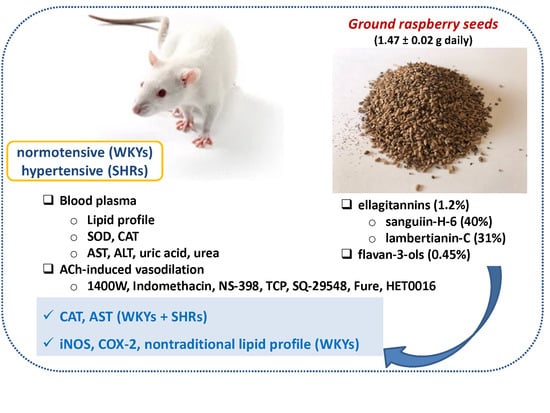
The Characterization of Ground Raspberry Seeds and the Physiological Response to Supplementation in Hypertensive and Normotensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Drugs and Chemicals
2.3. Ethical Statements
2.4. Animal Protocol and Dietary Treatment
2.5. Experimental Procedures
2.6. Analysis of Blood Plasma
2.7. Vascular Reactivity Studies
2.8. Data Analysis and Statistics
3. Results
3.1. Plant Material
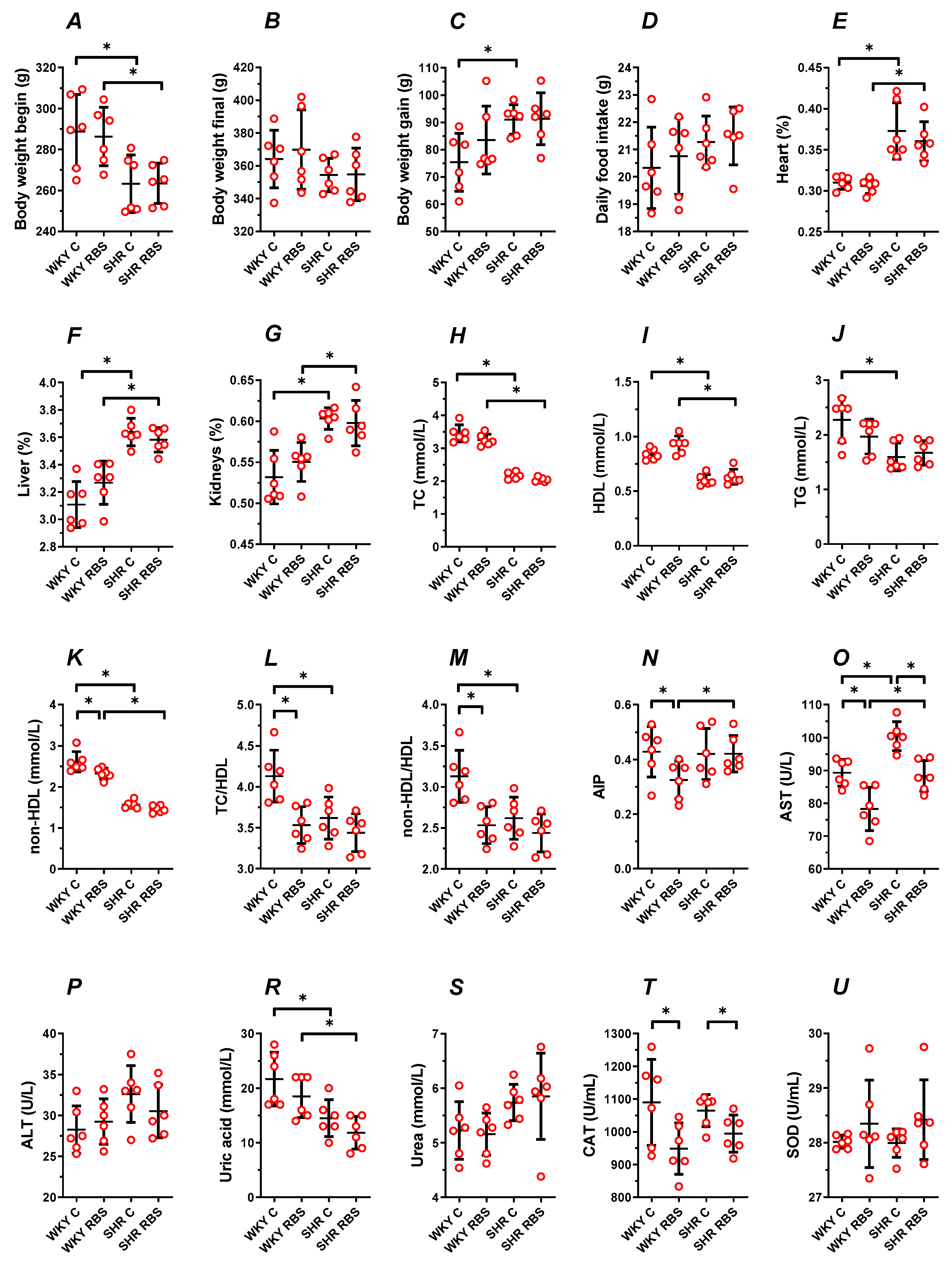
3.2. Animal Characteristics
3.3. Blood Plasma Lipid Profile
3.4. AST, ALT, Uric Acid, and Urea
3.5. The Enzymatic Antioxidant Status
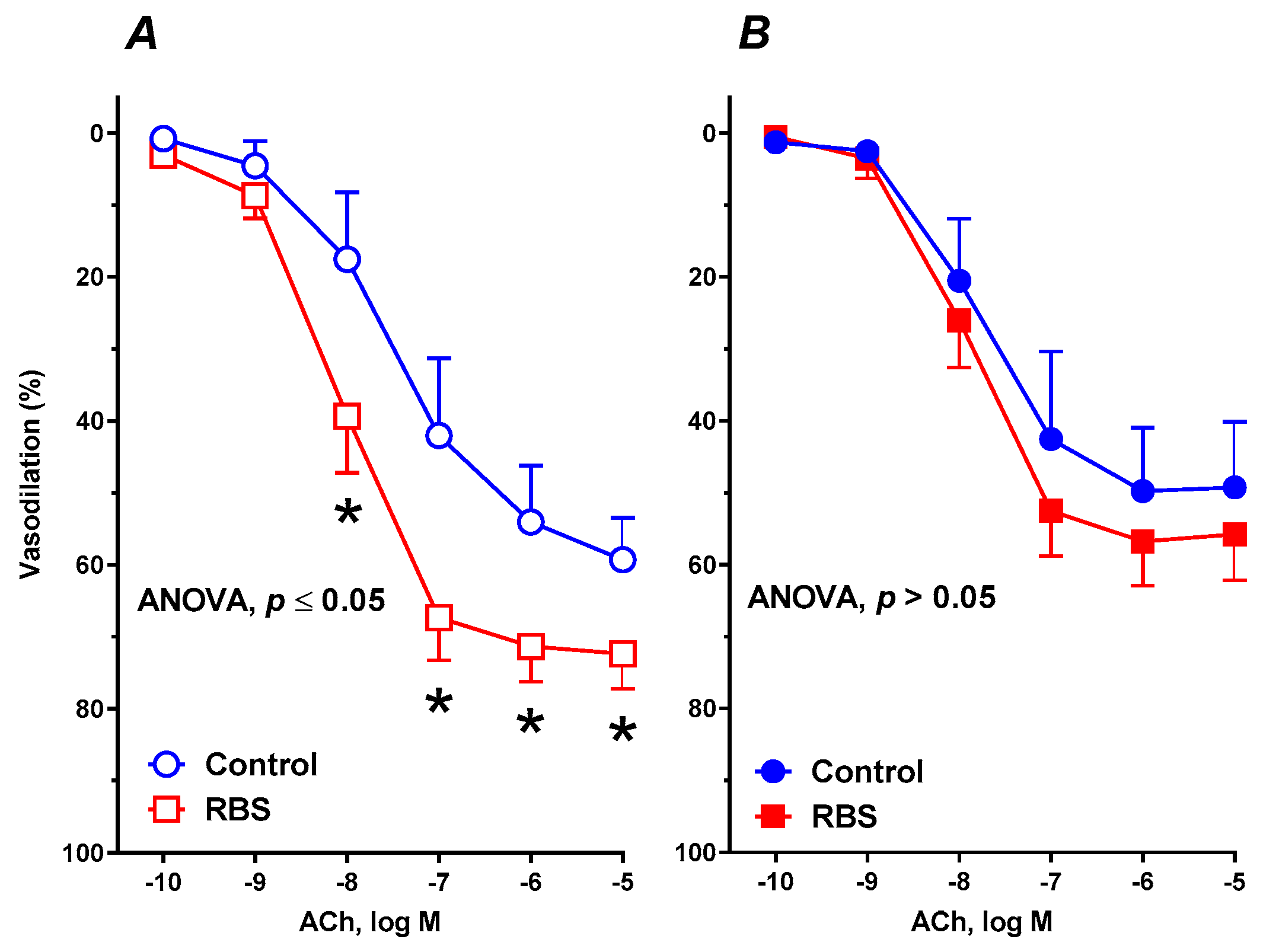
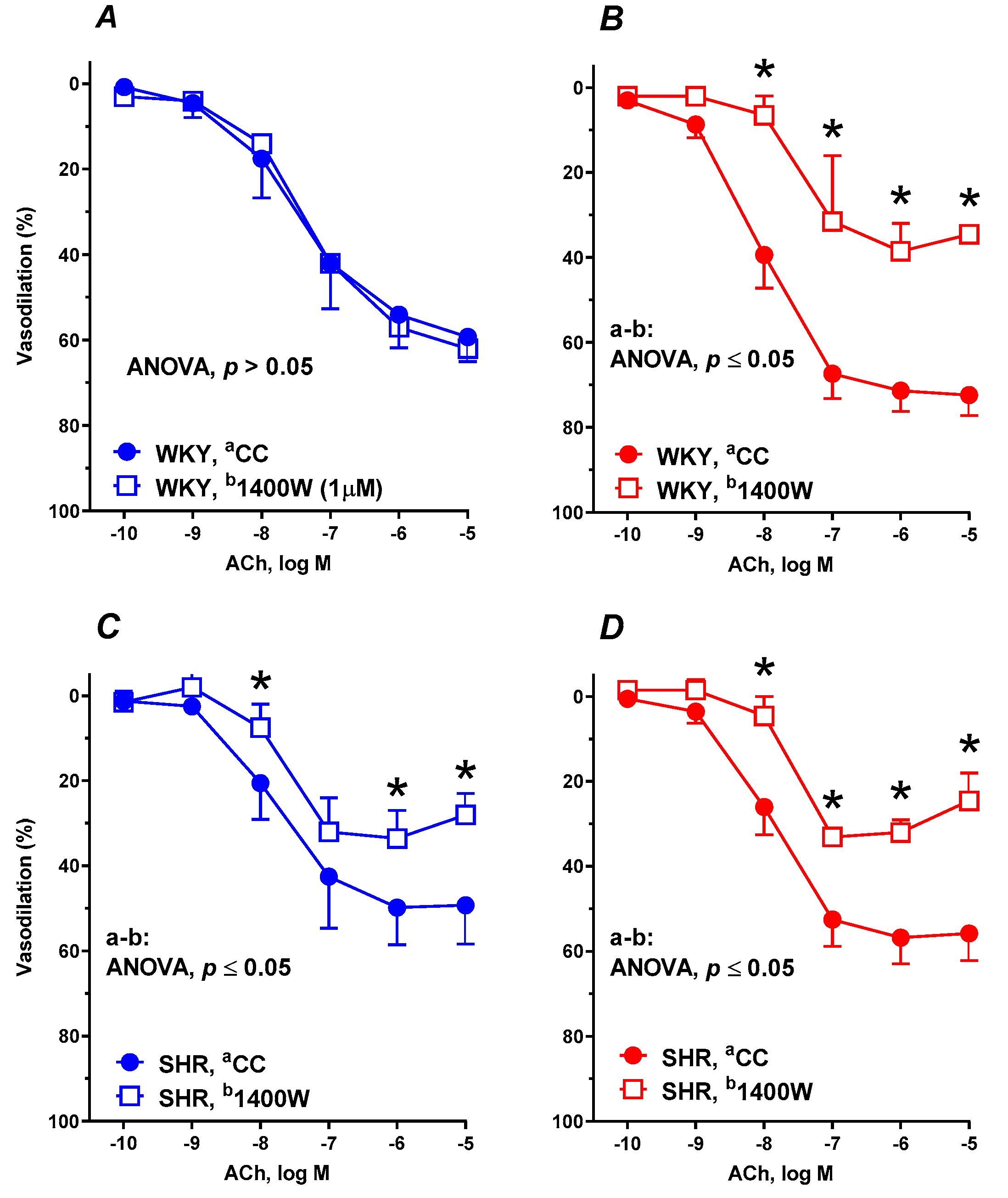
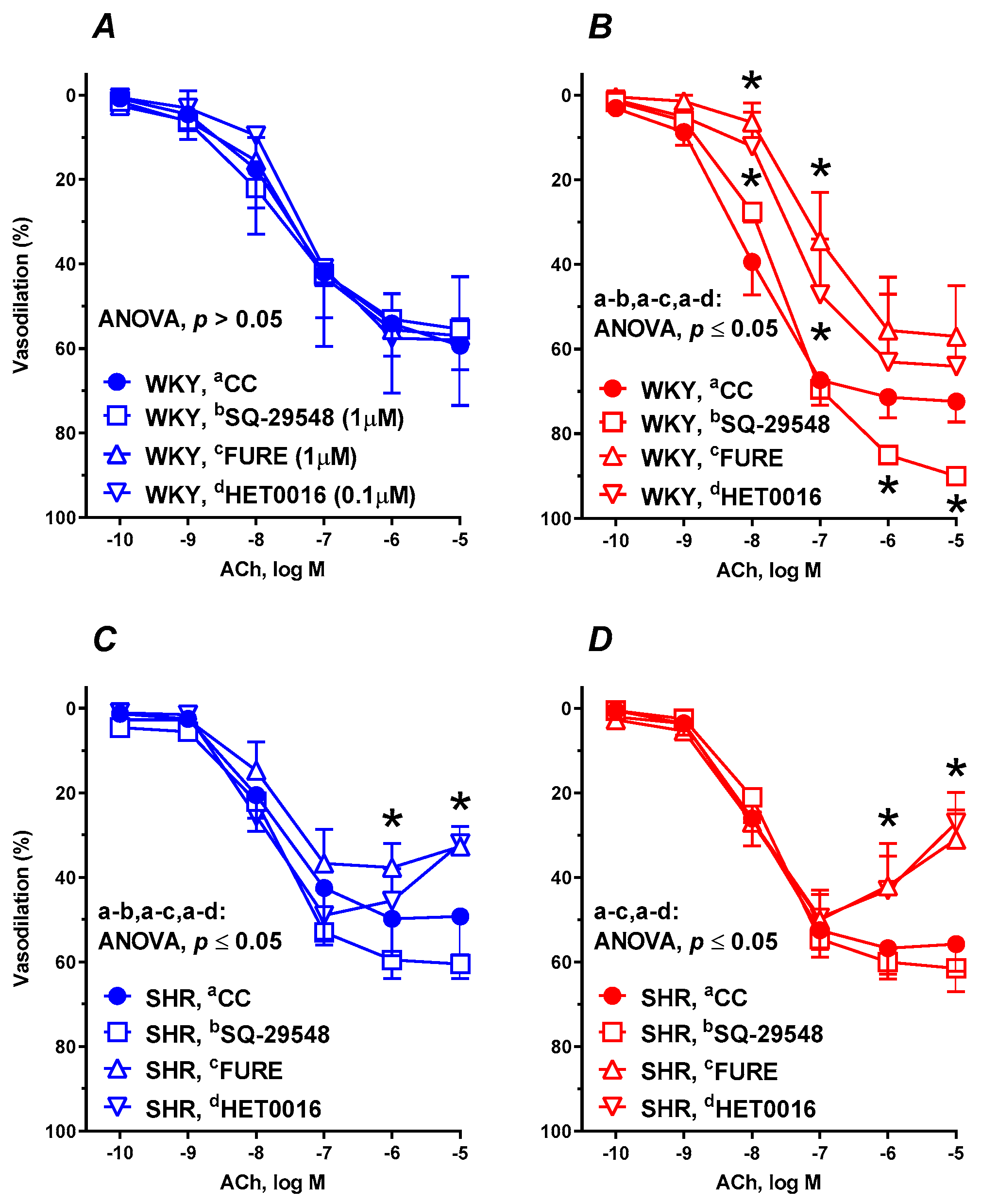
3.6. Vascular Reactivity Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wynne, B.M.; Labazi, H.; Lima, V.V.; Carneiro, F.S.; Webb, R.C.; Tostes, R.C.; Giachini, F.R. Mesenteric arteries from stroke–prone spontaneously hypertensive rats exhibit an increase in nitric–oxide–dependent vasorelaxation. Can. J. Physiol. Pharmacol. 2018, 96, 719–727. [Google Scholar] [CrossRef]
- Tunctan, B.; Korkmaz, B.; Cuez, T.; Kemal Buharalioglu, C.; Sahan–First, S.; Falck, J.; Malik, K.U. Contribution of vasoactive eicosanoids and nitric oxide production to the effect of selective cyclooxygenase–2 inhibitor, NS–398, on endotoxin–induced hypotension in rats. Basic Clin. Pharmacol. Toxicol. 2010, 107, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Blanco–Rivero, J.; Cachofeiro, V.; Lahera, V.; Aras–Lopez, R.; Márquez–Rodas, I.; Salaices, M.; Xavier, F.E.; Ferrer, M.; Balfagón, G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension 2005, 46, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Jung, H.; Lee, H.; Yi, H.C.; Kwak, H.K.; Hwang, K.T. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT–29 colon cancer cells. Food Funct. 