Comparison between Appendicular Skeletal Muscle Index DXA Defined by EWGSOP1 and 2 versus BIA Tengvall Criteria among Older People Admitted to the Post-Acute Geriatric Care Unit in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Assessment of Anthropometric Parameters and Body Composition
2.4. Food Intake Assessment
2.5. Handgrip
2.6. Health-Related Quality of Life
2.7. Barthel Index
2.8. Criteria Used for Appendicular Skeletal Muscle Index Estimation
- EWGSOP1 ASM/height2 (<7.23 kg/m2 for men and <5.67 kg/m2 for women);
- EWGSOP2 ASM/height2 (<7.0 kg/m2 for men and <5.5 kg/m2 for women);
- Tengvall SMI: moderate sarcopenia when SMI is between 8.51 and 10.75 kg/m2 (men) or 5.76 and 6.75 kg/m2 (women) and severe sarcopenia when SMI is ≤8.50 kg/m2 (men) or ≤5.75 kg/m2.
2.9. Statistical Analysis
3. Results
3.1. Study Sample
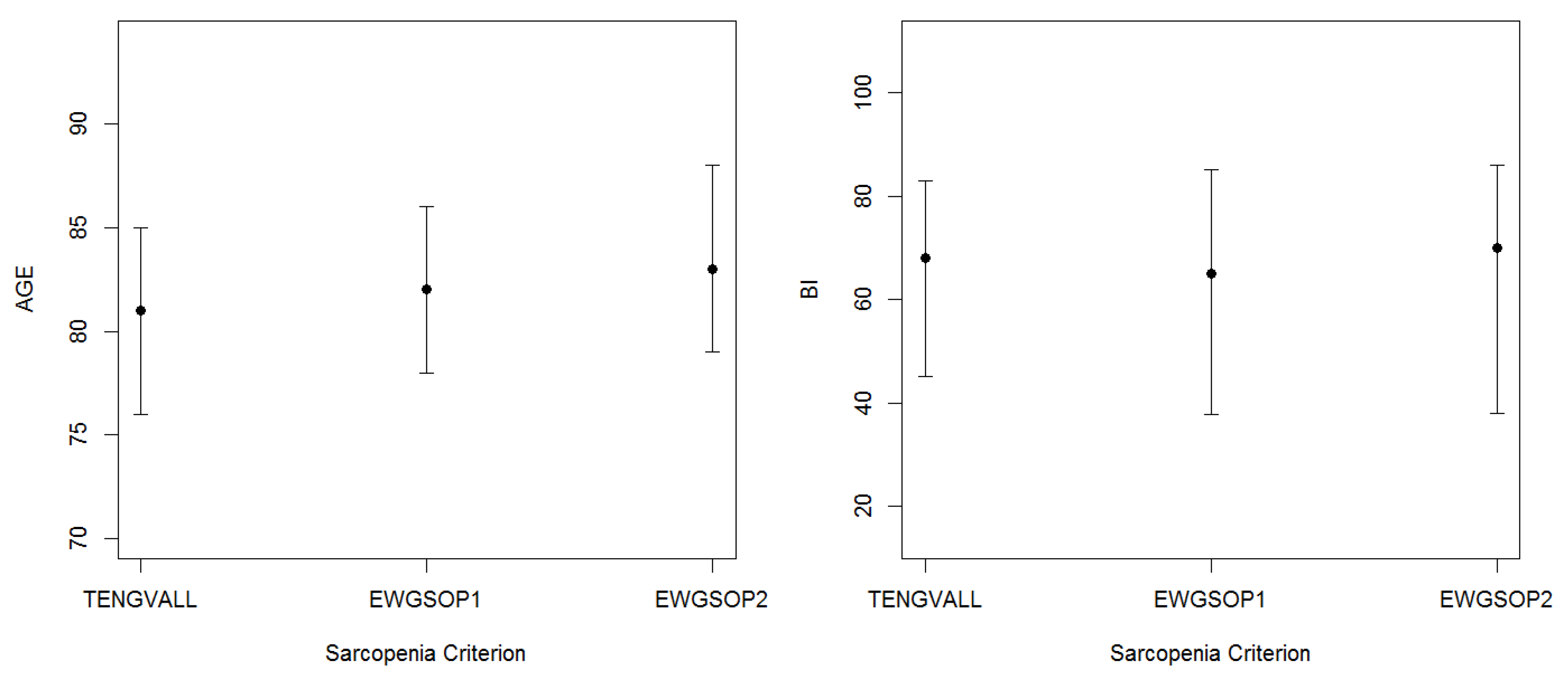
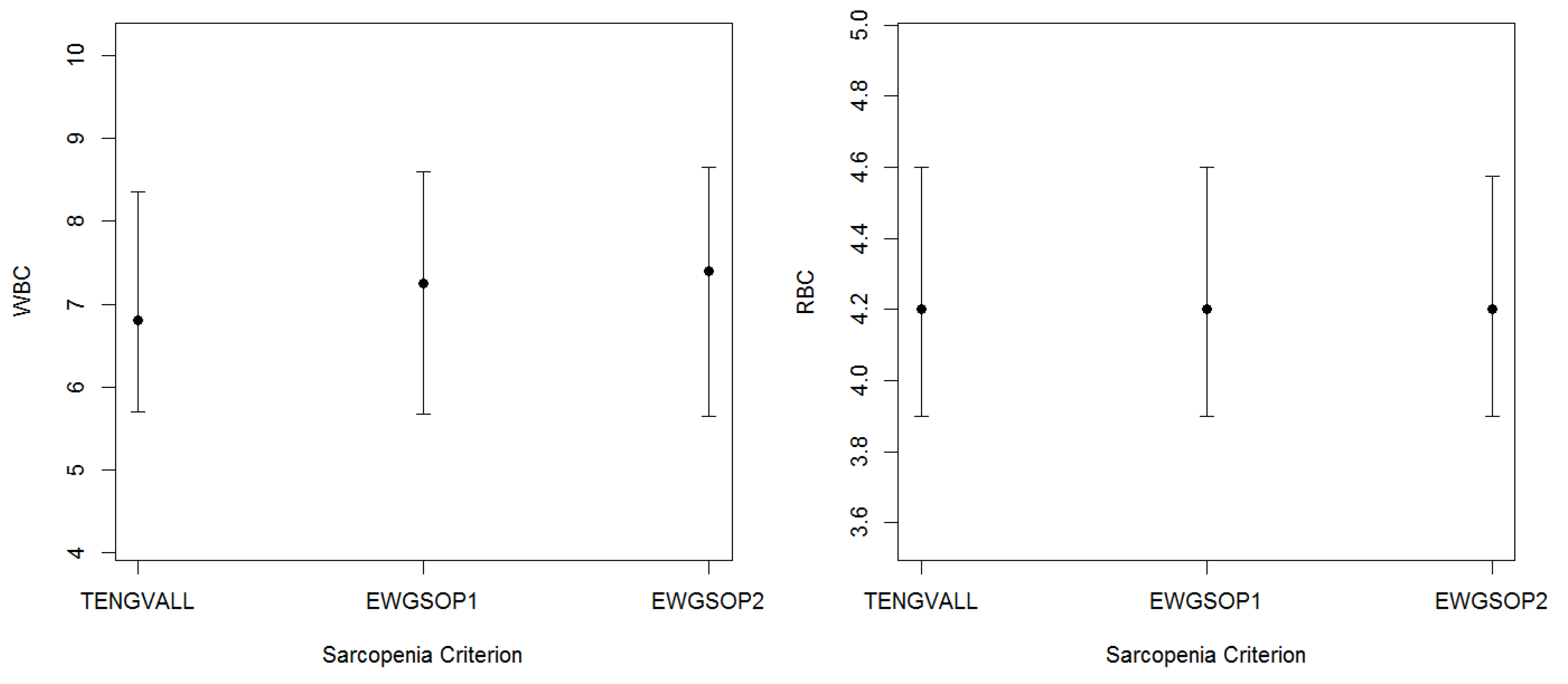
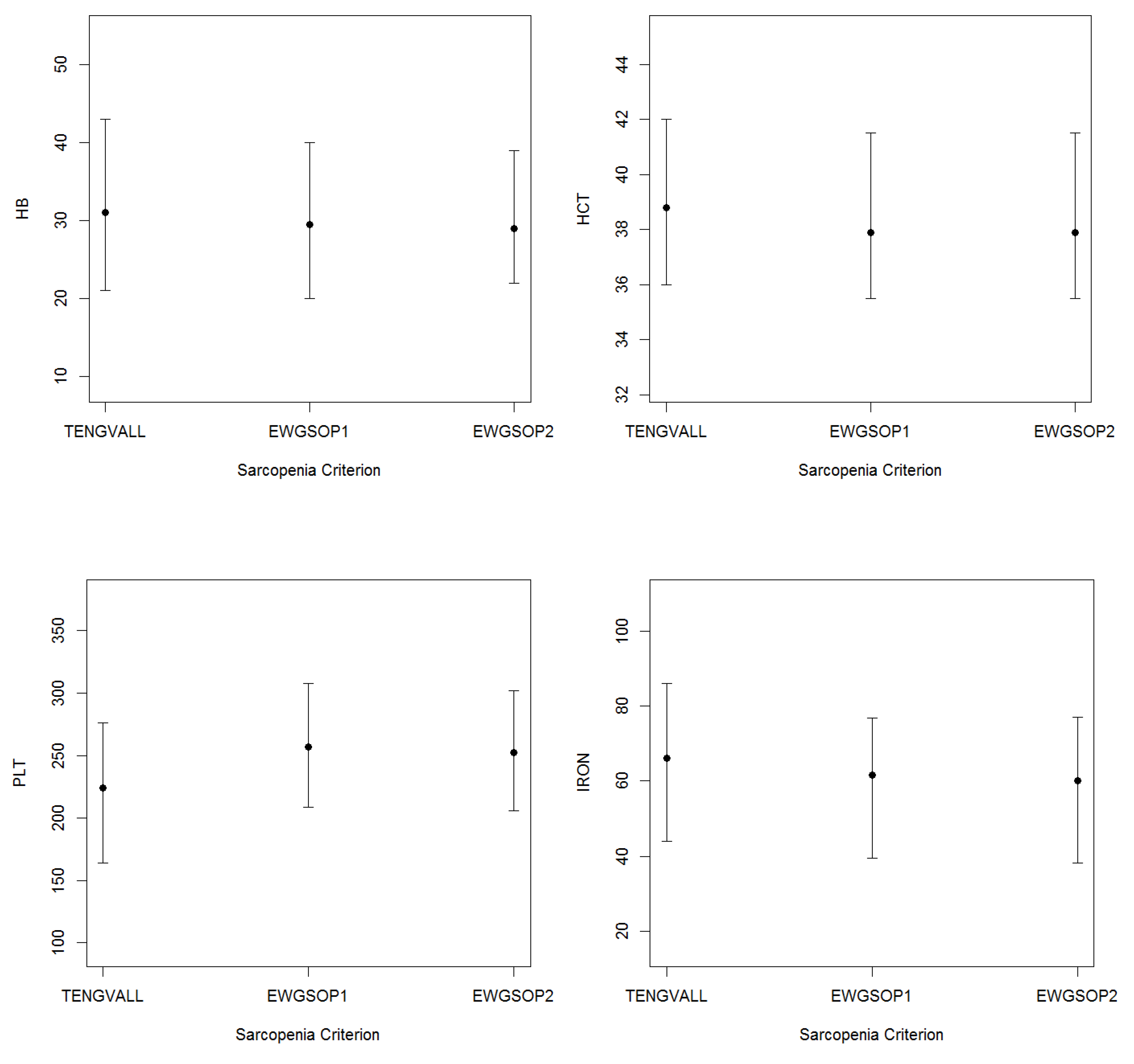
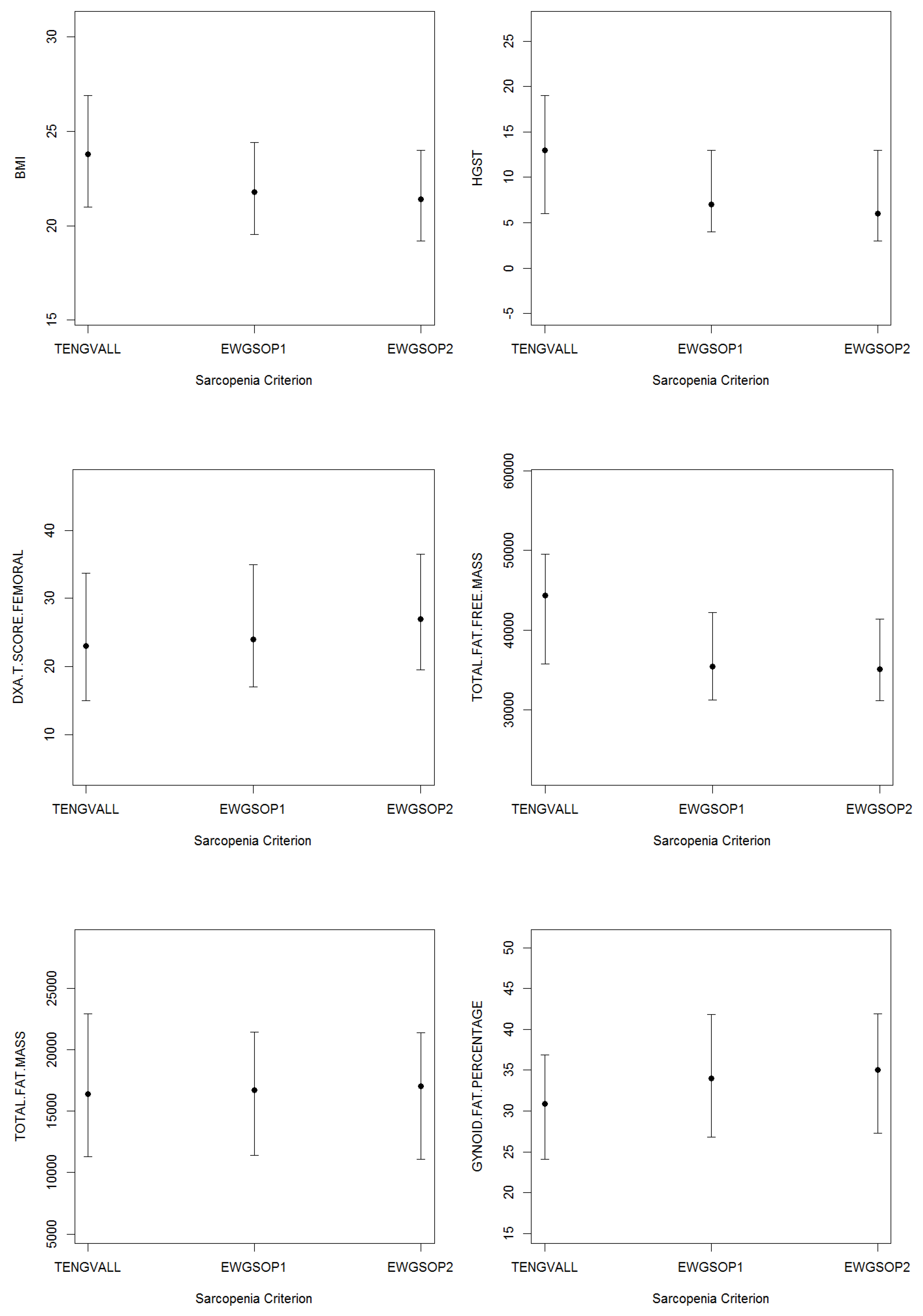
3.2. Patients’ Characteristics
3.3. Prevalence of Low ASMI
3.4. Agreement Analysis
3.5. ASMI Loss Risk Factors
3.6. Sarcopenic Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A





References
- Bahat, G.; Tufan, A.; Tufan, F.; Kilic, C.; Akpinar, T.S.; Kose, M.; Erten, N.; Karan, M.A.; Cruz-Jentoft, A.J. Cutoff points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin. Nutr. 2016, 35, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Sarcopenia: Alternative Definitions and Associations with Lower Extremity Function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Hirayama, K.; Han, T.-F.; Izutsu, M.; Yuki, M. Sarcopenia Prevalence and Risk Factors among Japanese Community Dwelling Older Adults Living in a Snow-Covered City According to EWGSOP2. J. Clin. Med. 2019, 8, 291. [Google Scholar] [CrossRef] [Green Version]
