumption was significantly associated with sarcopenia (adjusted OR 0.72, 95% CI 0.53–0.99, p < 0.05). No significant difference in regard to any of food groups was observed in men (with or without sarcopenia). |
| Perala et al. (2017) [35] | 1072 men and women (mean age 71) in the Helsinki Birth Cohort Study (born 1934–1944); Helsinki, Finland; | PS | Elements of the Nordic Diet Score: Nordic fruit and berries (apples, pears and berries), Nordic vegetables (tomatoes, cucumber, leafy vegetables, roots, cabbages and peas), Nordic cereals (rye, oats and barley), Nordic fish (salmon and freshwater fishes), red and processed meat assessed with a validate FFQ at mean age of 61 (2001–2004); | GS, isometric leg strength (knee extension), body composition (BIA) assessed at mean age of 71 years (2011–2013) | In women, Nordic cereals intake were positively related to leg strength change (p = 0.05), whereas red and processed meats were inversely related to GS change (p = 0.001). |
| Bradlee et al. (2018) [36] | 1016 men and 1333 women median age 52 years in the Framingham Offspring Study; Boston starting in 1972, USA; | PS | Red meats, poultry and fish, dairy, legumes, soy, nuts and seeds assessed at exam 3 and 5 from 3-day dietary records; servings/day derived using the standard United States Department of Agriculture serving size; | Skeletal muscle mass (SMM) assessed by BIA at exam 6 and 7 (approximately 9-year follow-up); selected functional tasks reflecting impaired muscle function from the Roscow–Breslau scale and the Nagi scale assessed at exam 5 through 8 (approximate 13-year follow-up); | Women who consumed ≥2 servings of red meats (beef, lamb, and pork)/day had an extra mean 1.2% SMM (p<0.001) compared with those consuming <0.85 servings/day (1 serving = 1 ounce, cooked). Men and women consuming ≥3 servings/day of poultry and fish compared to those consuming <1 had an extra mean % SMM of 0.8 (p = 0.02) and 1.2 (p = 0.001), respectively (1 serving = 1 ounce, cooked). Men and women consuming ≥1.25 servings/day of legumes, soy, nuts and seeds compared with those consuming < 0.25 had an extra mean % SMM of 0.7 (p = 0.02) and 0.8 (p = 0.02), respectively over 9 years (1 serving = 1 cup, cooked). A non-significant 20% reduction in developing functional decline in ≥2 tasks in those aged ≥ 50 years who consumed ≥1 serving/day of dairy (1 serving = 1 cup of milk or yoghurt, 1–1.5–ounce cheese) or ≥1 (women) or ≥2 (men) servings of poultry and fish versus less. |
| Dairy (including semi-solids and cheese) | |||||
| Radavelli-Bagatini et al. (2013) [37] | 1456 women aged 70 to 85 years in the Calcium Intake Fracture Outcome Study (CAIFOS); Western Australia; | CS | Dairy intake (milk, yogurt and cheese products) assessed by a validated FFQ at baseline in 1998; | Skeletal muscle mass assessed by DXA, GS, and TUG | Compared to those in the lowest tertile of dairy intake (≤1.5 serving/day), women in the highest tertile (≥2.2 servings/day) had significantly greater whole body lean mass (34.4 ± 03 vs. 32.9 ± 0.03 kg, p = 0.001), ASM (15.3 ± 0.2 vs. 14.5 ± 0.2 kg, p = 0.002), greater GS (20.9 ± 0.2 vs. 20.0 ± 0.2 kg, p = 0.02), and 26% lower odds of poor TUG (>10.2 s) (p = 0.04). |
| Radavelli-Bagatini et al. (2014) [38] | 564 women aged 80 to 92 years in the Calcium Intake Fracture Outcome Study (CAIFOS) Aged Extension Study (CAIFOS/CARES); Western Australia; | CS | Dairy intake (milk, yogurt and cheese products) assessed by a validated FFQ from the Cancer Council Victoria at 10-year follow-up in 2008; | Skeletal muscle mass assessed by DXA | Women in the highest tertile of dairy intake (≥2.2 servings/day) had 4.0% higher ASM (p = 0.04) compared with the lowest tertile (≤1.5 serving/day), which remained significant after multivariate adjustments (3.3%, p = 0.01) (1 serving = 250 g milk, or 200 g yogurt, or 40 g of hard, firm, soft, and low-fat cheese, or 120 g cottage/ricotta cheese). |
| Lana et al. (2015) [39] | 1871 adults aged ≥ 60 in the Study of Nutrition and Cardiovascular Risk (Seniors-ENRICA); Spain | PS | Dairy (milk, yogurt, cheese) assessed at baseline (2008–2010) using a validated diet history (developed form the European Prospective Investigation into Cancer and Nutrition); | Gait speed (defined as height and sex-adjusted lowest quintile in the sample), GS. | Those who consumed ≥7 servings/week of dairy (low-fat milk or yogurt) had lower risk of slow gait speed (OR = 0.64, 95% CI = 0.44–0.92, p = 0.01). No associations were found for GS. One standard serving was defined as 250 mL for milk, 150 mL for yogurt, and 40 g for cheese. |