2015, 6, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, M.; Zduńczyk, Z.; Juśkiewicz, J.; Jurgoński, A.; Karlińska, E.; Macierzyński, J.; Jańczak, R.; Rój, E. Chemical composition of defatted strawberry and raspberry seeds and the effect of these dietary ingredients on polyphenol metabolites, intestinal function, and selected serum parameters in rats. J. Agric. Food Chem. 2015, 63, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Fraser, P.D.; Martens, S. Carotenoids and tocopherols in yellow and red raspberries. Food Chem. 2013, 139, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M. Allium sativum: Facts and myths regarding human health. Rocz. Panstw. Zakl. Hig. 2014, 65, 1–8. [Google Scholar] [PubMed]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. The interaction between resveratrol and two forms of copper as carbonate and nanoparticles on antioxidant mechanisms and vascular function in Wistar rats. Pharmacol. Rep. 2019, 71, 862–869. [Google Scholar] [CrossRef]
- Majewski, M.; Ognik, K.; Thoene, M.; Rawicka, A.; Juśkiewicz, J. Resveratrol modulates the blood plasma levels of Cu and Zn, the antioxidant status and the vascular response of thoracic arteries in copper deficient Wistar rats. Toxicol. Appl. Pharmacol. 2020, 390, 114877. [Google Scholar] [CrossRef]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska–Sztachańska, M.; Jedrejek, D.; Stochmal, A.; Olas, B. Phenolic fractions from dandelion leaves and petals as modulators of the antioxidant status and lipid profile in an in vivo study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. The antioxidant status, lipid profile, and modulation of vascular function by fish oil supplementation in nano–copper and copper carbonate fed Wistar rats. J. Funct. Foods 2020, 64, 103595. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Sójka, M.; Jurgoński, A.; Zduńczyk, Z. Ellagitannins and flavan–3–ols from raspberry pomace modulate caecal fermentation processes and plasma lipid parameters in rats. Molecules 2015, 20, 22848–22862. [Google Scholar] [CrossRef] [Green Version]
