Diet and Lp(a): Does Dietary Change Modify Residual Cardiovascular Risk Conferred by Lp(a)?
Abstract
:1. Introduction
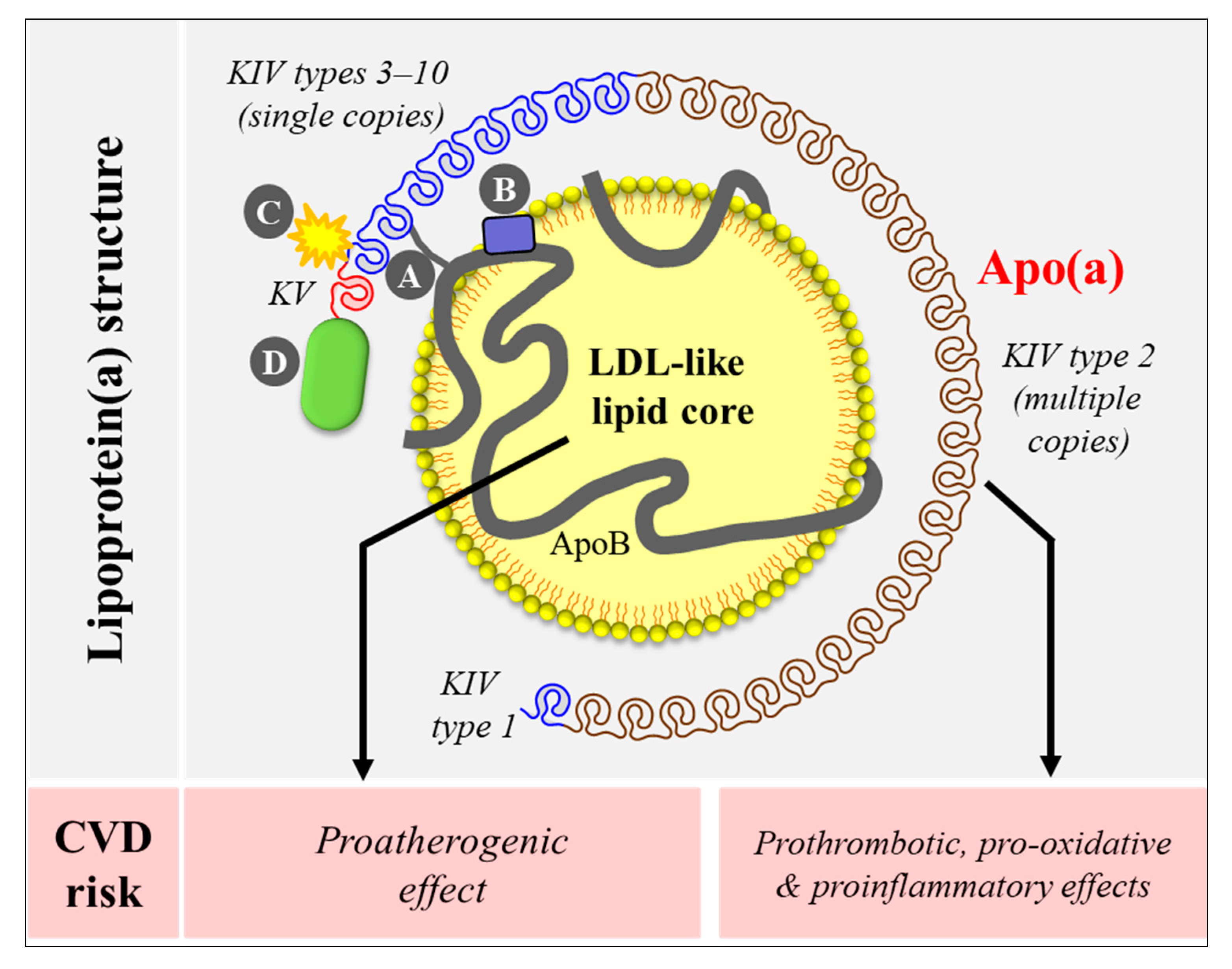
2. Lipoprotein(a) Structure and Unique Features
3. Lipoprotein(a) [Lp(a)] Is an Independent, Causal, Genetically Determined Cardiovascular Disease (CVD) Risk Factor
4. Public Health and Clinical Relevance of Lp(a) as a Contributor to Residual CVD Risk
5. Lp(a), Lipid-Lowering Therapeutics and Cardiovascular Benefit
6. The Effect of Dietary Intervention on Lp(a)
6.1. Saturated Fat Replacement
6.2. Diets with Different Macronutrient Compositions
7. Potential Mechanisms to Explain Pharmacological and Non-Pharmacological (e.g., Diet) Intervention-Induced Changes in Lp(a) Concentration
8. Measurement of Low-Density Lipoprotein Cholesterol (LDL-C) and Lp(a) Change
9. Future Direction
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Institute for Health Metrics and Evaluation (IHME). GBD Compare. Available online: https://vizhub.healthdata.org/gbd-compare/ (accessed on 1 May 2020).