- Locquet, M.; Beaudart, C.; Petermans, J.; Reginster, J.-Y.; Bruyère, O. EWGSOP2 Versus EWGSOP1: Impact on the Prevalence of Sarcopenia and Its Major Health Consequences. J. Am. Med. Dir. Assoc. 2019, 20, 384–385. [Google Scholar] [CrossRef]
- Reiss, J.; Iglseder, B.; Alzner, R.; Mayr-Pirker, B.; Pirich, C.; Kässmann, H.; Kreutzer, M.; Dovjak, P.; Reiter, R. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing 2019. [Google Scholar] [CrossRef]
- Gould, H.; Brennan, S.L.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Total and appendicular lean mass reference ranges for Australian men and women: The Geelong osteoporosis study. Calcif. Tissue Int. 2014, 94, 363–372. [Google Scholar] [CrossRef]
- Tengvall, M.; Ellegård, L.; Malmros, V.; Bosaeus, N.; Lissner, L.; Bosaeus, I. Body composition in the elderly: Reference values and bioelectrical impedance spectroscopy to predict total body skeletal muscle mass. Clin. Nutr. 2009, 28, 52. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, U.G.; Genton, L.; Hans, D.; Pichard, C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin. Nutr. 2003, 22, 537–543. [Google Scholar] [CrossRef]
- Frisancho, A.R. New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. Am. J. Clin. Nutr. 1984, 40, 808–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal Muscle Cutpoints Associated with Elevated Physical Disability Risk in Older Men and Women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Wang, Z.; Horlick, M.; Wang, J.; Heymsfield, S.B. Dual-energy X-ray absorptiometry lean soft tissue hydration Independent contributions of intra- and extracellular water. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E842–E847. [Google Scholar] [CrossRef]
