The Role of Vitamin D in Respiratory Allergies Prevention. Why the Effect Is so Difficult to Disentangle?
Abstract
:1. Introduction
2. Molecular Mechanisms of 25(OH)D Action in Asthma and Allergy Prevention
3. Clinical Background—Observational Studies
4. Clinical Background—Interventional Studies
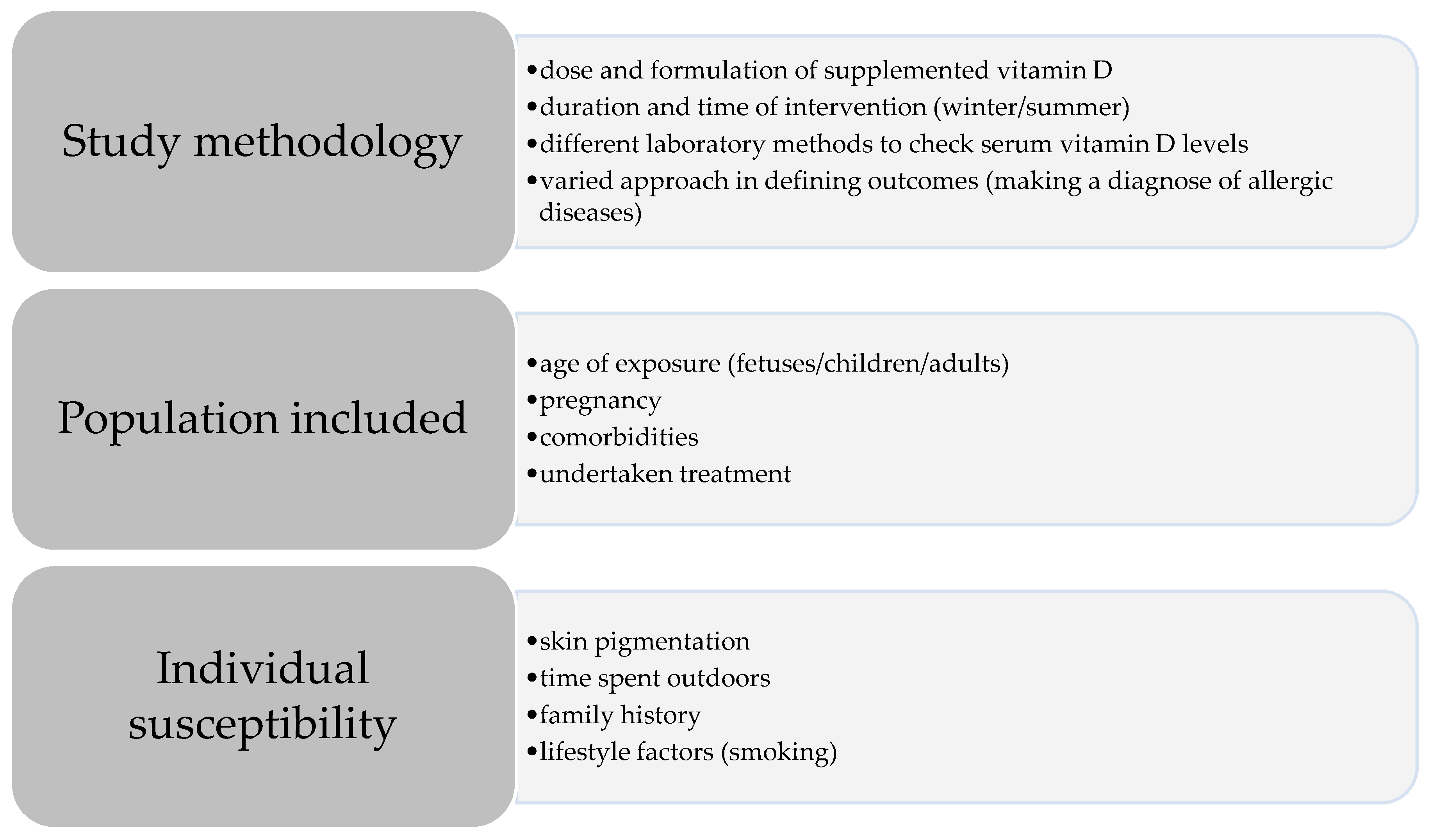
5. The Role of Vitamin D in Asthma and Respiratory Allergies Prevention: Where do the Different Conclusions Come from?
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Douros, K.; Boutopoulou, B.; Fouzas, S.; Loukou, I. Asthma and Allergy “Epidemic” and the Role of Vitamin D Deficiency. Adv. Exp. Med. Biol. 2017, 996, 169–183. [Google Scholar] [PubMed]
- Bunyavanich, S.; Rifas-Shiman, S.L.; Platts-Mills, T.A.; Workman, L.; Sordillo, J.E.; Camargo, C.A., Jr.; Gillman, M.W.; Gold, D.R.; Litonjua, A.A. Prenatal, perinatal, and childhood vitamin D exposure and their association with childhood allergic rhinitis and allergic sensitization. J. Allergy Clin. Immunol. 2016, 137, 1063–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef]
- Muehleisen, B.; Gallo, R.L. Vitamin D in allergic disease: Shedding light on a complex problem. J. Allergy Clin. Immunol. 2013, 131, 324–329. [Google Scholar] [CrossRef]
- Raby, B.A.; Silverman, E.K.; Lazarus, R.; Lange, C.; Kwiatkowski, D.J.; Weiss, S.T. Chromosome 12q harbors multiple genetic loci related to asthma and asthma-related phenotypes. Hum. Mol. Genet. 2003, 12, 1973–1979. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.S.; Hewison, M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matheu, V.; Back, O.; Mondoc, E.; Issazadeh-Navikas, S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: Enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J. Allergy Clin. Immunol. 2003, 112, 585–592. [Google Scholar] [CrossRef]
- Kamen, D.L.; Tangpricha, V. Vitamin D and molecular actions on the immune system: Modulation of innate and autoimmunity. J. Mol. Med. 2010, 88, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, B.; Heine, G.; Babina, M.; Steinmeyer, A.; Zügel, U.; Radbruch, A.; Worm, M. Targeting the vitamin D receptor inhibits the B cell-dependent allergic immune response. Allergy 2010, 66, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Jirapongsananuruk, O.; Melamed, I.; Leung, D.Y. Additive immunosuppressive effects of 1,25-dihydroxyvitamin D3 and corticosteroids on TH1, but not TH2, responses. J. Allergy Clin. Immunol. 2000, 106, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Damera, G.; Fogle, H.W.; Lim, P.; Goncharova, E.A.; Zhao, H.; Banerjee, A.; Tliba, O.; Krymskaya, V.P.; Panettieri, R.A., Jr. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br. J. Pharmacol. 2009, 158, 1429–1441. [Google Scholar] [CrossRef]