- Mokdad, A.H.; Ballestros, K.; Echko, M.; Glenn, S.; Olsen, H.E.; Mullany, E.; Lee, A.; Khan, A.R.; Ahmadi, A.; Ferrari, A.J. The state of US health, 1990–2016: Burden of diseases, injuries, and risk factors among US states. JAMA 2018, 319, 1444–1472. [Google Scholar] [CrossRef] [Green Version]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Nicklas, T.A.; O’Neil, C.E.; Fulgoni III, V.L. Diet quality is inversely related to cardiovascular risk factors in adults. J. Nutr. 2012, 142, 2112–2118. [Google Scholar] [CrossRef]
- Mensink, R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; De Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, T.A.; Maki, K.C.; Orringer, C.E.; Jones, P.H.; Kris-Etherton, P.; Sikand, G.; La Forge, R.; Daniels, S.R.; Wilson, D.P.; Morris, P.B.; et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. J. Clin. Lipidol. 2015, 9, S1–S122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef] [PubMed]
- Berg, K. A New Serum Type System in Man-the Lp System. Acta Pathol. Microbiol. Scand. 1963, 59, 369–382. [Google Scholar] [CrossRef]
- Miles, L.A.; Plow, E.F. Lp(a): An interloper into the fibrinolytic system? Thromb. Haemost. 1990, 63, 331–335. [Google Scholar] [CrossRef]
- McLean, J.W.; Tomlinson, J.E.; Kuang, W.J.; Eaton, D.L.; Chen, E.Y.; Fless, G.M.; Scanu, A.M.; Lawn, R.M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature 1987, 330, 132–137. [Google Scholar] [CrossRef]
- Koschinsky, M.L.; Beisiegel, U.; Henne-Bruns, D.; Eaton, D.L.; Lawn, R.M. Apolipoprotein(a) size heterogeneity is related to variable number of repeat sequences in its mRNA. Biochemistry 1990, 29, 640–644. [Google Scholar] [CrossRef]
- Lackner, C.; Cohen, J.C.; Hobbs, H.H. Molecular definition of the extreme size polymorphism in apolipoprotein(a). Hum. Mol. Genet. 1993, 2, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoek, Y.Y.; Wittekoek, M.E.; Beisiegel, U.; Kastelein, J.J.; Koschinsky, M.L. The apolipoprotein(a) kringle IV repeats which differ from the major repeat kringle are present in variably-sized isoforms. Hum. Mol. Genet. 1993, 2, 361–366. [Google Scholar] [CrossRef]
- Hobbs, H.H.; White, A.L. Lipoprotein(a): Intrigues and insights. Curr. Opin. Lipidol. 1999, 10, 225–236. [Google Scholar] [CrossRef]
- Leibundgut, G.; Scipione, C.; Yin, H.; Schneider, M.; Boffa, M.B.; Green, S.; Yang, X.; Dennis, E.; Witztum, J.L.; Koschinsky, M.L. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J. Lipid Res. 2013, 54, 2815–2830. [Google Scholar] [CrossRef] [Green Version]
- Anglés-Cano, E.; Rojas, G. Apolipoprotein (a): Structure-function relationship at the lysine-binding site and plasminogen activator cleavage site. Biol. Chem. 2002, 383, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Berg, K. The Lp system. Ser. Haematol. 1968, 1, 111–136. [Google Scholar]
- Utermann, G. The mysteries of lipoprotein(a). Science 1989, 246, 904–910. [Google Scholar] [CrossRef]
- Kostner, G.M.; Avogaro, P.; Cazzolato, G.; Marth, E.; Bittolo-Bon, G.; Qunici, G.B. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis 1981, 38, 51–61. [Google Scholar] [CrossRef]
- Rhoads, G.G.; Dahlen, G.; Berg, K.; Morton, N.E.; Dannenberg, A.L. Lp(a) lipoprotein as a risk factor for myocardial infarction. JAMA 1986, 256, 2540–2544. [Google Scholar] [CrossRef]
- Dahlen, G.H.; Guyton, J.R.; Attar, M.; Farmer, J.A.; Kautz, J.A.; Gotto, A.M., Jr. Association of levels of lipoprotein Lp(a), plasma lipids, and other lipoproteins with coronary artery disease documented by angiography. Circulation 1986, 74, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Zenker, G.; Koltringer, P.; Bone, G.; Niederkorn, K.; Pfeiffer, K.; Jurgens, G. Lipoprotein(a) as a strong indicator for cerebrovascular disease. Stroke 1986, 17, 942–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambillau, M.; Simon, A.; Amar, J.; Giral, P.; Atger, V.; Segond, P.; Levenson, J.; Merli, I.; Megnien, J.L.; Plainfosse, M.C.; et al. Serum Lp(a) as a discriminant marker of early atherosclerotic plaque at three extracoronary sites in hypercholesterolemic men. The PCVMETRA Group. Arterioscler. Thromb. 1992, 12, 1346–1352. [Google Scholar] [CrossRef] [Green Version]
- Orth-Gomer, K.; Mittleman, M.A.; Schenck-Gustafsson, K.; Wamala, S.P.; Eriksson, M.; Belkic, K.; Kirkeeide, R.; Svane, B.; Ryden, L. Lipoprotein(a) as a determinant of coronary heart disease in young women. Circulation 1997, 95, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Stampfer, M.J. A prospective study of lipoprotein(a) and the risk of myocardial infarction. JAMA 1993, 270, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J.; Collins, R.; Peto, R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation 2000, 102, 1082–1085. [Google Scholar] [CrossRef] [Green Version]
- Bennet, A.; Di Angelantonio, E.; Erqou, S.; Eiriksdottir, G.; Sigurdsson, G.; Woodward, M.; Rumley, A.; Lowe, G.D.; Danesh, J.; Gudnason, V. Lipoprotein(a) levels and risk of future coronary heart disease: Large-scale prospective data. Arch. Intern. Med. 2008, 168, 598–608. [Google Scholar] [CrossRef] [Green Version]