- Moon, J.R.; Stout, J.R.; Smith-Ryan, A.E.; Kendall, K.L.; Fukuda, D.H.; Cramer, J.T.; Moon, S.E. Tracking fat-free mass changes in elderly men and women using single-frequency bioimpedance and dual-energy X-ray absorptiometry A four-compartment model comparison. Eur. J. Clin. Nutr. 2013, 67 (Suppl. 1), S40–S46. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 1996, 54, S59–S65. [Google Scholar] [CrossRef]
- Spijkerman, D.C.; Snijders, C.J.; Stijnen, T.; Lankhorst, G.J. Standardization of grip strength measurements. Effects on repeatability and peak force. Scand. J. Rehabil. Med. 1991, 23, 203–206. [Google Scholar]
- Ware, J. SF-36 Health Survey Manual and Interpretation Guide; Health Institute New England Medical Center: Boston, MA, USA, 1993. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 56–61. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; 2017. [Google Scholar]
- Diz, J.B.M.; Leopoldino, A.A.O.; Moreira, B.d.S.; Henschke, N.; Dias, R.C.; Pereira, L.S.M.; Oliveira, V.C. Prevalence of sarcopenia in older Brazilians: A systematic review and meta-analysis. Geriatr. Gerontol. Int. 2017, 17, 5–16. [Google Scholar] [CrossRef]
- Chien, M.-Y.; Huang, T.-Y.; Wu, Y.-T. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J. Am. Geriatr. Soc. 2008, 56, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.W.; Auyeung, T.-W.; Kwok, T.; Lau, E.M.C.; Leung, P.