- Feresin, R.G.; Huang, J.; Klarich, D.S.; Zhao, Y.; Pourafshar, S.; Arjmandi, B.H.; Salazar, G. Blackberry, raspberry and black raspberry polyphenol extracts attenuate angiotensin II-induced senescence in vascular smooth muscle cells. Food Funct 2016, 7, 4175–4187. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.; Hein, S.; Heiss, C.; Williams, C.; Rodriguez-Mateos, A. Blueberries and cardiovascular disease prevention. Food Funct. 2019, 10, 7621–7633. [Google Scholar] [CrossRef]
- Zafra–Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef]
- Noratto, G.D.; Chew, B.P.; Atienza, L.M. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017, 227, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Zielińska–Wasielica, J.; Olkowicz, M. Raspberry (Rubus idaeus L.) fruit extract decreases oxidation markers, improves lipid metabolism and reduces adipose tissue inflammation in hypertrophied 3T3–L1 adipocytes. J. Funct. Foods 2019, 62, 103568. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Rigby, N.; Sójka, M.; Kołodziejczyk, K.; Mackie, A.; Zduńczyk, Z. Raspberry pomace alters cecal microbial activity and reduces secondary bile acids in rats fed a high–fat diet. J. Nutr. Biochem. 2017, 46, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. Dietary supplementation with raspberry seed oil modulates liver functions, inflammatory state, and lipid metabolism in rats. J. Nutr. 2015, 145, 1793–1799. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Chen, L.; Huang, Q.; Wang, J.; Lin, Q.; Liu, M.; Lee, W.Y.; Song, H. Ultrasonic–assisted extraction of raspberry seed oil and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. PLoS ONE 2016, 11, e0153457. [Google Scholar] [CrossRef]
- Teng, H.; Lin, Q.; Li, K.; Yuan, B.; Song, H.; Peng, H.; Yi, L.; Wei, M.C.; Yang, Y.C.; Battino, M.; et al. Hepatoprotective effects of raspberry (Rubus coreanus Miq.) seed oil and its major constituents. Food Chem. Toxicol. 2017, 110, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2007. [Google Scholar]