- Erqou, S.; Kaptoge, S.; Perry, P.L.; Di Angelantonio, E.; Thompson, A.; White, I.R.; Marcovina, S.M.; Collins, R.; Thompson, S.G.; Danesh, J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009, 302, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Kraft, H.G.; Lingenhel, A.; Kochl, S.; Hoppichler, F.; Kronenberg, F.; Abe, A.; Muhlberger, V.; Schonitzer, D.; Utermann, G. Apolipoprotein(a) kringle IV repeat number predicts risk for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 713–719. [Google Scholar] [CrossRef]
- Paultre, F.; Pearson, T.A.; Weil, H.F.; Tuck, C.H.; Myerson, M.; Rubin, J.; Francis, C.K.; Marx, H.F.; Philbin, E.F.; Reed, R.G.; et al. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2619–2624. [Google Scholar] [CrossRef] [Green Version]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Steffensen, R.; Nordestgaard, B.G. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009, 301, 2331–2339. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1732–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erqou, S.; Thompson, A.; Di Angelantonio, E.; Saleheen, D.; Kaptoge, S.; Marcovina, S.; Danesh, J. Apolipoprotein(a) isoforms and the risk of vascular disease: Systematic review of 40 studies involving 58,000 participants. J. Am. Coll. Cardiol. 2010, 55, 2160–2167. [Google Scholar] [CrossRef] [Green Version]
- Kraft, H.G.; Windegger, M.; Menzel, H.J.; Utermann, G. Significant impact of the +93 C/T polymorphism in the apolipoprotein(a) gene on Lp(a) concentrations in Africans but not in Caucasians: Confounding effect of linkage disequilibrium. Hum. Mol. Genet. 1998, 7, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Parson, W.; Kraft, H.G.; Niederstatter, H.; Lingenhel, A.W.; Kochl, S.; Fresser, F.; Utermann, G. A common nonsense mutation in the repetitive Kringle IV-2 domain of human apolipoprotein(a) results in a truncated protein and low plasma Lp(a). Hum. Mutat. 2004, 24, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Ogorelkova, M.; Kraft, H.G.; Ehnholm, C.; Utermann, G. Single nucleotide polymorphisms in exons of the apo(a) kringles IV types 6 to 10 domain affect Lp(a) plasma concentrations and have different patterns in Africans and Caucasians. Hum. Mol. Genet. 2001, 10, 815–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luke, M.M.; Kane, J.P.; Liu, D.M.; Rowland, C.M.; Shiffman, D.; Cassano, J.; Catanese, J.J.; Pullinger, C.R.; Leong, D.U.; Arellano, A.R.; et al. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2030–2036. [Google Scholar] [CrossRef]
- Schunkert, H.; Konig, I.R.; Kathiresan, S.; Reilly, M.P.; Assimes, T.L.; Holm, H.; Preuss, M.; Stewart, A.F.; Barbalic, M.; Gieger, C.; et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011, 43, 333–338. [Google Scholar] [CrossRef]
- Tregouet, D.A.; Konig, I.R.; Erdmann, J.; Munteanu, A.; Braund, P.S.; Hall, A.S.; Grosshennig, A.; Linsel-Nitschke, P.; Perret, C.; DeSuremain, M.; et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat. Genet. 2009, 41, 283–285. [Google Scholar] [CrossRef]
- Shiffman, D.; Kane, J.P.; Louie, J.Z.; Arellano, A.R.; Ross, D.A.; Catanese, J.J.; Malloy, M.J.; Ellis, S.G.; Devlin, J.J. Analysis of 17,576 potentially functional SNPs in three case-control studies of myocardial infarction. PLoS ONE 2008, 3, e2895. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.C.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef] [Green Version]
- Shiffman, D.; O’Meara, E.S.; Bare, L.A.; Rowland, C.M.; Louie, J.Z.; Arellano, A.R.; Lumley, T.; Rice, K.; Iakoubova, O.; Luke, M.M.; et al. Association of gene variants with incident myocardial infarction in the Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Luke, M.M.; Shiffman, D.; Devlin, J.J. Genetic variants in the apolipoprotein(a) gene and coronary heart disease. Circ. Cardiovasc. Genet. 2011, 4, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanassoulis, G.; Campbell, C.Y.; Owens, D.S.; Smith, J.G.; Smith, A.V.; Peloso, G.M.; Kerr, K.F.; Pechlivanis, S.; Budoff, M.J.; Harris, T.B.; et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 2013, 368, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helgadottir, A.; Gretarsdottir, S.; Thorleifsson, G.; Holm, H.; Patel, R.S.; Gudnason, T.; Jones, G.T.; Van Rij, A.M.; Eapen, D.J.; Baas, A.F.; et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J. Am. Coll. Cardiol. 2012, 60, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Kuroda, T.; Yamasawa, M.; Nishinaga, M.; Mitsuhashi, T.; Seino, Y.; Nagoh, N.; Kayaba, K.; Yamada, S.; Matsuo, H.; et al. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study). Am. J. Cardiol. 1995, 76, 928–932. [Google Scholar] [CrossRef]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef] [Green Version]