-C.; Woo, J. Associated Factors and Health Impact of Sarcopenia in Older Chinese Men and Women: A Cross-Sectional Study. Gerontology 2007, 53, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; Rizzo, M.R.; et al. Prevalence and Clinical Correlates of Sarcopenia, Identified According to the EWGSOP Definition and Diagnostic Algorithm, in Hospitalized Older People: The GLISTEN Study. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2017, 72, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nishizawa, M.; Uchiyama, T.; Kasahara, Y.; Shindo, M.; Miyachi, M.; Tanaka, S. Developing and Validating an Age-Independent Equation Using Multi-Frequency Bioelectrical Impedance Analysis for Estimation of Appendicular Skeletal Muscle Mass and Establishing a Cutoff for Sarcopenia. Int. J. Environ. Res. Public Health 2017, 14, 809. [Google Scholar] [CrossRef]
- Steihaug, O.M.; Gjesdal, C.G.; Bogen, B.; Ranhoff, A.H. Identifying low muscle mass in patients with hip fracture: Validation of bioelectrical impedance analysis and anthropometry compared to dual energy X-ray absorptiometry. J. Nutr. Heal. Aging 2016, 20, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.W.P.D.; Santos, M.R.; Souza, F.R.; Takayama, L.; Rodrigues Pereira, R.M.; Negrão, C.E.; Alves, M.D.N.N. Discriminating sarcopenia in overweight/obese male patients with heart failure The influence of body mass index. Esc Hear. Fail. 2019. [Google Scholar] [CrossRef]
- Kamijo, Y.; Kanda, E.; Ishibashi, Y.; Yoshida, M. Sarcopenia and frailty in PD: Impact on mortality, malnutrition, and inflammation. Perit. Dial. Int. 2018, 38, 447–454. [Google Scholar] [CrossRef]
- Du, Y.; No, J.K. Sarcopenia: Nutrition and Related Diseases. Culin. Sci. Hosp. Res. 2017, 23, 66–78. [Google Scholar]
