Imipramine Influences Body Distribution of Supplemental Zinc Which May Enhance Antidepressant Action
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Drug Administration
2.3. Forced Swim Test (FST)
2.4. Locomotor Activity
2.5. Determination of Zn (II) in Serum and Brain Homogenates
2.6. Determination of Zn Transport Using Caco-2 Cells
2.7. Zn (II) Analysis by F-AAS Method
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
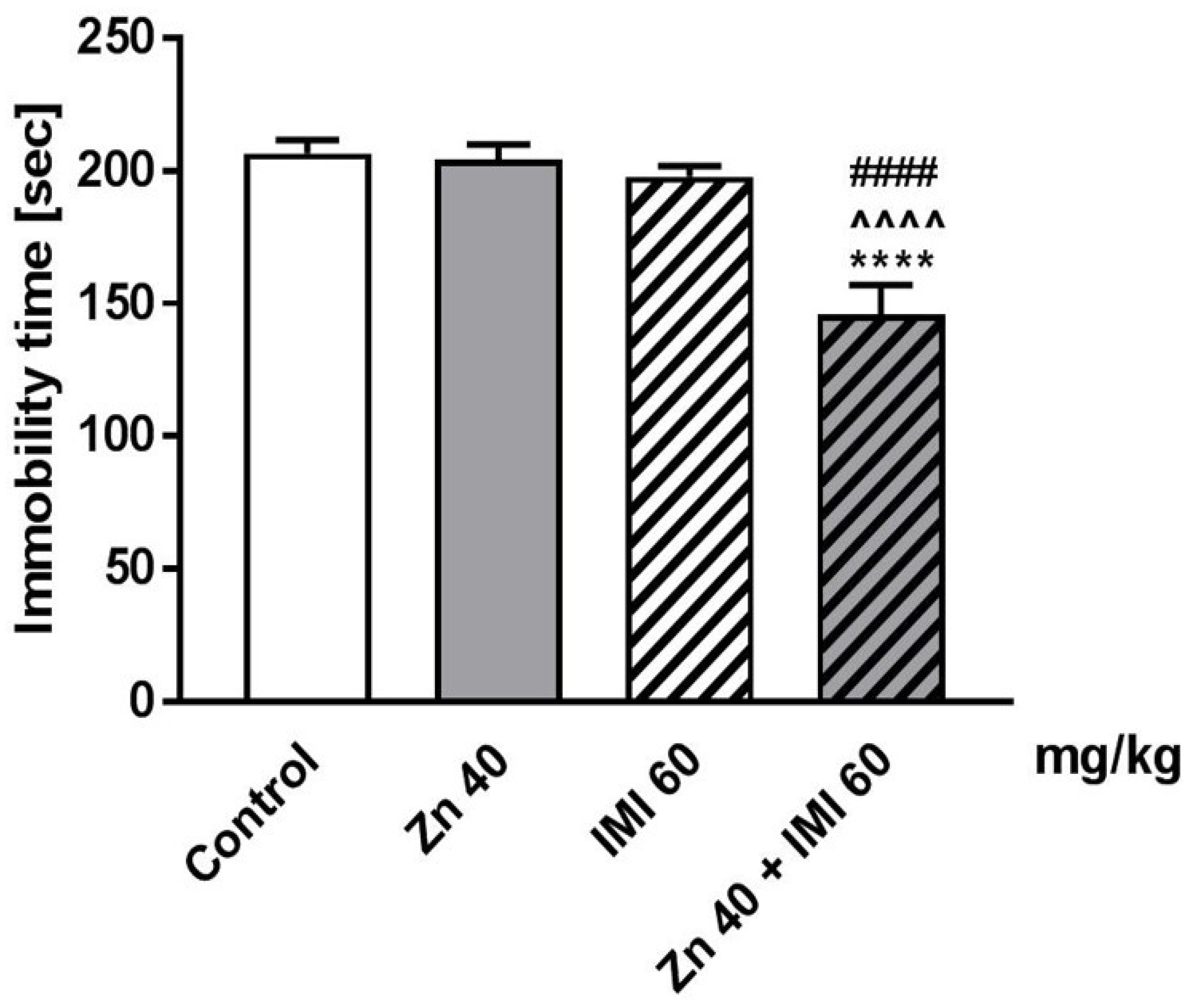
3.1. The Effect of Coadministration of Zn and IMI in the FST in Mice
3.2. The Effect of Coadministration of Zn and IMIon Locomotor Activity of Mice
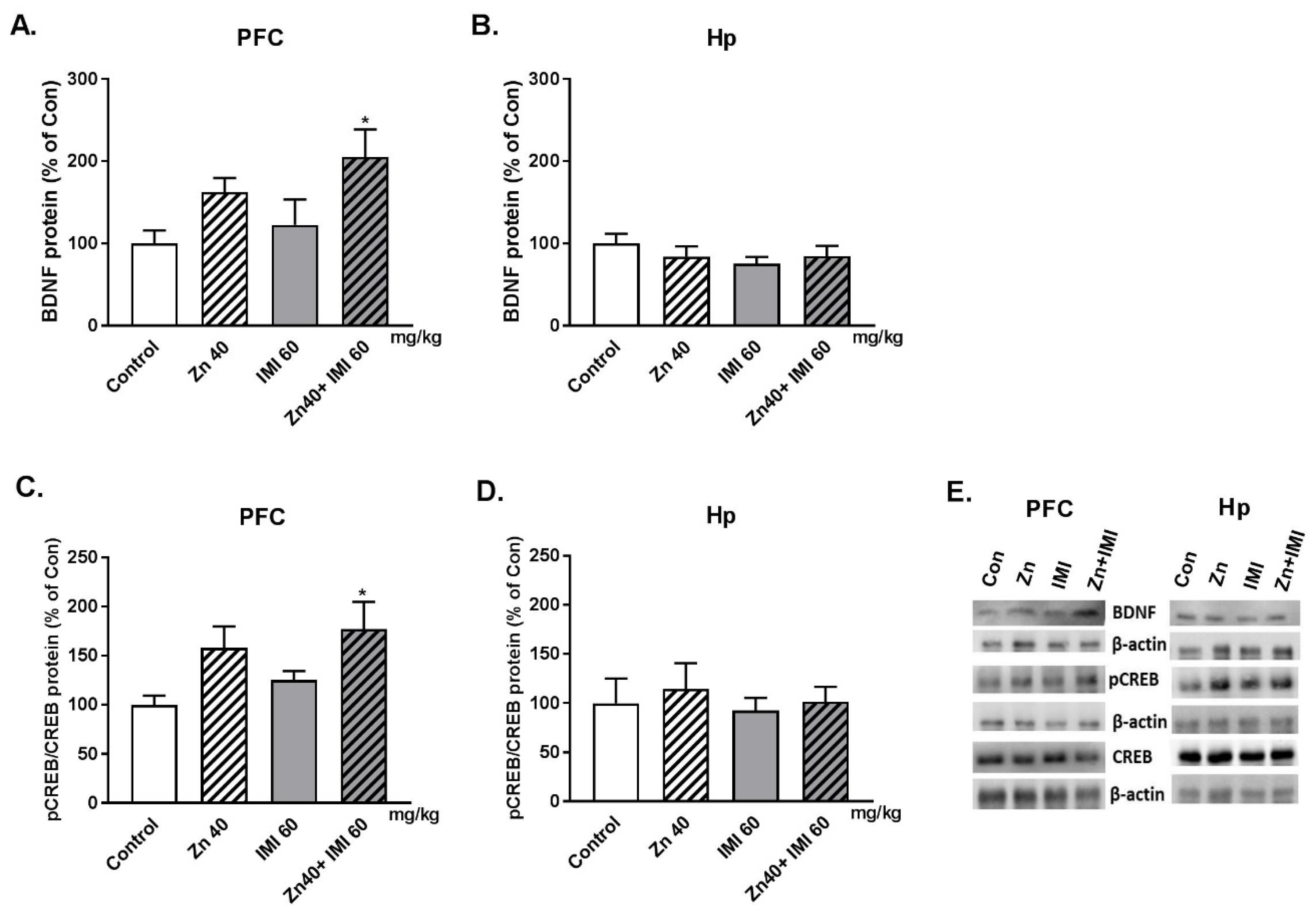