- Glader, C.A.; Birgander, L.S.; Soderberg, S.; Ildgruben, H.P.; Saikku, P.; Waldenstrom, A.; Dahlen, G.H. Lipoprotein(a), Chlamydia pneumoniae, leptin and tissue plasminogen activator as risk markers for valvular aortic stenosis. Eur. Heart J. 2003, 24, 198–208. [Google Scholar] [CrossRef]
- Bozbas, H.; Yildirir, A.; Atar, I.; Pirat, B.; Eroglu, S.; Aydinalp, A.; Ozin, B.; Muderrisoglu, H. Effects of serum levels of novel atherosclerotic risk factors on aortic valve calcification. J. Heart Valve Dis. 2007, 16, 387–393. [Google Scholar]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, B.J.; Boekholdt, S.M.; Dube, M.P.; Rheaume, E.; Wareham, N.J.; Khaw, K.T.; Sandhu, M.S.; Tardif, J.C. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: A prospective Mendelian randomization study and replication in a case-control cohort. Circ. Cardiovasc. Genet. 2014, 7, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Bergmark, C.; Dewan, A.; Orsoni, A.; Merki, E.; Miller, E.R.; Shin, M.J.; Binder, C.J.; Horkko, S.; Krauss, R.M.; Chapman, M.J.; et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 2008, 49, 2230–2239. [Google Scholar] [CrossRef] [Green Version]
- Taleb, A.; Witztum, J.L.; Tsimikas, S. Oxidized phospholipids on apoB-100-containing lipoproteins: A biomarker predicting cardiovascular disease and cardiovascular events. Biomark. Med. 2011, 5, 673–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimikas, S.; Brilakis, E.S.; Miller, E.R.; McConnell, J.P.; Lennon, R.J.; Kornman, K.S.; Witztum, J.L.; Berger, P.B. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 2005, 353, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bosse, Y.; Dumesnil, J.G.; Tam, J.W.; Teo, K.K.; Mahmut, A.; Yang, X.; et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 66, 1236–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanutti, C.; Julius, U.; Watts, G.F.; Harada-Shiba, M.; Cossu, M.; Schettler, V.J.; De Silvestro, G.; Soran, H.; Van Lennep, J.R.; Pisciotta, L.; et al. Toward an international consensus-Integrating lipoprotein apheresis and new lipid-lowering drugs. J. Clin. Lipidol. 2017, 11, 858–871. [Google Scholar] [CrossRef]
- Anderson, T.J.; Gregoire, J.; Pearson, G.J.; Barry, A.R.; Couture, P.; Dawes, M.; Francis, G.A.; Genest, J., Jr.; Grover, S.; Gupta, M.; et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can. J. Cardiol. 2016, 32, 1263–1282. [Google Scholar] [CrossRef]
- Wilson, D.P.; Jacobson, T.A.; Jones, P.H.; Koschinsky, M.L.; McNeal, C.J.; Nordestgaard, B.G.; Orringer, C.E. Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J. Clin. Lipidol. 2019, 13, 374–392. [Google Scholar] [CrossRef]
- Cegla, J.; Neely, R.D.G.; France, M.; Ferns, G.; Byrne, C.D.; Halcox, J.; Datta, D.; Capps, N.; Shoulders, C.; Qureshi, N.; et al. HEART UK consensus statement on Lipoprotein(a): A call to action. Atherosclerosis 2019, 291, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Willeit, P.; Ridker, P.M.; Nestel, P.J.; Simes, J.; Tonkin, A.M.; Pedersen, T.R.; Schwartz, G.G.; Olsson, A.G.; Colhoun, H.M.; Kronenberg, F.; et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: Individual patient-data meta-analysis of statin outcome trials. Lancet 2018, 392, 1311–1320. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, B.R.; Richter, Y.; Nagel, D.; Heigl, F.; Vogt, A.; Roeseler, E.; Parhofer, K.; Ramlow, W.; Koch, M.; Utermann, G.; et al. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Leebmann, J.; Roeseler, E.; Julius, U.; Heigl, F.; Spitthoever, R.; Heutling, D.; Breitenberger, P.; Maerz, W.; Lehmacher, W.; Heibges, A.; et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: Prospective observational multicenter study. Circulation 2013, 128, 2567–2576. [Google Scholar] [CrossRef] [Green Version]
- Cannon, C.P.; Shah, S.; Dansky, H.M.; Davidson, M.; Brinton, E.A.; Gotto, A.M.; Stepanavage, M.; Liu, S.X.; Gibbons, P.; Ashraf, T.B.; et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 2010, 363, 2406–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovingh, G.K.; Kastelein, J.J.; Van Deventer, S.J.; Round, P.; Ford, J.; Saleheen, D.; Rader, D.J.; Brewer, H.B.; Barter, P.J. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2015, 386, 452–460. [Google Scholar] [CrossRef]
- Albers, J.J.; Slee, A.; O’Brien, K.D.; Robinson, J.G.; Kashyap, M.L.; Kwiterovich, P.O., Jr.; Xu, P.; Marcovina, S.M. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: The AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J. Am. Coll. Cardiol. 2013, 62, 1575–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raal, F.J.; Giugliano, R.P.; Sabatine, M.S.; Koren, M.J.; Blom, D.; Seidah, N.G.; Honarpour, N.; Lira, A.; Xue, A.; Chiruvolu, P.; et al. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: An analysis of 10 clinical trials and the LDL receptor’s role. J. Lipid Res. 2016, 57, 1086–1096. [Google Scholar] [CrossRef] [Green Version]
- Enkhmaa, B.; Anuurad, E.; Zhang, W.; Yue, K.; Li, C.S.; Berglund, L. The roles of apo(a) size, phenotype, and dominance pattern in PCSK9-inhibition-induced reduction in Lp(a) with alirocumab. J. Lipid Res. 2017, 58, 2008–2016. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Ceska, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef]