- Du, Y.; Oh, C.; No, J. Associations between Sarcopenia and Metabolic Risk Factors: A Systematic Review and Meta-Analysis. J. Obes. Metab. Syndr. 2018, 27, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Uemura, K.; Doi, T.; Lee, S.; Shimada, H. Sarcopenia and Low Serum Albumin Level Synergistically Increase the Risk of Incident Disability in Older Adults. J. Am. Med. Dir. Assoc. 2019, 20, 90–93. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Heymsfield, S.B. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia What are we really estimating? J. Cachexia. Sarcopenia Muscle 2017, 8, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; De Rui, M.; Stubbs, B.; Veronese, N.; Manzato, E. Measurement of lean body mass using bioelectrical impedance analysis A consideration of the pros and cons. Aging Clin. Exp. Res. 2017, 29, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Yamada, Y.; Arai, H. Comparability of two representative devices for bioelectrical impedance data acquisition. Geriatr. Gerontol. Int. 2016, 16, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
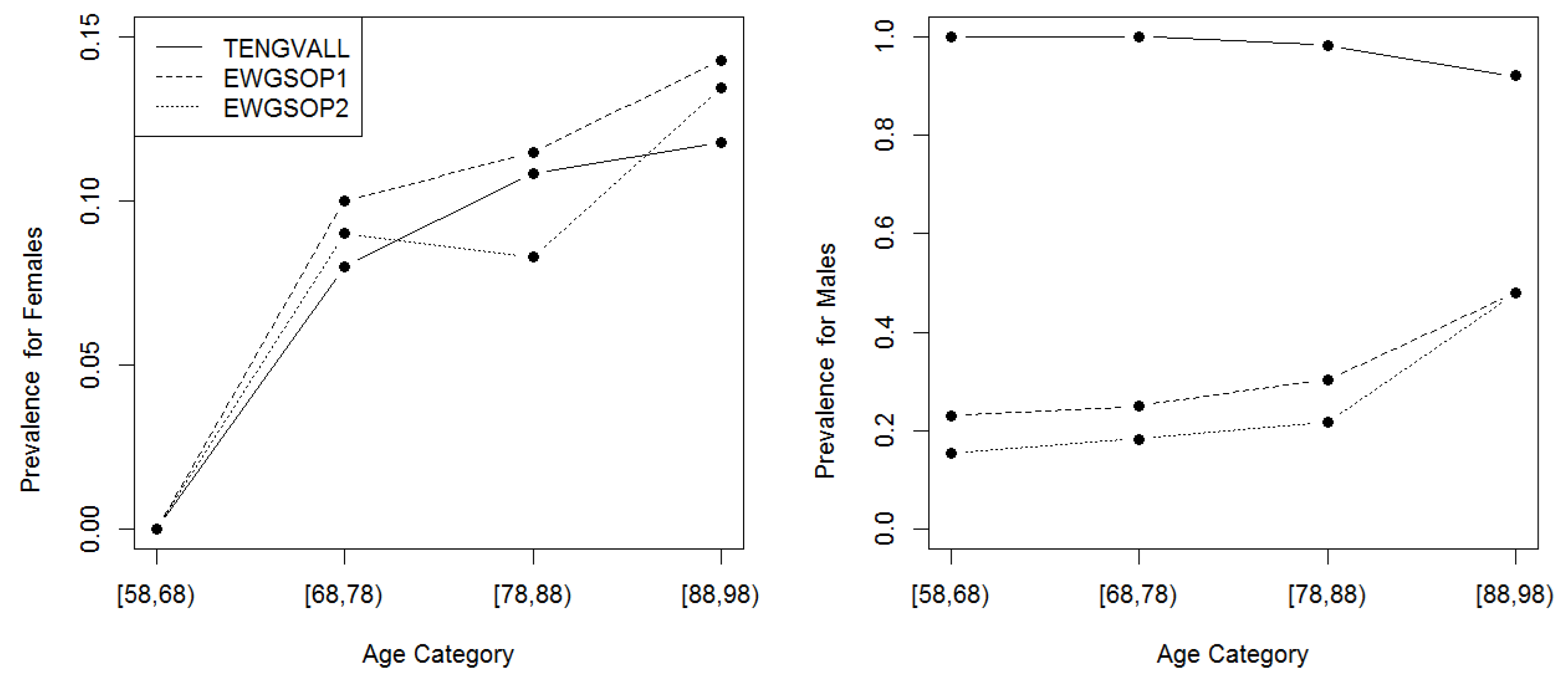
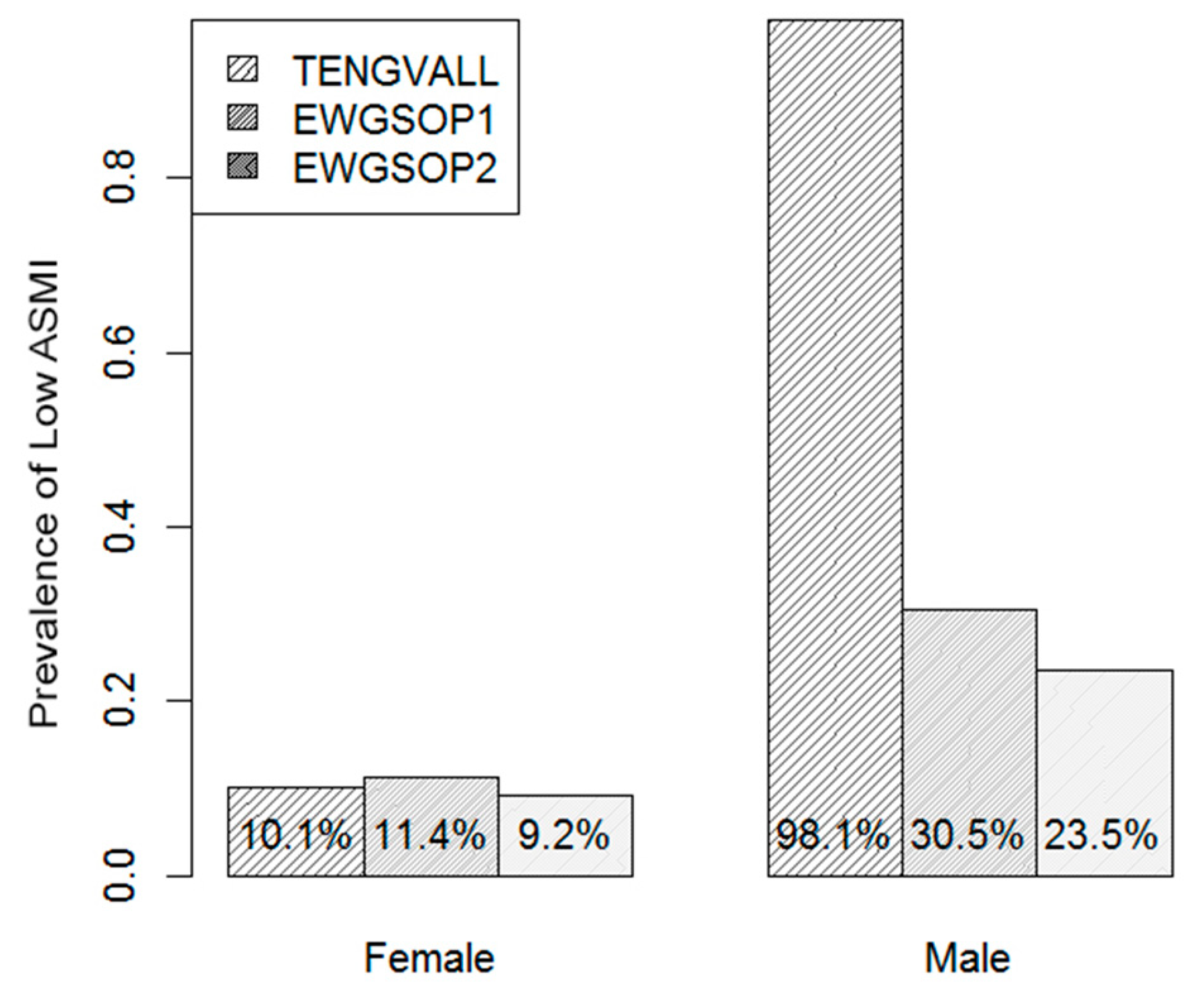
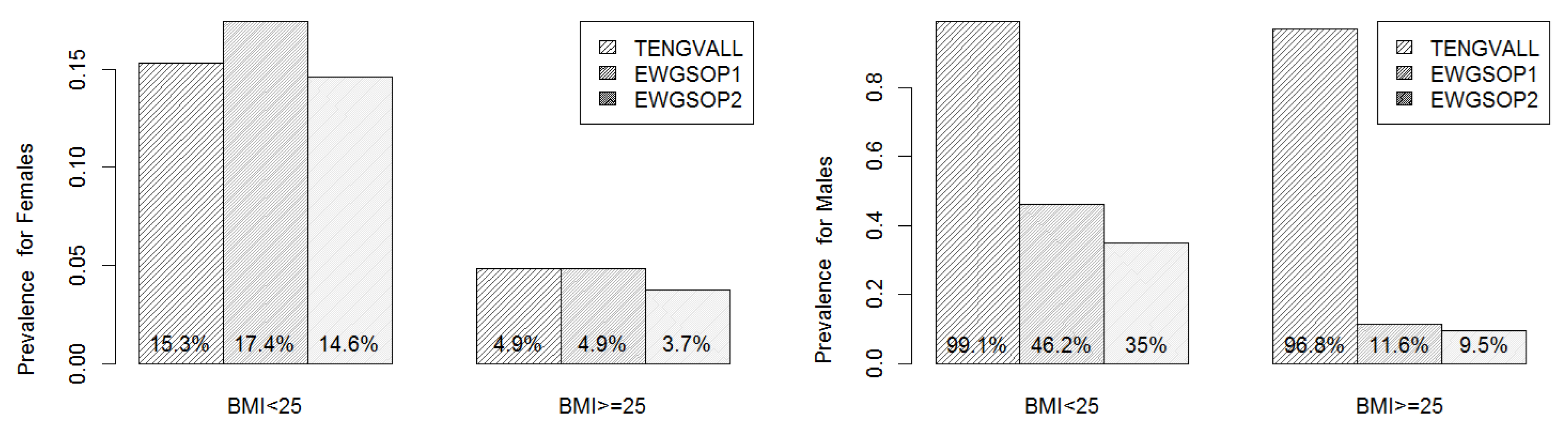
| Characteristics | Total (n = 765) | Male (n = 213, 27.8%) | Female (n = 552, 72.2%) | |||
|---|---|---|---|---|---|---|
| Smoke: Yes/No | 53/518 | 14/142 | 39/376 | |||
| Age (years) | 82.0 | (8.2) | 80.0 | (8.0) | 82.0 | (9.0) |
| BI (score) | 70.0 | (35.0) | 68.0 | (37.0) | 71.0 | (35.0) |
| WBC (K/µL) | 6.6 | (2.5) | 6.8 | (2.7) | 6.5 | (2.5) |
| RBC (M/µL) | 4.2 | (0.7) | 4.3 | (0.7) | 4.2 | (0.7) |
