Dietary Supplementation with Dunaliella Tertiolecta Prevents Whitening of Brown Fat and Controls Diet-Induced Obesity at Thermoneutrality in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Cultivation and Preparation of Algal Extract
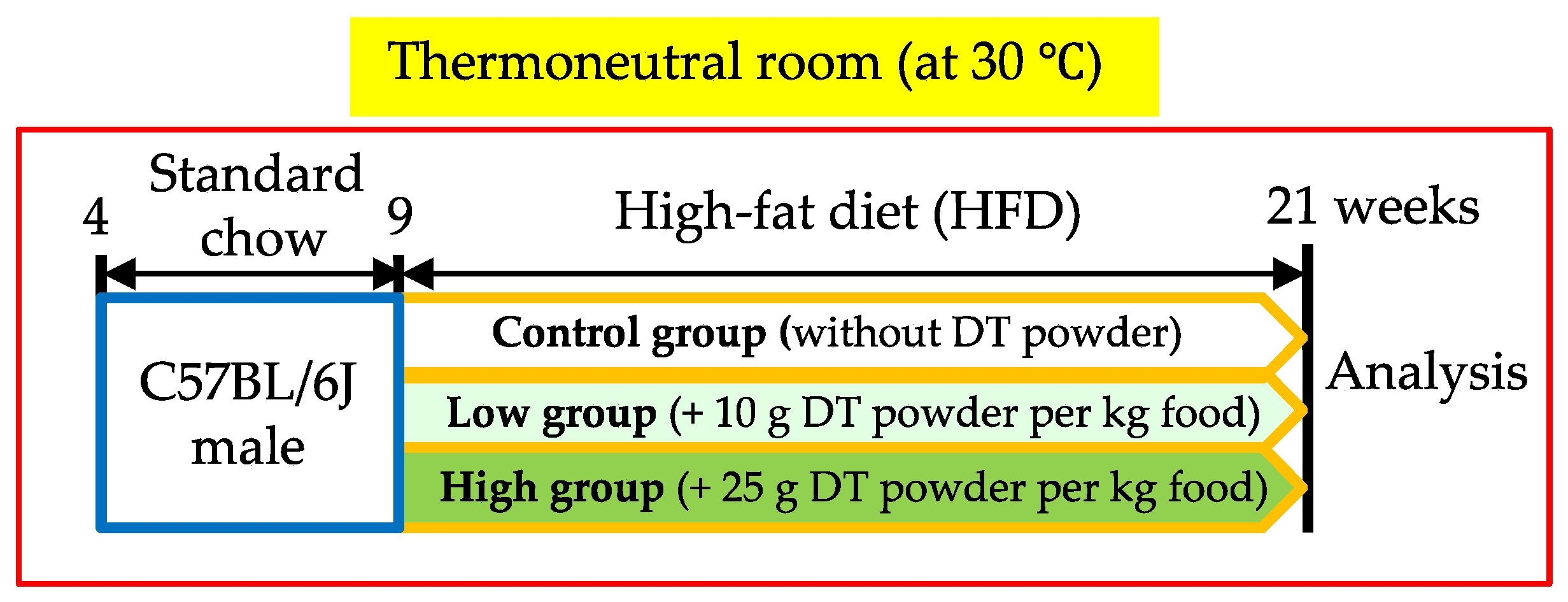
2.2. Experimental Animals
2.3. Blood Biochemistry
2.4. C3H10T1/2 Adipocyte Culture
2.5. Protein Analysis
2.6. Gene Expression Analysis
2.7. Histological Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of Evodiamine-Containing DT Supplementation on Diet-Induced Obesity at Thermoneutrality in Mice
3.2. Effect of DT Supplementation on Signal Transduction in WAT and Liver
3.3. DT Supplementation Stimulates Expression of Brown Fat-Associated Genes in IBAT of HFD-Fed Mice at Thermoneutrality
3.4. DT Extract Stimulates Brown Fat-Associated Gene Expression and FGF 21 Production in C3H10T1/2 Adipocytes
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolonin, M.G.; Saha, P.K.; Chan, L.; Pasqualini, R.; Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004, 625–632. [Google Scholar] [CrossRef]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.E.; Kozak, L.P. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brow adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Kontani, Y.; Wang, Y.; Kimura, K.; Inokuma, K.I.; Saito, M.; Suzuki-Miura, T.; Wang, Z.; Sato, Y.; Mori, N.; Yamashita, H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell 2005, 4, 147–155. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Tseng, Y.H.; Cypess, A.M.; Kahn, R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [Green Version]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Gene Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Alsenani, F.; Ahmed, F.; Schenk, P.M. Nutraceuticals from microalgae. In Nutraceuticals and Functional Foods in Human Health and Disease Prevention, 1st ed.; Bagchi, D., Preuss, H.G., Swaroop, A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 673–684. [Google Scholar]
- Hamed, I.; Özogul, F.; Özoğul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A Review. Compr. Rev. Food. Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Kumar, S.A.; Magnusson, M.; Ward, L.C.; Paul, N.A.; Brown, L. A green algae mixture of Scenedesmus and Schroederiella attenuates obesity-linked metabolic syndrome in rats. Nutrients 2015, 7, 2771–2787. [Google Scholar] [CrossRef]
- Gille, A.; Stojnic, B.; Derwenskus, F.; Trautmann, A.; Schmid-Staiger, U.; Posten, C.; Briviba, K.; Palou, A.; Bonet, M.L.; Ribot, J. A lipophilic fucoxanthin-rich Phaeodactylum tricornutum extract ameliorates effects of diet-induced obesity in C57BL/6J Mice. Nutrients 2019, 11, 796. [Google Scholar] [CrossRef] [Green Version]
- Bauer, R.; Xiao, P.G. Fructus Evodiae Wuzhuyu. In Chromatographic Fingerprint Analysis of Herbal Medicines; Wagner, H., Bauer, R., Melchart, D., Xiao, P.G., Staudinger, A., Eds.; Springer Nature: Vienna, Austria, 2011; pp. 391–401. [Google Scholar]
- Chiou, W.F.; Chou, C.J.; Shum, A.Y.; Chen, C.F. The vasorelaxant effect of evodiamine in rat isolated mesenteric arteries: Mode of action. Eur. J. Pharmacol. 1992, 215, 277–283. [Google Scholar] [CrossRef]