- Marston, N.A.; Gurmu, Y.; Melloni, G.E.M.; Bonaca, M.; Gencer, B.; Sever, P.S.; Pedersen, T.R.; Keech, A.C.; Roselli, C.; Lubitz, S.A.; et al. The Effect of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Inhibition on the Risk of Venous Thromboembolism. Circulation 2020, 141, 1600–1607. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bittner, V.A.; Diaz, R.; Goodman, S.G.; Kim, Y.U.; Jukema, J.W.; Pordy, R.; Roe, M.T.; et al. Peripheral Artery Disease and Venous Thromboembolic Events After Acute Coronary Syndrome: Role of Lipoprotein(a) and Modification by Alirocumab: Prespecified Analysis of the ODYSSEY OUTCOMES Randomized Clinical Trial. Circulation 2020, 141, 1608–1617. [Google Scholar] [CrossRef]
- Ray, K.K.; Stoekenbroek, R.M.; Kallend, D.; Leiter, L.A.; Landmesser, U.; Wright, R.S.; Wijngaard, P.; Kastelein, J.J.P. Effect of an siRNA Therapeutic Targeting PCSK9 on Atherogenic Lipoproteins: Prespecified Secondary End Points in ORION 1. Circulation 2018, 138, 1304–1316. [Google Scholar] [CrossRef]
- Santos, R.D.; Raal, F.J.; Catapano, A.L.; Witztum, J.L.; Steinhagen-Thiessen, E.; Tsimikas, S. Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein(a) in various populations with hypercholesterolemia: Results of 4 phase III trials. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 689–699. [Google Scholar] [CrossRef] [Green Version]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.C.; Baum, S.J.; Steinhagen-Thiessen, E.; Shapiro, M.D.; Stroes, E.S.; Moriarty, P.M.; Nordestgaard, B.G.; et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef]
- Burgess, S.; Ference, B.A.; Staley, J.R.; Freitag, D.F.; Mason, A.M.; Nielsen, S.F.; Willeit, P.; Young, R.; Surendran, P.; Karthikeyan, S.; et al. Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis. JAMA Cardiol. 2018, 3, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Lamina, C.; Kronenberg, F.; Lp, G.C. Estimation of the Required Lipoprotein(a)-Lowering Therapeutic Effect Size for Reduction in Coronary Heart Disease Outcomes: A Mendelian Randomization Analysis. JAMA Cardiol. 2019, 4, 575–579. [Google Scholar] [CrossRef] [Green Version]
- Madsen, C.M.; Kamstrup, P.R.; Langsted, A.; Varbo, A.; Nordestgaard, B.G. Lipoprotein(a)-Lowering by 50 mg/dL (105 nmol/L) May Be Needed to Reduce Cardiovascular Disease 20% in Secondary Prevention: A Population-Based Study. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Hornstra, G.; Van Houwelingen, A.; Kester, A.; Sundram, K. A palm oil-enriched diet lowers serum lipoprotein (a) in normocholesterolemic volunteers. Atherosclerosis 1991, 90, 91–93. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Kris-Etherton, P.; Dennis, B.; Elmer, P.J.; Ershow, A.; Lefevre, M.; Pearson, T.; Roheim, P.; Ramakrishnan, R.; Reed, R. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: The DELTA Study, protocol 1. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Berglund, L.; Lefevre, M.; Ginsberg, H.N.; Kris-Etherton, P.M.; Elmer, P.J.; Stewart, P.W.; Ershow, A.; Pearson, T.A.; Dennis, B.H.; Roheim, P.S. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: Studies in the fasting and postprandial states. Am. J. Clin. Nutr. 2007, 86, 1611–1620. [Google Scholar] [CrossRef]
- Clevidence, B.A.; Judd, J.T.; Schaefer, E.J.; Jenner, J.L.; Lichtenstein, A.H.; Muesing, R.A.; Wittes, J.; Sunkin, M.E. Plasma lipoprotein (a) levels in men and women consuming diets enriched in saturated, cis-, or trans-monounsaturated fatty acids. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1657–1661. [Google Scholar] [CrossRef]
- Muller, H.; Lindman, A.S.; Blomfeldt, A.; Seljeflot, I.; Pedersen, J.I. A diet rich in coconut oil reduces diurnal postprandial variations in circulating tissue plasminogen activator antigen and fasting lipoprotein (a) compared with a diet rich in unsaturated fat in women. J. Nutr. 2003, 133, 3422–3427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, H.; Lindman, A.S.; Brantsæter, A.L.; Pedersen, J.I. The serum LDL/HDL cholesterol ratio is influenced more favorably by exchanging saturated with unsaturated fat than by reducing saturated fat in the diet of women. J. Nutr. 2003, 133, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Mensink, R.P.; Zock, P.L.; Katan, M.B.; Hornstra, G. Effect of dietary cis and trans fatty acids on serum lipoprotein[a] levels in humans. J. Lipid Res. 1992, 33, 1493–1501. [Google Scholar] [PubMed]
- Tindall, A.M.; Kris-Etherton, P.M.; Petersen, K.S. Replacing Saturated Fats with Unsaturated Fats from Walnuts or Vegetable Oils Lowers Atherogenic Lipoprotein Classes Without Increasing Lipoprotein (a). J. Nutr. 2020, 150, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bordi, P.L.; Fleming, J.A.; Hill, A.M.; Kris-Etherton, P.M. Effect of a moderate fat diet with and without avocados on lipoprotein particle number, size and subclasses in overweight and obese adults: A randomized, controlled trial. J. Am. Heart Assoc. 2015, 4, e001355. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, K.R. Cholesterol profile measurement by vertical auto profile method. Clin. Lab. Med. 2006, 26, 787–802. [Google Scholar] [CrossRef]