| HB (g/dL) | 30.0 | (22.0) | 33.0 | (23.0) | 29.0 | (21.5) |
| HCT (%) | 38.2 | (5.7) | 39.4 | (6.2) | 37.9 | (5.4) |
| PLT (K/µL) | 236.8 | (97.1) | 217.5 | (103.5) | 242.0 | (94.1) |
| Iron (µg/dL) | 66.0 | (43.0) | 66.0 | (40.0) | 67.0 | (43.0) |
| Triglyceride (mmol/L) | 106.0 | (58.0) | 99.5 | (47.2) | 110.0 | (62.0) |
| Cholesterol (mmol/L) | 182.0 | (62.0) | 161.0 | (50.0) | 190.0 | (60.0) |
| HDL (mmol/L) | 49.0 | (19.0) | 44.0 | (15.0) | 51.0 | (19.0) |
| LDL (mmol/L) | 108.0 | (48.2) | 96.2 | (46.2) | 113.0 | (49.3) |
| Total Proteins (g/dL) | 6.6 | (0.8) | 6.6 | (0.7) | 6.6 | (0.8) |
| Albumin (%) | 56.5 | (6.2) | 55.7 | (7.5) | 56.6 | (5.9) |
| Albumin (g/dL) | 3.7 | (0.6) | 3.7 | (0.7) | 3.8 | (0.6) |
| Creatinine (mg/dL) | 0.8 | (0.3) | 0.9 | (0.5) | 0.8 | (0.3) |
| Azotemia (mg/dL) | 40.0 | (21.0) | 41.0 | (22.0) | 40.0 | (22.0) |
| Sodium (mmol/L) | 140.0 | (4.0) | 139.0 | (4.0) | 140.0 | (4.0) |
| Potassium (mmol/L) | 16.0 | (6.0) | 17.0 | (6.0) | 16.0 | (5.0) |
| Chloride (mmol/L) | 104.0 | (5.0) | 104.0 | (5.0) | 104.0 | (5.0) |
| Calcium (mmol/L) | 9.1 | (0.7) | 9.1 | (0.7) | 9.1 | (0.7) |
| Blood Amylase (U/L) | 32.0 | (30.8) | 33.0 | (28.5) | 31.0 | (31.0) |
| Uric Acid (mg/dL) | 4.9 | (2.1) | 5.4 | (2.3) | 4.8 | (1.9) |
| Bilirubin (mg/dL) | 0.6 | (0.4) | 0.6 | (0.5) | 0.6 | (0.4) |
| AST (IU/L) | 17.0 | (8.0) | 16.0 | (7.2) | 17.0 | (7.0) |
| ALT (IU/L) | 17.0 | (32.0) | 18.0 | (46.2) | 17.0 | (30.0) |
| GGT (U/L) | 19.0 | (17.0) | 20.5 | (20.2) | 19.0 | (16.0) |
| GLIC (mg/dL) | 96.0 | (33.5) | 98.5 | (33.0) | 95.0 | (35.0) |
| ESR (mm/h) | 32.0 | (36.0) | 28.0 | (46.0) | 33.0 | (33.0) |