- Sójka, M.; Klimczak, E.; Macierzyński, J.; Kołodziejczyk, K. Nutrient and polyphenolic composition of industrial strawberry Press cake. Eur. Food Res. Technol. 2013, 237. [Google Scholar] [CrossRef] [Green Version]
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from raspberry (Rubus idaeus L.) fruit as natural inhibitors of Geotrichum candidum. Molecules 2016, 21, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majewski, M.; Lepczyńska, M.; Dzika, E.; Grzegorzewski, W.; Markiewicz, W.; Mendel, M.; Chłopecka, M. Evaluation of the time stability of aortic rings in young Wistar rats during an eight–hour incubation period. J. Elem. 2019, 24, 677–686. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Kosmala, M.; Milala, J.; Zduńczyk, Z.; Markowski, J. Grinding levels of raspberry pomace affect intestinal microbial activity, lipid and glucose metabolism in Wistar rats. Food Res. Int. 2019, 120, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Pieszka, M.; Tombarkiewicz, B.; Roman, A.; Migdał, W.; Niedziółka, J. Effect of bioactive substances found in rapeseed, raspberry and strawberry seed oils on blood lipid profile and selected parameters of oxidative status in rats. Environ. Toxicol. Pharmacol. 2013, 36, 1055–1062. [Google Scholar] [CrossRef]
- Ash, M.M.; Wolford, K.A.; Carden, T.J.; Hwang, K.T.; Carr, T.P. Unrefined and refined black raspberry seed oils significantly lower triglycerides and moderately affect cholesterol metabolism in male Syrian hamsters. J. Med. Food 2011, 14, 1032–1038. [Google Scholar] [CrossRef]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine (Baltimore) 2017, 96, e8058. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, L.; Zhou, H.; Ma, Q.; Zhou, X.; Lei, T.; Hu, J.; Xu, W.; Yi, N.; Lei, S. Atherogenic index of plasma is a novel and better biomarker associated with obesity: A population–based cross–sectional study in China. Lipids Health Dis. 2018, 17, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Zentella, M.L.; Hernández-Muñoz, R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxid. Med. Cell. Longev. 