- Schauber, J.; Dorschner, R.A.; Yamasaki, K.; Brouha, B.; Gallo, R.L. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 2006, 118, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Li, X.X.; Qiu, S.Q.; Yu, Y.; Li, M.G.; Yang, L.T.; Li, L.J.; Wang, S.; Zheng, P.Y.; Liu, Z.G.; et al. Vitamin D contributes to mast cell stabilization. Allergy 2017, 72, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Xystrakis, E.; Kusumakar, S.; Boswell, S.; Peek, E.; Urry, Z.; Richards, D.F.; Adikibi, T.; Pridgeon, C.; Dallman, M.; Loke, T.K.; et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J. Clin. Investig. 2006, 116, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Hollams, E.M.; Hart, P.H.; Holt, B.J.; Serralha, M.; Parsons, F.; de Klerk, N.H.; Zhang, G.; Sly, P.D.; Holt, P.G. Vitamin D and atopy and asthma phenotypes in children: A longitudinal cohort study. Eur. Respir. J. 2011, 38, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sjoukes, A.; Richards, D.; Banya, W.; Hawrylowicz, C.; Bush, A.; Saglani, S. Relationship between serum vitamin D, disease severity, and airway remodelling in children with asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.M.; Lucas, R.M.; Ponsonby, A.L.; Chapman, C.; Coulthard, A.; Dear, K.; Dwyer, T.; Kilpatrick, T.J.; McMichael, A.J.; Pender, M.P.; et al. The role of latitude, ultraviolet radiation exposure and vitamin D in childhood asthma and hayfever: An Australian multicenter study. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2011, 22, 327–333. [Google Scholar] [CrossRef]
- Searing, D.A.; Zhang, Y.; Murphy, J.R.; Hauk, P.J.; Goleva, E.; Leung, D.Y.M. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J. Allergy Clin. Immunol. Pract. 2010, 125, 995–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, E.R.; Goleva, E.; Jackson, L.P.; Stevens, A.D.; Leung, D.Y.M. Vitamin D levels, lung function, and steroid response in adult asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joudi, M.; Farid Hosseini, R.; Khoshkhui, M.; Salehi, M.; Kouzegaran, S.; Ahoon, M.; Jabbari Azad, F. Effects of Serum Vitamin D and Efficacy of Subcutaneous Immunotherapy in Adult Patients with Allergic Rhinitis. Allergy Asthma Immunol. Res. 2019, 11, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, S.; Carraro, S.; Giordano, G.; Boner, A.; Baraldi, E. Asthma, allergy and respiratory infections: The vitamin D hypothesis. Allergy 2012, 67, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xun, P.; Pike, K.; Wills, A.K.; Chawes, B.L.; Bisgaard, H.; Cai, W.; Wan, Y.; He, K. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: A meta-analysis of birth cohort studies. J. Allergy Clin. Immunol. 