- Yeang, C.; Clopton, P.C.; Tsimikas, S. Lipoprotein (a)-cholesterol levels estimated by vertical auto profile correlate poorly with Lp (a) mass in hyperlipidemic subjects: Implications for clinical practice interpretation of Lp (a)-mediated risk. J. Clin. Lipidol. 2016, 10, 1389–1396. [Google Scholar] [CrossRef]
- Haring, B.; Von Ballmoos, M.C.; Appel, L.J.; Sacks, F.M. Healthy dietary interventions and lipoprotein (a) plasma levels: Results from the Omni Heart Trial. PLoS ONE 2014, 9, e114859. [Google Scholar] [CrossRef] [Green Version]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R.; Conlin, P.R.; Erlinger, T.P.; Rosner, B.A.; Laranjo, N.M. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef]
- Faghihnia, N.; Tsimikas, S.; Miller, E.R.; Witztum, J.L.; Krauss, R.M. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J. Lipid Res. 2010, 51, 3324–3330. [Google Scholar] [CrossRef] [Green Version]
- Berryman, C.E.; West, S.G.; Fleming, J.A.; Bordi, P.L.; Kris-Etherton, P.M. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: A randomized controlled trial. J. Am. Heart Assoc. 2015, 4, e000993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Parker, T.L.; Connelly, P.W.; Qian, W.; Haight, J.S.; Faulkner, D.; Vidgen, E.; Lapsley, K.G. Dose response of almonds on coronary heart disease risk factors: Blood lipids, oxidized low-density lipoproteins, lipoprotein (a), homocysteine, and pulmonary nitric oxide: A randomized, controlled, crossover trial. Circulation 2002, 106, 1327–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajaram, S.; Burke, K.; Connell, B.; Myint, T.; Sabate, J. A monounsaturated fatty acid–rich pecan-enriched diet favorably alters the serum lipid profile of healthy men and women. J. Nutr. 2001, 131, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Zambón, D.; Sabaté, J.; Munoz, S.; Campero, B.; Casals, E.; Merlos, M.; Laguna, J.C.; Ros, E. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women: A randomized crossover trial. Arch. Intern. Med. 2000, 132, 538–546. [Google Scholar] [CrossRef]
- Lee, Y.; Berryman, C.E.; West, S.G.; Chen, C.Y.O.; Blumberg, J.B.; Lapsley, K.G.; Preston, A.G.; Fleming, J.A.; Kris-Etherton, P.M. Effects of dark chocolate and almonds on cardiovascular risk factors in overweight and obese individuals: A randomized controlled-feeding trial. J. Am. Heart Assoc. 2017, 6, e005162. [Google Scholar] [CrossRef] [Green Version]
- Santos, H.O.; Kones, R.; Rumana, U.; Earnest, C.P.; Izidoro, L.F.; Macedo, R.C. Lipoprotein (a): Current evidence for a physiologic role and the effects of nutraceutical strategies. Clin. Ther. 2019, 41, 1780–1797. [Google Scholar] [CrossRef]
- Yahya, R.; Berk, K.; Verhoeven, A.; Bos, S.; Van der Zee, L.; Touw, J.; Erhart, G.; Kronenberg, F.; Timman, R.; Sijbrands, E.; et al. Statin treatment increases lipoprotein(a) levels in subjects with low molecular weight apolipoprotein(a) phenotype. Atherosclerosis 2019, 289, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Enkhmaa, B.; Berglund, L. Statins and Lp(a): The plot thickens. Atherosclerosis 2019, 289, 173–175. [Google Scholar] [CrossRef] [Green Version]
- Brautbar, A.; Ballantyne, C.M. Pharmacological strategies for lowering LDL cholesterol: Statins and beyond. Nat. Rev. Cardiol. 2011, 8, 253–265. [Google Scholar] [CrossRef]
- Brousseau, M.E.; Ordovas, J.M.; Nicolosi, R.J.; Schaefer, E.J. Effects of dietary fat saturation on plasma lipoprotein (a) and hepatic apolipoprotein (a) mRNA concentrations in cynomolgus monkeys. Atherosclerosis 1994, 106, 109–118. [Google Scholar] [CrossRef]
- Azrolan, N.; Gavish, D.; Breslow, J.L. Plasma lipoprotein (a) concentration is controlled by apolipoprotein (a)(apo (a)) protein size and the abundance of hepatic apo (a) mRNA in a cynomolgus monkey model. J. Biol. Chem. 1991, 266, 13866–13872. [Google Scholar] [PubMed]
- Rainwater, D.L.; Manis, G.S.; Kushwaha, R.S. Characterization of an unusual lipoprotein similar to human lipoprotein a isolated from the baboon, Papio sp. Biochim. et Biophys. Acta (BBA)-Lipids Lipid Metab. 1986, 877, 75–78. [Google Scholar] [CrossRef]
- Laplaud, P.; Beaubatie, L.; Rall, S.; Luc, G.; Saboureau, M. Lipoprotein [a] is the major apoB-containing lipoprotein in the plasma of a hibernator, the hedgehog (Erinaceus europaeus). J. Lipid Res. 1988, 29, 1157–1170. [Google Scholar] [PubMed]