2016, 3529149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinnuga, A.M.; Siboto, A.; Khumalo, B.; Sibiya, N.H.; Ngubane, P.; Khathi, A. Bredemolic acid ameliorates selected liver function biomarkers in a diet–induced prediabetic rat model. Can. J. Gastroenterol. Hepatol. 2020, 2475301. [Google Scholar] [CrossRef] [PubMed]
- Godevac, D.; Tesević, V.; Vajs, V.; Milosavljević, S.; Stanković, M. Antioxidant properties of raspberry seed extracts on micronucleus distribution in peripheral blood lymphocytes. Food Chem. Toxicol. 2009, 47, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Jurgoński, A.; Fotschki, B.; Juśkiewicz, J. The toxic effects of monosodium glutamate (MSG) – The involvement of nitric oxide, prostanoids and potassium channels in the reactivity of thoracic arteries in MSG–obese rats. Toxicol. Appl. Pharmacol. 2018, 359, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Lis, B.; Olas, B.; Ognik, K.; Juśkiewicz, J. Dietary supplementation with copper nanoparticles influences the markers of oxidative stress and modulates vasodilation of thoracic arteries in young Wistar rats. PLoS ONE 2020, 15, e0229282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majewski, M.; Ognik, K.; Juśkiewicz, J. Copper nanoparticles modify the blood plasma antioxidant status and modulate the vascular mechanisms with nitric oxide and prostanoids involved in Wistar rats. Pharmacol. Rep. 2019, 71, 509–516. [Google Scholar] [CrossRef]
- Majewski, M.; Ognik, K.; Zdunczyk, P.; Juskiewicz, J. Effect of dietary copper nanoparticles versus one copper (II) salt: Analysis of vasoreactivity in a rat model. Pharmacol. Rep. 2017, 69, 1282–1288. [Google Scholar] [CrossRef]
- Tunctan, B.; Sari, A.N.; Kacan, M.; Unsal, D.; Buharalioglu, C.K.; Sahan–First, S.; Korkmaz, B.; Falck, J.R.; Malik, K.U. NS–398 reverses hypotension in endotoxemic rats: Contribution of eicosanoids, NO, and peroxynitrite. Prostaglandins Other Lipid Mediat. 2013, 104–105, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharmacother. 2017, 93, 370–375. [Google Scholar] [CrossRef]