2017, 139, 1508–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennessy, A.; Hourihane, J.O.; Malvisi, L.; Irvine, A.D.; Kenny, L.C.; Murray, D.M.; Kiely, M.E. Antenatal vitamin D exposure and childhood eczema, food allergy, asthma and allergic rhinitis at 2 and 5 years of age in the atopic disease-specific Cork BASELINE Birth Cohort Study. Allergy 2018, 73, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Maretzke, F.; Bechthold, A.; Egert, S.; Ernst, J.B.; Melo van Lent, D.; Pilz, S.; Reichrath, J.; Stangl, G.I.; Stehle, P.; Volkert, D.; et al. Role of Vitamin D in Preventing and Treating Selected Extraskeletal Diseases-An Umbrella Review. Nutrients 2020, 12, 969. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-González, R.M.; García-Marcos, L.; Morales, E. Prenatal vitamin D status and respiratory and allergic outcomes in childhood: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 243–253. [Google Scholar] [CrossRef]
- Van Oeffelen, A.A.; Bekkers, M.B.; Smit, H.A.; Kerkhof, M.; Koppelman, G.H.; Haveman-Nies, A.; van der A, D.L.; Jansen, E.H.; Wijga, A.H. Serum micronutrient concentrations and childhood asthma: The PIAMA birth cohort study. Pediatr. Allergy Immunol. 2011, 22, 784–793. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.W.; Kim, M.J.; Sol, I.S.; Yoon, S.H.; Ahn, H.S.; Kim, H.J.; Sohn, M.H.; Kim, K.-E. Vitamin D levels in allergic rhinitis: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2016, 27, 580–590. [Google Scholar] [CrossRef]
- Rothers, J.; Wright, A.L.; Stern, D.A.; Halonen, M.; Camargo, C.A., Jr. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J. Allergy Clin. Immunol. 2011, 128, 1093–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyppönen, E.; Berry, D.J.; Wjst, M.; Power, C. Serum 25-hydroxyvitamin D and IgE—A significant but nonlinear relationship. Allergy 2009, 64, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Jerzyńska, J.; Stelmach, W.; Rychlik, B.; Majak, P.; Podlecka, D.; Woicka-Kolejwa, K.; Stelmach, I. Clinical and immunological effects of vitamin D supplementation during the pollen season in children with allergic rhinitis. Arch. Med. Sci. 2018, 14, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Majak, P.; Olszowiec-Chlebna, M.; Smejda, K.; Stelmach, I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J. Allergy Clin. Immunol. 2011, 127, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Arikoglu, T.; Kuyucu, S.; Karaismailoglu, E.; Batmaz, S.B.; Balci, S. The association of vitamin D, cathelicidin, and vitamin D binding protein with acute asthma attacks in children. Allergy Asthma Proc. 2015, 36, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Sovio, U.; Wjst, M.; Patel, S.; Pekkanen, J.; Hartikainen, A.L.; Järvelinb, M.R. Infant Vitamin D supplementation and allergic conditions in adulthood: Northern Finland birth cohort 1966. Ann. N. Y. Acad. Sci. 2004, 1037, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E., Jr.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of prenatal supplementation with vitamin d on asthma or recurrent wheezing in offspring by age 3 years: The VDAART randomized clinical trial. JAMA 2016, 315, 362–370. [Google Scholar] [CrossRef]