- Simons, K.; Ehnholm, C.; Renkonen, O.; Bloth, B. Characterization of the Lp(a) lipoprotein in human plasma. Acta Pathol. Microbiol. Scand. Sect. B Microbiol. Immunol. 1970, 78, 459–466. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Anuurad, E.; Zhang, W.; Berglund, L. Significant associations between lipoprotein(a) and corrected apolipoprotein B-100 levels in African-Americans. Atherosclerosis 2014, 235, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Marcovina, S.M.; Albers, J.J.; Scanu, A.M.; Kennedy, H.; Giaculli, F.; Berg, K.; Couderc, R.; Dati, F.; Rifai, N.; Sakurabayashi, I.; et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a). Clin. Chem. 2000, 46, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]


| Study | Design | Study Duration | Participants | N | Test Diets | Macronutrient Profiles of the Test Diets 1 | Lp(a) mg/dL (Mean ± SEM) | LDL-C (Measured) mg/Dl (Mean ± SEM) | SFA Replacement Effect Summary | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHO | PRO | Total Fat | SFA | MUFA | PUFA | |||||||||
| Ginsberg et al. 1998 (USA) DELTA 1 [80] | 4-site multicenter, randomized, 3 period crossover, controlled feeding trial | 8-week diet periods (4–6 washout) | Normolipidemic, aged 22–65 years; 55% women (30% black; 32% postmenopausal) and 45% men (20% black) | 103 | AAD | 48 | 15 | 34.3 | 15.0 | 12.8 | 6.5 | 15.5 ± 1.8 a | 131.4 ± 2.7 2,a | SFA→CHO: ↑ Lp(a) ↓LDL-C |
| Step 1 | 55 | 15 | 28.6 | 9 | 12.9 | 6.7 | 17.0 ± 1.8 b | 122.2 ± 2.6 2,b | ||||||
| Low-SFA | 59 | 15 | 25.3 | 6.1 | 12.4 | 6.7 | 18.2 ± 1.9 c | 116.9 ± 2.6 2,c | ||||||
| Berglund et al. 2007 (USA) DELTA 2 [81] | 4-site multicenter, randomized, 3 period crossover, controlled feeding trial | 7-week diet periods (4–6 washout) | Low HDL-C, moderately elevated triglycerides and insulin;aged 21–65 years; 39% women (18% black) and 61% men (8% black) | 85 | AAD | 49 | 15.3 | 35.8 | 15.6 | 14.4 | 5.8 | 9.9 ± 1.4 a | 128 ± 3.1 a | SFA→CHO: ↑ Lp(a) ↓LDL-C SFA→MUFA: ↑ Lp(a) ↓LDL-C |
| MUFA | 48.8 | 15.5 | 35.7 | 8.7 | 20.8 | 6.2 | 11.0 ± 1.5 b | 120 ± 3.1 b | ||||||
| Step 1 | 54.9 | 16.1 | 29 | 8 | 15.5 | 5.5 | 11.9 ± 1.6 b | 119 ± 3.1 b | ||||||
| Clevidence et al. 1997 (USA) [82] | Randomized, 4 period, crossover, controlled feeding trial | 6-week diet periods (no washout) | 80–120% of desirable BMI; aged 25–65 years; total cholesterol 50th–75th percentile; HDL-C > 35 mg/dl (men) or >40 mg/dL (women) | 58 | SFA | 45 | 15 | 40 | 19.3 4 | 10.9 5 | 6.1 6 | 21.9 ± 0.4 a | 141 ± 9.3 2,a | SFA→MUFA: ↑ Lp(a) ↓LDL-C |
| Oleic | 46 | 15 | 39 | 13.4 4 | 16.7 5 | 6.1 6 | 23.8 ± 0.4 b | 129 ± 9.3 2,b | ||||||
| Moderate trans fat 7 | 46 | 15 | 39 | 13.0 4 | 14.1 5 | 6.0 6 | 23.8 ± 0.4 b | 137 ± 9.3 2,c | ||||||
| High trans fat 8 | 46 | 15 | 39 | 12.7 4 | 11.4 5 | 6.2 6 | 24.7 ± 0.4 b | 139 ± 9.3 2,a,c | ||||||
| Mensink et al. 1992 (The Netherlands) Experiment 1 [85] | 2 group parallel, controlled feeding trial | 17 days–control run-in diet 36 days–MUFA or PUFA | Young, normolipidemic (mean total cholesterol 193 ± 31 mg/dL), non-obese (mean BMI 21.6 ± 2.0 kg/m2) students | 58 | High SFA (control) | 48–49 | 13 | 36.7 | 19.3 | 11.5 | 4.6 | Pre MUFA: 8.4 (0–34.0) 9 Pre PUFA: 3.7 (0–23.5) 9 | Pre MUFA: 128 ± 29 2,3 Pre PUFA: 129 ± 26 2,3 | SFA→MUFA: ←→ Lp(a) ↓LDL-C SFA→PUFA: ←→ Lp(a) ↓LDL-C |
| 29 | MUFA | 48–49 | 13 | 37.4 | 12.9 | 15.1 | 7.9 | 9.1 (0–33.6) 9 | 104 ± 26 2,3,a | |||||
| 29 | PUFA | 48–49 | 13 | 37.6 | 12.6 | 10.8 | 12.7 | 4.0 (0–24.0) 9 | 111 ± 23 2,3,b | |||||
| Mensink et al. 1992 (The Netherlands) Experiment 2 [85] | Randomized, 3 period, crossover, controlled feeding trial | 3-week diet periods (washout not reported) | Mean total cholesterol 184 ± 31 mg/dL; Mean BMI 21.5 ± 2.1 kg/m2 | 59 | SFA | 46 | 13–14 | 38.8 | 19.4 | 14.7 | 3.4 | 2.6 (0–44.7) 9,a | 121 ± 22 2,3,a | SFA→MUFA: ↑ Lp(a) ↓LDL-C |
| Oleic acid 10 | 46 | 13–14 | 39.6 | 9.5 | 24.1 | 4.6 | 3.2 (0–48.4) 9,b | 103 ± 21 2,3,b | ||||||
| Trans fat | 46 | 13–14 | 40.2 | 10.0 | 13.3 | 4.6 | 4.5 (0–51.0) 9,c | 118 ± 24 2,3c | ||||||
| Mensink et al. 1992 (The Netherlands) Experiment 3 [85] | Randomized, 3 period, crossover, controlled feeding trial | 3-week diet periods (washout not reported) | Mean total cholesterol 195 ± 25 mg/dL; Mean BMI 22.0 ± 2.3 kg/m2 | 56 | Stearate | 44–47 | 12–13 | 43.5 | 20.1 (11.8 stearic acid) | 16.3 | 4.3 | 6.9 (0–74.9) 9,a | 116 ± 27 2,3,a | SFA→PUFA: ←→ Lp(a) ↓LDL-C |
| Linoleate | 44–47 | 12–13 | 41.1 | 11.0 (2.8 stearic acid) | 15.7 | 12.5 | 6.9 (0–78.2) 9,a | 109 ± 24 2,3,b | ||||||
| Trans fat 11 | 44–47 | 12–13 | 39.7 | 10.3 (3.0 stearic acid) | 15.6 | 3.8 | 8.5 (0–89.1) 9,b | 119 ± 25 2,3,a | ||||||
| Muller et al. 2003 (Norway) [83,84] | Randomized, 3 period, crossover, controlled feeding trial | 3-week diet periods (1-week washout) | Female students, aged 31 ± 10, BMI 24.5 ± 3.2 kg/m2 | 25 | High saturated fat | 46.7 | 14.9 | 38.4 | 22.7 12 | 5.5 | 3.9 | 31.6 ± 48.7 3,a | 124 ± 30 2,3,a | SFA 12→MUFA/PUFA: ↑ Lp(a) ↓LDL-C SFA 12→CHO: ←→ Lp(a) ←→ LDL-C |
| Low saturated fat | 63.8 | 16.5 | 19.7 | 10.5 12 | 3.5 | 2.3 | 34.0 ± 49.3 3,a,b | 121 ± 26 2,3,a | ||||||
| High MUFA/PUFA | 46.8 | 15 | 38.2 | 2.4 12 | 14.1 | 15.6 | 35.8 ± 51.5 3,b | 97 ± 25 2,3,b | ||||||