| CRP (mg/dL) | 0.3 | (0.7) | 0.4 | (1.4) | 0.2 | (0.6) |
| Height (cm) | 154.0 | (12.0) | 165.0 | (10.0) | 152.0 | (8.0) |
| Weight (kg) | 59.9 | (17.3) | 66.2 | (17.0) | 57.3 | (17.0) |
| BMI (kg/m2) | 24.6 | (6.2) | 24.4 | (5.9) | 24.6 | (6.7) |
| HGST (kg) | 9.0 | (10.0) | 15.0 | (12.5) | 8.0 | (8.8) |
| Femoral T-Score (DXA) | 24.0 | (17.0) | 20.0 | (16.8) | 25.0 | (16.0) |
| Total Fat Mass (g) | 19,830.0 | (13,258.2) | 17,051.0 | (11,221.0) | 20,576.0 | (13,775.8) |
| Gynoid Fat (%) | 38.9 | (16.0) | 28.8 | (10.8) | 42.4 | (12.8) |
| Android Fat (%) | 37.8 | (20.4) | 31.9 | (17.5) | 39.9 | (20.3) |
| Visceral Adipose Tissue (g) | 888.5 | (871.7) | 1199.1 | (971.9) | 785.3 | (735.0) |
| Subcutaneous Fat (g) | 698.5 | (938.7) | 441.8 | (553.2) | 858.9 | (987.2) |
| Diagnostic Criteria | TENGVALL | EWGSOP1 |
|---|---|---|
| EWGSOP1 | 84.6%, 0.200 (<0.001) | |
| EWGSOP2 | 86.4%, 0.224 (<0.001) | 97.8%, 0.883 (<0.001) |
| Diagnostic Criteria | TENGVALL | EWGSOP1 |
|---|---|---|
| EWGSOP1 | 32.4%, 0.017 (0.181) | |
| EWGSOP2 | 25.4%, 0.012 (0.263) | 93.0%, 0.822 (<0.001) |
| Predictors | TENGVALL | EWGSOP1 | EWGSOP2 | |||
|---|---|---|---|---|---|---|
| Azotemia | −0.0780 | (0.0316) * | −0.0512 | (0.0423) * | ||
| Height | −0.1634 | (0.0277) * | ||||
| Weight | −0.1931 | (0.0002) * | +0.1337 | (0.0232) * | +0.1130 | (0.0880) |
| BMI | −0.3610 | (0.0077) * | −0.3362 | (0.0278) * | ||
| Predictors | TENGVALL | EWGSOP1 | EWGSOP2 | ||
|---|---|---|---|---|---|
| ESR | +0.0818 | (0.0130) * | |||
| Height | |||||
| Weight | |||||
| BMI | −0.2598 | (0.0373) * | |||
| HGST | −0.0691 | (0.1560) | |||