- Takada, Y.; Kobayashi, Y.; Aggarwal, B.B. Evodiamine abolishes constitutive and inducible NF-kappaB activation by inhibiting IkappaBalpha kinase activation, thereby suppressing NF-kappaB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J. Biol. Chem. 2005, 280, 17203–17212. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, Y.; Kontani, Y.; Kobayashi, Y.; Sato, Y.; Mori, N.; Yamashita, H. Evodiamine improves diet-induced obesity in a uncoupling protein-1-independent manner: Involvement of antiadipogenic mechanism and extracellularly regulated kinase/mitogen-activated protein kinase signaling. Endocrinology 2008, 149, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Kusudo, T.; Takeuchi, T.; Yamashita, Y.; Kontani, Y.; Okamatsu, Y.; Saito, M.; Mori, N.; Yamashita, H. Evodiamine inhibits insulin-stimulated mTOR-S6K activation and IRS1 serine phosphorylation in adipocytes and improves glucose tolerance in obese/diabetic mice. PLoS ONE 2013, 8, e83264. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Kusudo, T.; Takeuchi, T.; Qiao, S.; Tsutsumiuchi, K.; Wang, T.; Wang, Y. Dietary supplementation with evodiamine prevents obesity and improves insulin resistance in ageing mice. J. Funct. Foods 2015, 19, 320–329. [Google Scholar] [CrossRef]
- Sakaki, S.; Tsutsumiuchi, K.; Yamaguchi, Y.; Yamashita, H.; Takenaka, H. Identification of evodiamine in the microalga Dunaliella tertiolecta (Chlorophyceae). New Food Indust. 2018, 60, 13–18. [Google Scholar]
- Berner, T.; Dubinsky, Z.; Wyman, K.; Falkowski, P.G. Photo-adaptation and the “package” effect in Dunaliella tertiolecta (Chloropyneae). J. Phycol. 1989, 25, 70–78. [Google Scholar] [CrossRef]
- Villar, R.; Laguna, M.R.; Calleja, J.M.; Cadavid, I. Effects of Phaedactylum tricornutum and Dunaliella tertiolecta extracts on the central nervous system. Planta Med. 1992, 58, 405–409. [Google Scholar] [CrossRef]
- Fabregas, J.; Herrero, C.; Gamallo, Y.; Otero, A.; Paz, M.H.; Vecino, E. Decrease of plasma cholesterol with the marine microalga Dunaliella tertiolecta in hyper cholesterolemic rats. J. Gen. Appl. Microbiol. 1994, 40, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Villar, R.; Laguna, R.; Martinez, D.; Nunez, L.; Jimenez, C. Anti-aggregant effects on human platelets of the crude aqueous extract and polar fractions of the microalga Dunlaiella tertiolecta. Phytother. Res. 1997, 11, 70–72. [Google Scholar] [CrossRef]
- Kusudo, T.; Wang, Z.; Mizuno, A.; Suzuki, M.; Yamashita, H. TRPV4 deficiency increases skeletal muscle metabolic capacity and resistance against diet-induced obesity. J. Appl. Physiol. 2012, 112, 1223–1232. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunmeir, R.; Wu, J.; Peng, X.; Kim, S.Y.; Julien, S.G.; Zhang, Q.; Xie, W.; Xu, F. Comparative transcriptomic and epigenomic analyses reveal new regulators of murine brown adipogenesis. PLoS Genet. 2016, 12, e1006474. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Rossmeisl, M.; McClaine, J.; Leslie, P.K. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J. Clin. Invest. 2003, 111, 399–407. [Google Scholar] [CrossRef]
- Kataoka, N.; Takeuchi, T.; Kusudo, T.; Li, Y.; Endo, Y.; Yamashita, H. Lack of UCP1 stimulates fatty liver but mediates UCP1-independent action of beige fat to improve hyperlipidemia in Apoe knockout mice. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165762. [Google Scholar] [CrossRef]
