Growth Assessment in Preterm Children from Birth to Preschool Age
Abstract
:1. Background
2. Materials and Methods
- Gestational age at birth ≥ 37+0 weeks;
- Syndromes, conditions or diseases known to affect growth and/or requiring specific charts, e.g., Down’s syndrome or other genetic syndromes, short bowel syndrome, etc.;
- Chronic diseases which required prolonged parenteral nutrition.
3. Results
3.1. Population Characteristics and in-Hospital Growth
3.2. Follow-Up at Preschool Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SGA | small for gestational age |
| IUGR | intrauterine growth restriction |
| EUGR | extrauterine growth restriction |
| WHO | World Health Organization |
References
- Greer, F.R.; Olsen, I.E. How fast should the preterm infant grow? Curr. Pediatr. Rep. 2013, 1, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Cole, T.J.; Statnikov, Y.; Santhakumaran, S.; Pan, H.; Modi, N.; Neonatal Data Analysis Unit and the Preterm Growth Investigator Group. Birth weight and longitudinal growth in infants born below 32 weeks’ gestation: A UK population study. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F34–F40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, K.M.; Ramsingh, L.; Gunn, E.; Streiner, D.; Van Lieshout, R.; Boyle, M.; Gerstein, H.; Schmidt, L.; Saigal, S. Cardiometabolic Health in Adults Born Premature with Extremely Low Birth Weight. Pediatrics 2016, 138, e20160515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 210, 69–80. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics, Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics 1977, 60, 519–530. [Google Scholar]
- Villar, J.; Giuliani, F.; Barros, F.; Roggero, P.; Coronado Zarco, I.A.; Rego, M.A.S.; Ochieng, R.; Gianni, M.L.; Rao, S.; Lambert, A.; et al. Monitoring the postnatal growth of preterm infants: A paradigm change. Pediatrics 2018, 141, e20172467. [Google Scholar] [CrossRef] [Green Version]