- Bisgaard, H.; Vissing, N.H.; Carson, C.G.; Bischoff, A.L.; Følsgaard, N.V.; Kreiner-Møller, E.; Chawes, B.L.; Stokholm, J.; Pedersen, L.; Bjarnadóttir, E.; et al. Deep phenotyping of the unselected COPSAC2010 birth cohort study. Clin. Exp. Allergy 2013, 43, 1384–1394. [Google Scholar] [CrossRef] [Green Version]
- Wolsk, H.M.; Chawes, B.L.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS ONE 2017, 12, e0186657. [Google Scholar] [CrossRef]
- Von Mutius, E.; Martinez, F.D. Vitamin D Supplementation during Pregnancy and the Prevention of Childhood Asthma. N. Engl. J. Med. 2020, 382, 574–575. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhshaee, M.; Sharifian, M.; Esmatinia, F.; Rasoulian, B.; Mohebbi, M. Therapeutic effect of vitamin D supplementation on allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2019, 276, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, F.; Cardoso, I.; Keller, A.; Stougaard, M.; Frederiksen, P.; Cohen, A.S.; Maslova, E.; Jacobsen, R.; Backer, V.; Heitmann, B.L. Neonatal Vitamin D Status and Risk of Asthma in Childhood: Results from the D-Tect Study. Nutrients 2020, 12, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skversky, A.L.; Kumar, J.; Abramowitz, M.K.; Kaskel, F.J.; Melamed, M.L. Association of glucocorticoid use and low 25-hydroxyvitamin D levels: Results from the National Health and Nutrition Examination Survey (NHANES): 2001–2006. J. Clin. Endocrinol. Metab. 2011, 96, 3838–3845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoxha, M.; Zoto, M.; Deda, L.; Vyshka, G. Vitamin D and Its Role as a Protective Factor in Allergy. Int. Sch. Res. Not. 2014, 2014, 951946. [Google Scholar] [CrossRef] [Green Version]
- Nwosu, B.U.; Kum-Nji, P. Tobacco smoke exposure is an independent predictor of vitamin D deficiency in US children. PLoS ONE 2018, 13, e0205342. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska-Szaflik, H.; Sozańska, B. The Role of Vitamin D in Respiratory Allergies Prevention. Why the Effect Is so Difficult to Disentangle? Nutrients 2020, 12, 1801. https://doi.org/10.3390/nu12061801
Sikorska-Szaflik H, Sozańska B. The Role of Vitamin D in Respiratory Allergies Prevention. Why the Effect Is so Difficult to Disentangle? Nutrients. 2020; 12(6):1801. https://doi.org/10.3390/nu12061801
Chicago/Turabian StyleSikorska-Szaflik, Hanna, and Barbara Sozańska. 2020. "The Role of Vitamin D in Respiratory Allergies Prevention. Why the Effect Is so Difficult to Disentangle?" Nutrients 12, no. 6: 1801. https://doi.org/10.3390/nu12061801
APA StyleSikorska-Szaflik, H., & Sozańska, B. (2020). The Role of Vitamin D in Respiratory Allergies Prevention. Why the Effect Is so Difficult to Disentangle? Nutrients, 12(6), 1801. https://doi.org/10.3390/nu12061801