- Véniant, M.M.; Sivits, G.; Helmering, J.; Komorowski, R.; Lee, J.; Fan, W.; Moyer, C.; Lloyd, D.J. Pharmacologic effects of FGF21 are independent of the “Browning” of white adipose tissue. Cell Metab. 2015, 21, 731–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, M.M.; O’Dwyer, S.M.; Baker, R.K.; Covey, S.D.; Kieffer, T.J. FGF21-mediated improvements in glucose clearance require uncoupling protein 1. Cell Rep. 2015, 13, 1521–1527. [Google Scholar] [CrossRef] [Green Version]
- Fisher, F.M.; Maratos-Flier, E. Understanding the physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [Green Version]
- Ni, B.; Farrar, J.S.; Vaitkus, J.A.; Celi, F.S. Metabolic Effects of FGF-21: Thermoregulation and Beyond. Front. Endocrinol. Lausanne 2015, 6, 148. [Google Scholar] [CrossRef] [Green Version]
- Serra, F.; Bonet, M.L.; Puigserver, P.; Oliver, J.; Palou, A. Stimulation of uncoupling protein 1 expression in brown adipocytes by naturally occurring carotenoids. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 650–655. [Google Scholar] [CrossRef] [Green Version]
- Coronel, J.; Pinos, I.; Amengual, J. β-carotene in Obesity Research: Technical Considerations and Current Status of the Field. Nutrients 2019, 11, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercader, J.; Palou, A.; Bonet, M.L. Induction of uncoupling protein-1 in mouse embryonic fibroblast-derived adipocytes by retinoic acid. Obes. Silver Spring 2010, 18, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.J.; Reginato, M.J.; Shao, D.; Krakow, S.L.; Lazar, M.A. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol. Cell Biol. 1997, 17, 1552–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Gene Symbol | Sense | Antisense |
|---|---|---|
| 36b4 | TCATCCAGCAGGTGTTTGACA | CCCATTGATGATGGAGTGTGG |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT |
| Fgf21 | GTGTCAAAGCCTCTAGGTTTCTT | GGTACACATTGTAACCGTCCTC |
| Ppargc1a | TAGGCCCAGGTACGACAGC | GCTCTTTGCGGTATTCATCC |
| Prdm16 | GACATTCCAATCCCACCAGA | CACCTCTGTATCCGTCAGCA |
| Ucp1 | GTGAAGGTCAGAATGCAAGC | AGGGCCCCCTTCATGAGGTC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, Y.; Takeuchi, T.; Endo, Y.; Goto, A.; Sakaki, S.; Yamaguchi, Y.; Takenaka, H.; Yamashita, H. Dietary Supplementation with Dunaliella Tertiolecta Prevents Whitening of Brown Fat and Controls Diet-Induced Obesity at Thermoneutrality in Mice. Nutrients 2020, 12, 1686. https://doi.org/10.3390/nu12061686
Yamashita Y, Takeuchi T, Endo Y, Goto A, Sakaki S, Yamaguchi Y, Takenaka H, Yamashita H. Dietary Supplementation with Dunaliella Tertiolecta Prevents Whitening of Brown Fat and Controls Diet-Induced Obesity at Thermoneutrality in Mice. Nutrients. 2020; 12(6):1686. https://doi.org/10.3390/nu12061686
Chicago/Turabian StyleYamashita, Yukari, Tamaki Takeuchi, Yuki Endo, Ayumi Goto, Setsuko Sakaki, Yuji Yamaguchi, Hiroyuki Takenaka, and Hitoshi Yamashita. 2020. "Dietary Supplementation with Dunaliella Tertiolecta Prevents Whitening of Brown Fat and Controls Diet-Induced Obesity at Thermoneutrality in Mice" Nutrients 12, no. 6: 1686. https://doi.org/10.3390/nu12061686