- Villar, J.; Papageorghiou, A.T.; Pang, R.; Ohuma, E.O.; Cheikh Ismail, L.; Barros, F.C.; Lambert, A.; Carvalho, M.; Jaffer, Y.A.; Bertino, E.; et al. The likeness of fetal growth and newborn size across non-isolated populations in the INTERGROWTH-21st Project: The Fetal Growth Longitudinal Study and Newborn Cross-Sectional Study. Lancet. Diabetes Endocrinol. 2014, 2, 781–792. [Google Scholar] [CrossRef]
- Villar, J.; Giuliani, F.; Figueras-Aloy, J.; Barros, F.; Bertino, E.; Bhutta, Z.A.; Kennedy, S.H. Growth of preterm infants at the time of global obesity. Arch. Dis. Child. 2019, 104, 725–727. [Google Scholar] [CrossRef]
- Villar, J.; Giuliani, F.; Fenton, T.R.; Ohuma, E.O.; Ismail, L.C.; Kennedy, S.H. INTERGROWTH-21st very preterm size at birth reference charts. Lancet 2016, 387, 844–845. [Google Scholar] [CrossRef] [Green Version]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Villar, J.; Giuliani, F.; Bhutta, Z.A.; Bertino, E.; Ohuma, E.O.; Ismail, L.C.; Barros, F.C.; Altman, D.G.; Victora, C.; Noble, J.A.; et al. Postnatal growth standards for preterm infants: The Preterm Postnatal Follow-up Study of the INTERGROWTH-21st Project. Lancet Glob. Health 2015, 3, e681–e691. [Google Scholar] [CrossRef] [Green Version]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Onis, M.; Garza, C.; Onyango, A.W.; Martorell, R. WHO Child Growth Standards. Acta Paediatr. 2006, 450, 1–101. [Google Scholar] [CrossRef] [Green Version]
- de Onis, M.; Onyango, A.; Borghi, E.; Siyam, A.; Blössner, M.; Lutter, C.; WHO Multicentre Growth Reference Study Group. Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr. 2012, 15, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics and the Society forMaternal-FetalMedicin. ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 2019, 133, e97–e109. [Google Scholar]