| Ref. | Study Participants & SETTING | Study Design | Intervention/Exposure | Outcome Measures | Summary of Main Findings |
|---|---|---|---|---|---|
| Meats | |||||
| Daly et al. (2014) [40] | 91 women aged 60–90 years; Melbourne, Australia; | Cluster RCT | PRT twice a week and allocated to either 160 g/day (cooked) lean red meat consumed across 2 meals/day for 6 days/week or ≥1 serving/day (25–30 g) carbohydrates (pasta or rice) for 4 months; | LTM, FSST, TUG, STS; | The intervention group experienced great gains in total body LTM and muscle strength compared with control group. Increases were demonstrated in a 10% greater increase in serum insulin-like growth factor I and a 16% greater reduction in the proinflammatory marker. |
| Charlton et al. (2016) [41] | Healthy older people aged ≥ 60 years (n = 48); mean age 78.2 ± 6.2 years; New South Wales, Australia; | Quasi-experimental study | Participants were instructed to continue with their habitual diet but to substitute 4 meals/week with either (1) pork (intervention) or (2) chicken (control)-containing meals for 12 weeks; | GS, sit-to-stand test, get-up-and-go test and 6MWT; | Provision of 4 pork meals/week did not result in improvements in cognitive function, nor measures of muscle strength or physical performance, compared to those consuming chicken meals (control) in healthy older adults. |
| Torres et al. (2017) [42] | 91 women aged 60–90 years; Melbourne, Australia; | Cluster RCT | PRT twice a week and allocated to either 160 g/day (cooked) lean red meat consumed across 2 meals/day for 6 days/week or ≥1 serving/day (25–30 g) carbohydrates (pasta or rice) over 4 months; | LTM, muscle strength (1-RM); | PRT combined with diet enriched with lean red meat induced changes in lower limb muscle strength not but LTM. |
| Fruit and vegetables | |||||
| Neville et al. (2013) [43] | 80 healthy, community-dwelling older adults aged 65–85 years; Belfast, Ireland; | RCT | Participants randomised to continue their normal diet (≥2 portions FV/day), or to consume ≥5 portions of FV/day for 16 weeks. | GS, SPPB; | Increased FV to 5 portions/day resulted in a modest increase in GS, but no effect on physical performance (SPPB) in healthy older adults. |
| Multiple whole foods | |||||
| Haub et al. (2002) [44] | 21 men (mean age 65 ± 5 years); AR, USA; | RCT | Men consumed habitual diets during the first week. During the next two weeks, all participants consumed a self-select LOV diet (textured vegetable protein (soy) products)). For the remaining 12 weeks, men were randomly assigned to either continue the LOV diet or begin a beef-containing diet (a self-selected LOV diet supplemented with beef); | Body density (plethysmography), biopsy, cross-sectional muscle area (computed tomography scans performed on a General Electric scanner); | There were no differences between the two groups in terms of muscle strength and size. |
| Haub et al. (2005) [45] | 21 men (mean age 65 ± 5 years); AR, USA | RCT | Men consumed habitual diets during the first week. During the next two weeks, all participants consumed a self-select LOV diet, including textured vegetable protein (soy) products. For the remaining 12 weeks, men were randomly assigned to either continue the LOV diet or begin a beef-containing diet (a self-selected LOV diet supplemented with beef). RT 3 day/week during the 12-week period; | Muscle strength and muscle power (3 maximal repetitions at 4 intensities relative to their 1-RM at the time of resting); | There were no differences between groups for upper body or lower body output at baseline or 12 weeks post RT and no differences in strength gains. |
| Dairy (including semi- solids and cheese) | |||||
| Aleman-Mateo et al. (2012) [46] | Older women (n = 23) and men (n = 17) with sarcopenia, mean age 76 ± 5.4 years; Hermosillo, Sonora, Mexico; | RCT | Participants in the intervention group were asked to consume their HD but add 210 g of ricotta cheese a day over 3 months (HD + RCH; divided into three equal portions of 70 g, ingested at breakfast, lunch and dinner). Subjects in the control group were instructed to consume only their habitual diet; | TASM, GS; | No differences between groups for changes in TASM and GS. Sarcopenic men in HD+RCD gained 260 g of TASM compared with 220 g of TASM in control group (2.7 versus 1.2% relative change), and lean body mass in arms (4.7 versus 1.3% relative change). |