- Diebolt, M.; Bucher, B.; Andriantsitohaina, R. Wine Polyphenols Decrease Blood Pressure, Improve NO Vasodilatation, and Induce Gene Expression. Hypertension 2001, 38, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossoni, G.; Grande, S.; Galli, C.; Visioli, F. Wild artichoke prevents the age-associated loss of vasomotor function. J. Agric. Food Chem. 2005, 53, 10291–10296. [Google Scholar] [CrossRef] [PubMed]
- Mavangira, V.; Brown, J.; Gandy, J.C.; Sordillo, L.M. 20–hydroxyeicosatetraenoic acid alters endothelial cell barrier integrity independent of oxidative stress and cell death. Prostaglandins Other Lipid Mediat. 2020, 149, 106425. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.J.; Ceravolo, G.S.; Echem, C.; Hashimoto, C.M.; Costa, B.P.; Santos–Eichler, R.A.; Oliveira, M.A.; Jiménez–Altayó, F.; Akamine, E.H.; Dantas, A.P.; et al. Detrimental effects of testosterone addition to estrogen therapy involve cytochrome p–450–induced 20–hete synthesis in aorta of ovariectomized spontaneously hypertensive rat (SHR), a Model of Postmenopausal Hypertension. Front. Physiol. 2018, 9, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visioli, F.; de la Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étienne-Selloum, N.; Li, H.; Martínez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis Versus Free Radical Scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Item | Value (%) |
|---|---|
| Dry matter content | 96.52 ± 0.18 |
| Ash | 1.71 ± 0.06 |
| Protein | 10.51 ± 0.45 |
| Fat | 14.14 ± 0.22 |
| Carbohydrates * | 68.50 |
| Total dietary fiber | 63.92 ± 0.38 |
| Soluble dietary fiber | 1.23 ± 0.02 |
| Insoluble fiber | 62.69 ± 0.40 |
| Total polyphenols | 1.66 ± 43.6 |
| Total polyphenols | mg/100 g |
| Ellagitannins | 1211.3 ± 34.4 |
| bis-HHDP-glucose isomer 1 | 34.6 ± 3.2 |
| bis-HHDP-glucose isomer 2 | 38.9 ± 2.4 |
| Sanguiin H-10 isomer 1 | 31.8 ± 0.5 |