- Tanner, J.M.; Goldstein, H.; Whitehouse, R.H. Standards for Children’s Height at Ages 2–9 Years Allowing for Height of Parents. Arch. Dis. Child. 1970, 45, 755–762. [Google Scholar] [CrossRef] [Green Version]
- WHO Expert Committee. Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Zeve, D.; Regelmann, M.O.; Holzman, I.R.; Rapaport, R. Small at Birth, but How Small? The Definition of SGA Revisited. Horm. Res. Paediatr. 2016, 86, 357–360. [Google Scholar] [CrossRef]
- Tuzun, F.; Yucesoy, E.; Baysal, B.; Kumral, A.; Duman, N.; Ozkan, H. Comparison of INTERGROWTH-21 and Fenton growth standards to assess size at birth and extrauterine growth in very preterm infants. J. Matern. Fetal Neonatal Med. 2018, 31, 2252–2257. [Google Scholar] [CrossRef]
- Hack, M.B.; Schluchter, M.; Cartar, L.; Rahman, M.; Cuttler, L.; Borawski, E. Growth of very low birth weight infants to age 20 years. Pediatrics 2003, 112, e30–e38. [Google Scholar] [CrossRef] [Green Version]
- Hollanders, J.J.; van der Pal, S.M.; van Dommelen, P.; Rotteveel, J.; Finken, M.J.J. Growth pattern and final height of very preterm vs. very low birth weight infants. Pediatr. Res. 2017, 82, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, M.C.; McGrath, M.M.; Hawes, K.; Lester, B.M. Growth trajectories of preterm infants: Birth to 12 years. J. Pediatr. Health Care 2008, 22, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Baseline Characteristics of Study Population (n = 80) | |
|---|---|
| Male gender (n, %) | 46 (57.5%) |
| Cesarean section (n, %) | 37 (67.2%) |
| Maternal smoking in pregnancy | 1 (1.25%) |
| Hypertension in pregnancy | 8 (10%) |
| Gestational diabetes | 10 (12.5%) |
| Average age at follow-up ±SD | 4.21±0.28 years |