| Femoral T-Score | +0.0507 | (0.0373) * | +0.1377 | (0.0056) * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilal, S.; Perna, S.; Gasparri, C.; Alalwan, T.A.; Vecchio, V.; Fossari, F.; Peroni, G.; Riva, A.; Petrangolini, G.; Rondanelli, M. Comparison between Appendicular Skeletal Muscle Index DXA Defined by EWGSOP1 and 2 versus BIA Tengvall Criteria among Older People Admitted to the Post-Acute Geriatric Care Unit in Italy. Nutrients 2020, 12, 1818. https://doi.org/10.3390/nu12061818
Hilal S, Perna S, Gasparri C, Alalwan TA, Vecchio V, Fossari F, Peroni G, Riva A, Petrangolini G, Rondanelli M. Comparison between Appendicular Skeletal Muscle Index DXA Defined by EWGSOP1 and 2 versus BIA Tengvall Criteria among Older People Admitted to the Post-Acute Geriatric Care Unit in Italy. Nutrients. 2020; 12(6):1818. https://doi.org/10.3390/nu12061818
Chicago/Turabian StyleHilal, Sawsan, Simone Perna, Clara Gasparri, Tariq A. Alalwan, Viviana Vecchio, Federica Fossari, Gabriella Peroni, Antonella Riva, Giovanna Petrangolini, and Mariangela Rondanelli. 2020. "Comparison between Appendicular Skeletal Muscle Index DXA Defined by EWGSOP1 and 2 versus BIA Tengvall Criteria among Older People Admitted to the Post-Acute Geriatric Care Unit in Italy" Nutrients 12, no. 6: 1818. https://doi.org/10.3390/nu12061818
APA StyleHilal, S., Perna, S., Gasparri, C., Alalwan, T. A., Vecchio, V., Fossari, F., Peroni, G., Riva, A., Petrangolini, G., & Rondanelli, M. (2020). Comparison between Appendicular Skeletal Muscle Index DXA Defined by EWGSOP1 and 2 versus BIA Tengvall Criteria among Older People Admitted to the Post-Acute Geriatric Care Unit in Italy. Nutrients, 12(6), 1818. https://doi.org/10.3390/nu12061818