| Lambertianin C without ellagic acid | 29.1 ± 1.4 |
| Sanguiin H-10 isomer 2 | 31.1 ± 1.0 |
| Lambertianin C isomer 1 | 21.5 ± 0.7 |
| Lambertianin C isomer 2 | 41.9 ± 0.0 |
| Lambertianin C isomer 3 | 14.4 ± 0.8 |
| Lambertianin D | 113.5 ± 3.0 |
| Lambertianin C | 375.8 ± 7.0 |
| Sanguiin H-6 | 478.8 ± 14.5 |
| Ellagic acid | 106.9 ± 4.8 |
| Flavan-3-ols, including | 453.95 ± 9.22 |
| Procyanidins | 439.03 ± 8.76 |
| Free catechins | 14.92 ± 0.46 |
| Experimental Group | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WKYs, Control | WKYs, RBS | SHRs, Control | SHRs, RBS | |||||||||||||
| m | AUC | Emax (%) | pEC50 | m | AUC | Emax (%) | pEC50 | m | AUC | Emax (%) | pEC50 | m | AUC | Emax (%) | pEC50 | |
| ACh * | 8 | 148.0 ± 24.12 | 57.08 ± 4.917 | 7.502 ± 0.2742 | 8 | 224.3 ± 14.66 # | 72.43 ± 2.908 # | 8.055 ± 0.1353 # | 8 | 140.5 ± 26.21 | 49.66 ± 4.965 | 7.823 ± 0.3193 | 8 | 166.9 ± 17.33 #€ | 57.1 ± 3.235 € | 7.942 ± 0.1798 |
| +1400W | 6 | 149.5 ± 21.3 | 60.95 ± 0.9386 | 7.322 ± 0.04617 | 6 | 96.75 ± 17.46 *# | 37.77 ± 4.492 *# | 7.50 ± 0.3692 * | 6 | 87.31 ± 12.19 * | 32.64 ± 3.688 * | 7.717 ± 0.3405 | 6 | 82.88 ± 7.028 * | 30.93 ± 3.191 * | 7.739 ± 0.295 |
| +Indo | 6 | 165.3 ± 9.624 | 70.09 ± 3.564 | 7.184 ± 0.1264 | 6 | 94.25 ± 13.02 *# | 54.38 ± 4.446 *# | 6.633 ± 0.1993 *# | 6 | 100.8 ± 10.57 * | 54.3 ± 3.673 | 6.827 ± 0.1419 * | 6 | 136.2 ± 21.52 *# | 59.97 ± 5.512 | 7.215 ± 0.255 * |
| +NS-398 | 6 | 99.50 ± 18.98 * | 49.57 ± 3.583 | 6.913 ± 0.1835 * | 6 | 87.67 ± 16.44 * | 42.72 ± 2.794 * | 7.091 ± 0.1647 * | 6 | 135.1 ± 11.34 | 52.07 ± 2.476 | 7.687 ± 0.1463 | 6 | 162.5 ± 21.11 # | 52.18 ± 1.568 | 8.159 ± 0.09929 |
| +TCP | 6 | 118.2 ± 25.26 * | 47.54 ± 5.896 * | 7.377 ± 0.3778 | 6 | 123.3 ± 10.88 * | 52.33 ± 2.62 * | 7.316 ± 0.1437 * | 6 | 130.7 ± 10.98 | 49.26 ± 2.667 | 7.675 ± 0.1672 | 6 | 166.2 ± 13.10 # | 56.21 ± 3.457 | 7.855 ± 0.2007 |