| Twins (n,%) | 50 (62.5%) |
| Average gestational age at birth ± SD | 33.3 ± 2.2 weeks, range 30–36 weeks |
| Average birthweight ± SD | 1835 ± 486 g |
| Average weight z-score at birth | −0.61 ± 0,97 |
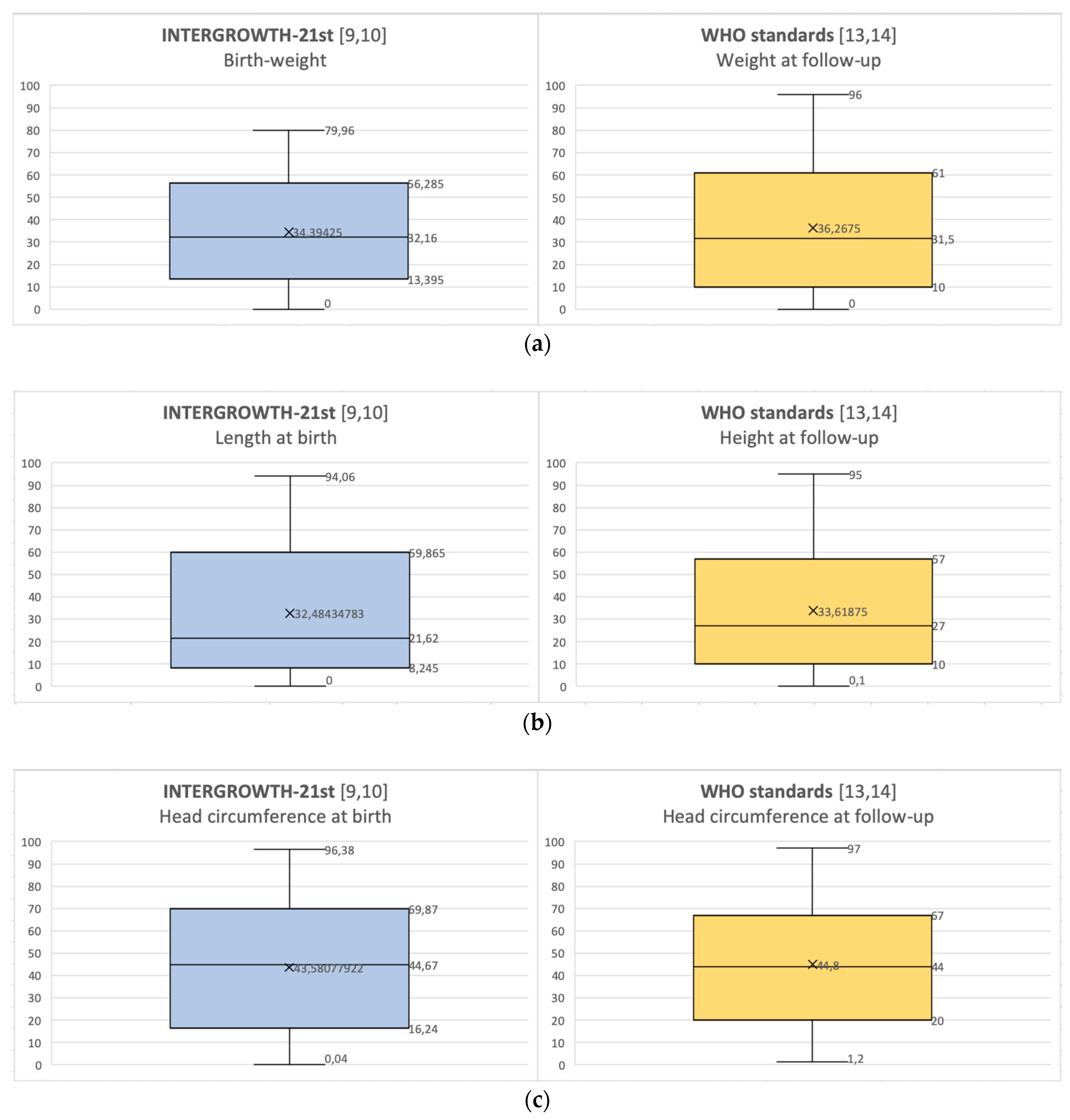
| Average percentile at birth [9,10] ± SD | Weight: 34.39 ± 23.88 |
| Length: 32.48 ± 26.91 | |
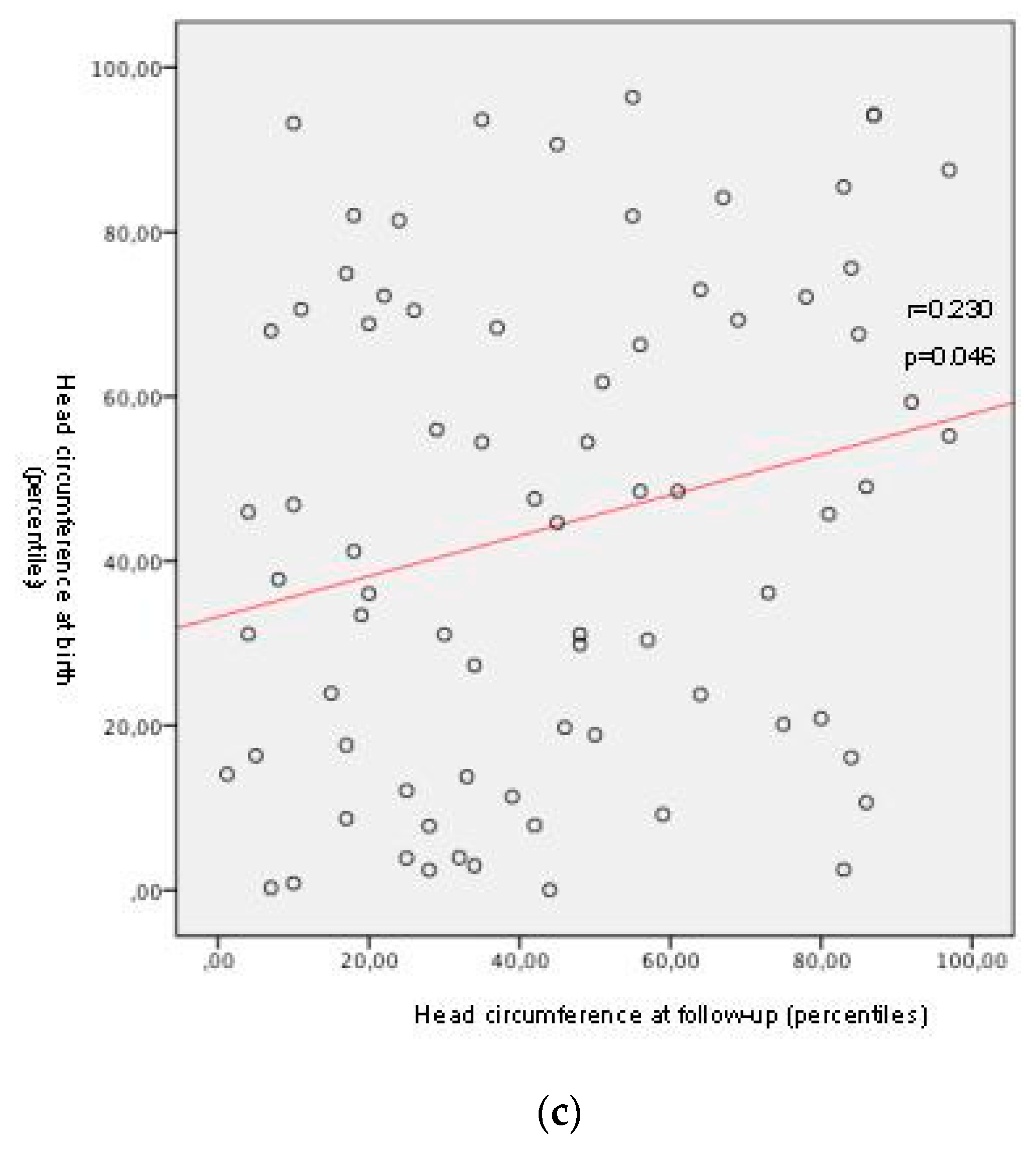
| Average percentile at birth [9,10] ± SD SGA at birth (n, %) IUGR history (n, %) | Head circumference: 43.58 ± 29.82 |
| 15 (18.75%) | |
| 13 (16.25%) | |
| Length of stay, average ± SD | 25.68 ± 20.45 days |
| Average percentile at follow-up [13,14] ± SD | Weight: 36.73 ± 28.10 |
| Length of stay, average ± SD | Height: 33.62 ± 27.69 |
| Average percentile at follow-up [13,14] ± SD Average weight z-score at follow-up Average height z-score at follow-up | Head circumference: 44.80 ± 27.21 |
| −0.42 ±1.04 | |
| −0.63 ± 1.06 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceratto, S.; Savino, F.; Vannelli, S.; De Sanctis, L.; Giuliani, F. Growth Assessment in Preterm Children from Birth to Preschool Age. Nutrients 2020, 12, 1941. https://doi.org/10.3390/nu12071941
Ceratto S, Savino F, Vannelli S, De Sanctis L, Giuliani F. Growth Assessment in Preterm Children from Birth to Preschool Age. Nutrients. 2020; 12(7):1941. https://doi.org/10.3390/nu12071941
Chicago/Turabian StyleCeratto, Simone, Francesco Savino, Silvia Vannelli, Luisa De Sanctis, and Francesca Giuliani. 2020. "Growth Assessment in Preterm Children from Birth to Preschool Age" Nutrients 12, no. 7: 1941. https://doi.org/10.3390/nu12071941
APA StyleCeratto, S., Savino, F., Vannelli, S., De Sanctis, L., & Giuliani, F. (2020). Growth Assessment in Preterm Children from Birth to Preschool Age. Nutrients, 12(7), 1941. https://doi.org/10.3390/nu12071941