| Aleman-Mateo et al. (2014) [47] | 100 healthy older men (n = 50) and women (n = 50) age 70.2 ± 7.0 years; Hermosillo, Sonora, Mexico; | RCT | Participants in the intervention group were asked to consume their HD but add 210 g of ricotta cheese a day over 12 weeks (HD+RCH; divided into three equal portions of 70 g, ingested at breakfast, lunch and dinner). Subjects in the control group were instructed to consume only their HD; | GS, SPPB, ASM; | Difference between groups for ASM (p = 0.009), intervention group (0.6 ± 3.5 kg) vs. control (−1.0 ± 2.6). No significant difference for GS, SPPB score, gait speed, and 5-chair rises, whilst balance scores were higher in intervention group. |
| Eggs | |||||
| Wright et al. (2018) [48] | 22 adults aged 50–80 years; West Lafayette, Indiana, USA; | Parallel-design RCT | A 12-week diet with three whole eggs per day versus a HD void of eggs; | muscle composition (lean mass, IMAT, MSCA) | A 12-week high-protein diet with whole eggs did not improve muscle composition in older adults with overweight or obesity. |
| Whole Foods | Muscle Health Outcome | Who? | Study Design and Quality of Evidence | Ref. |
|---|---|---|---|---|
| Meats | Observational studies | |||
 | mid-arm muscle area | older adults | cross-sectional; low RoB | [21] |
| ASM index | older adults | cross-sectional; low RoB | [22] | |
| SMM | women | prospective; medium RoB | [36] | |
| Fruit and vegetables | ||||
 | lean muscle mass | men | cross-sectional; medium RoB | [24] |
| walking speed | older adults | prospective; medium RoB | [25] | |
 | lean muscle mass | women | cross-sectional; medium RoB | [24] |
| walking speed | older adults | perspective; medium RoB | [25] | |
| grip strength | women | prospective; low RoB | [27] | |
| sarcopenia | older adults, women | cross-sectional; low RoB | [28] | |
| physical performance (SFT) | women | prospective; low RoB | [35] | |
 | walking speed | older adults, women | prospective, cross-sectional; medium RoB | [25,30] |
| grip strength | older adults, women | prospective; high & low RoB, respectively | [26,27] | |
| knee extension strength | women | prospective; low RoB | [32] | |
| physical performance (TUG) | women | prospective; low RoB | [27] | |
| chair rise | women | cross-sectional; medium RoB | [30] | |
| ASM | women | cross-sectional; low RoB | [31] | |
| Dairy products | ||||
 | ASM | women | cross-sectional; low & medium RoB | [37,38] |
| grip strength | women | cross-sectional; low RoB | [37] | |
| physical performance (TUG) | women | cross-sectional; low RoB | [37] | |
| walking speed | older adults | prospective; low RoB | [39] | |
| Meats | Intervention studies | |||
 | lean tissue mass | older women | RCT; low RoB | [40] |
| muscle strength (leg extension) | older women | RCT; low RoB | [40] | |
| Physical Component Score of SF-36 | older women | RCT; low RoB | [42] | |
| Fruit and vegetables | ||||
 | grip strength | older adults | RCT; high RoB | [44] |
| Supplemented LOV 1 | ||||
 | muscle strength (upper & lower body) | older men | RCT; high RoB | [44,45] |
| muscle power | older men | RCT; high RoB | [45] | |
| muscle size | older men | RCT; high RoB | [45] | |
| Dairy products | ||||
 | total ASM | sarcopenic men | RCT; some concerns for RoB | [46] |
| lean body mass in arms | sarcopenic men | RCT; some concerns for RoB | [46] | |
| grip strength | sarcopenic men & healthy older adults | RCT; some concerns; low RoB | [46,47] | |
| lean tissue in legs | healthy older adults | RCT; low RoB | [47] | |
| total muscle mass | healthy older adults | RCT; Low RoB | [47] | |
| Eggs | ||||
 | lean body mass (trunk and total) | overweight/obese older adults | RCT; some concerns for RoB | [48] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granic, A.; Dismore, L.; Hurst, C.; Robinson, S.M.; Sayer, A.A. Myoprotective Whole Foods, Muscle Health and Sarcopenia: A Systematic Review of Observational and Intervention Studies in Older Adults. Nutrients 2020, 12, 2257. https://doi.org/10.3390/nu12082257
Granic A, Dismore L, Hurst C, Robinson SM, Sayer AA. Myoprotective Whole Foods, Muscle Health and Sarcopenia: A Systematic Review of Observational and Intervention Studies in Older Adults. Nutrients. 2020; 12(8):2257. https://doi.org/10.3390/nu12082257
Chicago/Turabian StyleGranic, Antoneta, Lorelle Dismore, Christopher Hurst, Sian M. Robinson, and Avan A. Sayer. 2020. "Myoprotective Whole Foods, Muscle Health and Sarcopenia: A Systematic Review of Observational and Intervention Studies in Older Adults" Nutrients 12, no. 8: 2257. https://doi.org/10.3390/nu12082257