| Study | Design | Study Duration | Participants | N | Test diets | Macronutrient Profiles of the Test Diets 1 | Lp(a) mg/dL (Mean ± SEM) | LDL-C (Measured) mg/dL (Mean ± SEM) | Effect Summary | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHO | PRO | Total Fat | SFA | MUFA | PUFA | |||||||||
| Omni Heart (USA) [90,91] | Randomized, 3 period, crossover, controlled feeding trial | 6-week diet periods (2–4 week washout) | Systolic blood pressure 120–159 mmHg or diastolic blood pressure 80–99 mmHg, aged > 30 years; 46% women (70% black) and 54% men (44% black) | 155 | CHO | 58 | 15 | 27 | 6 | 13 | 8 | 3.2 (2.2, 4.2) 2,*,a | -11.6 (-14.6, -8.6) 2,3,*,a | CHO→PRO ↑ Lp(a) ↓LDL-C CHO→MUFA/PUFA ↑ Lp(a) ↓LDL-C MUFA/PUFA→PRO ↑ Lp(a) ↓LDL-C |
| Protein | 48 | 25 | 27 | 6 | 13 | 8 | 4.7 (3.7, 5.7) 2, *,b | -14.2 (-17.5 -10.9) 2,3,*,b | ||||||
| Unsaturated fat | 48 | 15 | 37 | 6 | 21 | 10 | 2.1 (1.1, 3.1) 2,*,c | -13.1 (-16.4, -9.8) 2,3,*,a,b | ||||||
| Faghihnia et al. 2010 (USA) [92] | Randomized, 2 period, crossover, trial | 4-week diet periods (no washout) | Body weight <130% of ideal; aged >20 years; 97% men | 63 | High-fat, low-CHO | 45 | 15 | 40 | 13 | 11 | 14 | 17.8 ± 12.8 4,a | 124.0 ± 31.5 3,4,a | High-fat, low-CHO → Low-fat, high-CHO ↑ Lp(a) ↓LDL-C |
| Low-fat, high-CHO | 65 | 15 | 20 | 5 | 10 | 5 | 19.9 ± 13.7 4,b | 117.3 ± 30.7 3,4,b | ||||||
| Berryman et al. 2015 (USA)) [93] | Randomized, 2 period crossover, controlled feeding trial | 6-week diet periods (2-week washout) | LDL-C 121–190 mg/dL women or 128–194 mg/dL men; aged 30–65 years; BMI 20–35 kg/m2; 54% women | 48 | Almond | 51.3 | 16.4 | 32.3 | 7.7 | 13.9 | 8.4 | 7.7 ± 0.8 a | 129 ± 3 a | Lower fat, higher CHO → Higher fat, lower CHO diet with almonds ↑ Lp(a) ↓LDL-C |
| Control | 58.4 | 15.2 | 26.4 | 7.8 | 10.4 | 6.2 | 6.7 ± 0.8 b | 135 ± 3 b | ||||||
| Jenkins et al. 2002 (Canada) [94] | Randomized, 3 period crossover, trial | 4-week diet periods (>2-week washout) | Hyperlipidemic (LDL-C >159 mg/dL); aged 48–86 years; BMI 20.5–31.5 kg/m2; 56% men and 44% postmenopausal women | 27 | Full-dose almond | 44.8 | 17.4 | 36.0 | 7.2 | 18.9 | 8.2 | 14.2 ± 2.9 a | 155 ± 4.6 3,a | Lower fat, higher CHO → Higher fat, lower CHO diet with almonds ↓ Lp(a) ↓LDL-C |
| Half-dose almond | 48.4 | 17.6 | 32.1 | 7.5 | 14.5 | 8.0 | 15.4 ± 3.2 | 159 ± 4.6 3,a | ||||||
| Control | 54.5 | 17.5 | 26.3 | 7.0 | 9.0 | 8.0 | 15.5 ± 3.2 b | 163 ± 5.0 3,b | ||||||
| Lee et al. 2017 (USA) [97] | Randomized, 4 period crossover, controlled feeding trial | 4-week diet periods (2-week washout) | Overweight or obese; aged 30–70 years; LDL-C 25th–95th percentile | 31 | AAD | 49 | 17 | 34 | 13 | 13 | 7 | 4.9 (4.1, 5.8) 5 | 135.6 ± 2.8 a | Average American diet → Higher fat, lower saturated fat diet with almonds or almonds + chocolate ←→ Lp(a) ↓LDL-C Average American diet → higher carbohydrate diet with chocolate ←→ Lp(a) ←→LDL-C |
| Almond | 48 | 16 | 36 | 8 | 16 | 9 | 5.3 (4.5, 6.3) 5 | 126.4 ± 2.8 b | ||||||
| CHOC | 51 | 16 | 33 | 12 | 12 | 6 | 4.6 (3.9, 5.5) 5 | 136.1 ± 2.8 a | ||||||
| Almond+ CHOC | 49 | 16 | 35 | 9 | 9 | 8 | 5.1 (4.3, 6.1) 5 | 128.9 ± 2.8 b | ||||||
| Rajaram et al. 2001 (USA) [95] | Randomized, 2 period crossover, controlled feeding trial | 4-week diet periods (no washout) | Healthy; total cholesterol 15th–80th percentile | 23 | Step 1 | 56.8 | 14.5 | 28.3 | 8.2 | 11.0 | 6.3 | 25 ± 22 4,a | 117.9 ± 21.7 4,6,a | Pecan-enriched higher fat, lower carbohydrate diet → lower fat, higher carbohydrate diet ↓ Lp(a) ↓LDL-C |
| Pecan-enriched | 47.2 | 13.1 | 39.6 | 8.1 | 18.9 | 10.7 | 21 ± 18 4,b | 105.6 ± 19.7 4,6,b | ||||||
| Zambon et al. (Spain) [96] | Randomized, 2 period crossover, controlled feeding trial | 4-week diet periods (no washout) | Polygenic hypercholesterolemia | 49 | Control (Mediterranean) | 49.8 | 19.0 | 31.2 | 6.9 | 17.5 | 4.8 | 34 ± 24 4,a | 185 ± 25 4,a | Mediterranean diet → Mediterranean diet with walnuts (35% of total fat; 41–56 g/day) ↓ Lp(a) ↓LDL-C |
| Walnut (Mediterranean) | 48 | 17.9 | 33.2 | 6.0 | 13.5 | 11.7 | 32 ± 22 4,b | 174 ± 30 4,b | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enkhmaa, B.; Petersen, K.S.; Kris-Etherton, P.M.; Berglund, L. Diet and Lp(a): Does Dietary Change Modify Residual Cardiovascular Risk Conferred by Lp(a)? Nutrients 2020, 12, 2024. https://doi.org/10.3390/nu12072024
Enkhmaa B, Petersen KS, Kris-Etherton PM, Berglund L. Diet and Lp(a): Does Dietary Change Modify Residual Cardiovascular Risk Conferred by Lp(a)? Nutrients. 2020; 12(7):2024. https://doi.org/10.3390/nu12072024
Chicago/Turabian StyleEnkhmaa, Byambaa, Kristina S. Petersen, Penny M. Kris-Etherton, and Lars Berglund. 2020. "Diet and Lp(a): Does Dietary Change Modify Residual Cardiovascular Risk Conferred by Lp(a)?" Nutrients 12, no. 7: 2024. https://doi.org/10.3390/nu12072024
APA StyleEnkhmaa, B., Petersen, K. S., Kris-Etherton, P. M., & Berglund, L. (2020). Diet and Lp(a): Does Dietary Change Modify Residual Cardiovascular Risk Conferred by Lp(a)? Nutrients, 12(7), 2024. https://doi.org/10.3390/nu12072024