| +SQ-29548 | 6 | 177.9 ± 37.19 | 63.03 ± 7.603 | 7.641 ± 0.396 | 6 | 233.8 ± 32.669 *# | 88.34 ± 0.9784 *# | 7.592 ± 0.03549 * | 6 | 172.5 ± 7.331 | 61.09 ± 1.841 | 7.704 ± 0.1008 | 6 | 169.0 ± 8.703 | 62.01 ± 2.238 | 7.749 ± 0.1134 |
| +FURE | 6 | 149.8 ± 16.27 | 57.18 ± 4.771 | 7.457 ± 0.2676 | 6 | 126.2 ± 21.36 * | 58.39 ± 6.11 * | 7.147 ± 0.2344 * | 6 | 109.3 ± 14.76 * | 36.86 ± 3.14 * | 7.867 ± 0.2796 | 6 | 141.2 ± 17.23 *# | 41.21 ± 4.321 * | 8.319 ± 0.3902 *# |
| +HET0016 | 6 | 140.3 ± 7.080 | 59.31 ± 2.119 | 7.322 ± 0.1028 | 6 | 159.5 ± 26.36 * | 65.13 ± 7.552 * | 7.367 ± 0.3457 * | 6 | 138.0 ± 10.64 | 42.66 ± 3.8 | 8.231 ± 0.2997 * | 6 | 137.0 ± 11.57 * | 40.07 ± 4.368 * | 8.331 ± 0.3965 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewski, M.; Kucharczyk, E.; Kaliszan, R.; Markuszewski, M.; Fotschki, B.; Juśkiewicz, J.; Borkowska-Sztachańska, M.; Ognik, K. The Characterization of Ground Raspberry Seeds and the Physiological Response to Supplementation in Hypertensive and Normotensive Rats. Nutrients 2020, 12, 1630. https://doi.org/10.3390/nu12061630
Majewski M, Kucharczyk E, Kaliszan R, Markuszewski M, Fotschki B, Juśkiewicz J, Borkowska-Sztachańska M, Ognik K. The Characterization of Ground Raspberry Seeds and the Physiological Response to Supplementation in Hypertensive and Normotensive Rats. Nutrients. 2020; 12(6):1630. https://doi.org/10.3390/nu12061630
Chicago/Turabian StyleMajewski, Michał, Ewa Kucharczyk, Roman Kaliszan, Michał Markuszewski, Bartosz Fotschki, Jerzy Juśkiewicz, Małgorzata Borkowska-Sztachańska, and Katarzyna Ognik. 2020. "The Characterization of Ground Raspberry Seeds and the Physiological Response to Supplementation in Hypertensive and Normotensive Rats" Nutrients 12, no. 6: 1630. https://doi.org/10.3390/nu12061630
APA StyleMajewski, M., Kucharczyk, E., Kaliszan, R., Markuszewski, M., Fotschki, B., Juśkiewicz, J., Borkowska-Sztachańska, M., & Ognik, K. (2020). The Characterization of Ground Raspberry Seeds and the Physiological Response to Supplementation in Hypertensive and Normotensive Rats. Nutrients, 12(6), 1630. https://doi.org/10.3390/nu12061630