3.3. The Effect of Zn and IMI on the BDNF and pCREB/CREB Protein Level in the PFC and Hp of Mice Brain
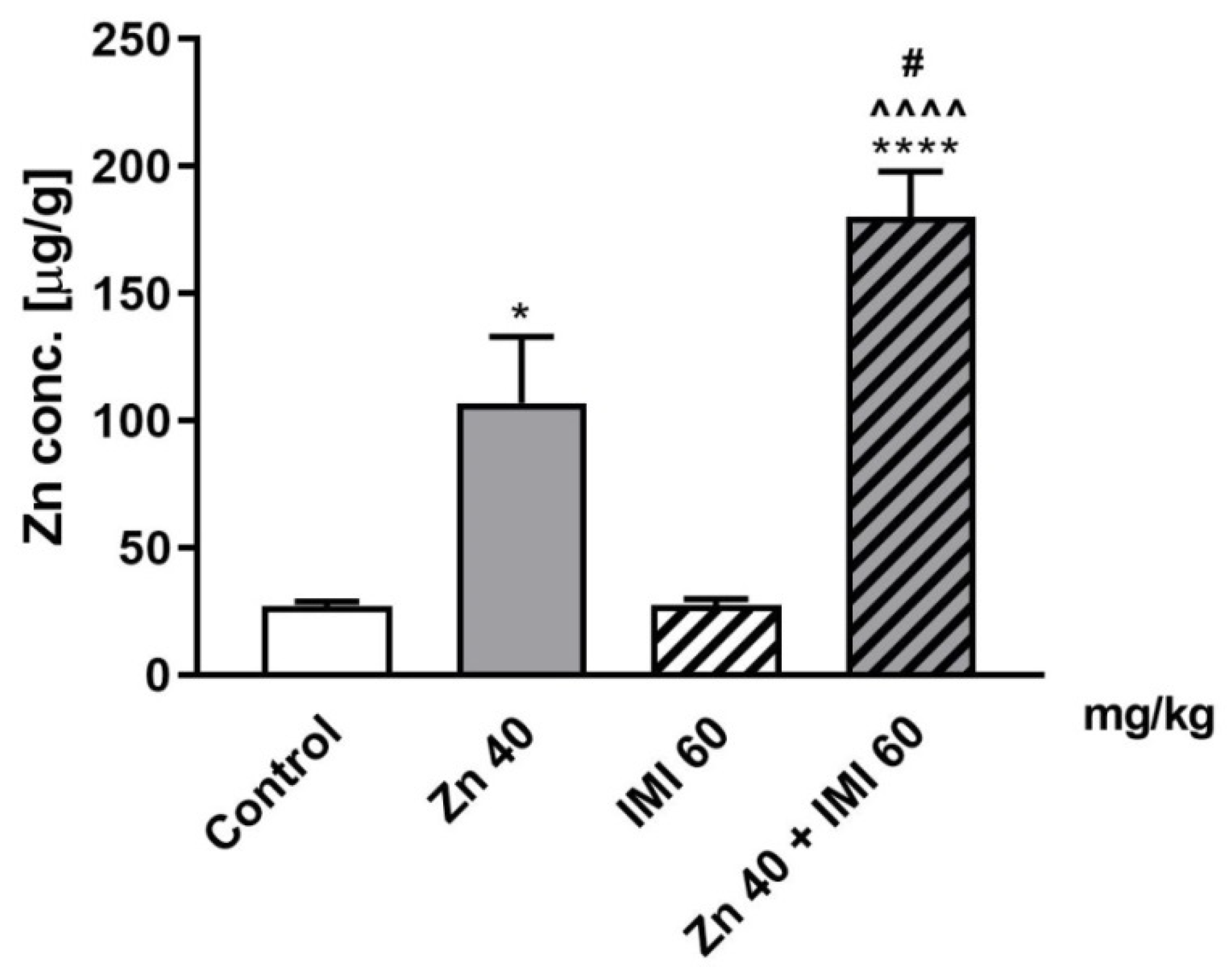
3.4. The Effect of Zn and IMI on the Concentrations of Zn in Mouse Serum and Brain
3.5. The Effect of Zn + IMI on Zn Transport in Human Colon Epithelial Cells (In Vitro Studies)
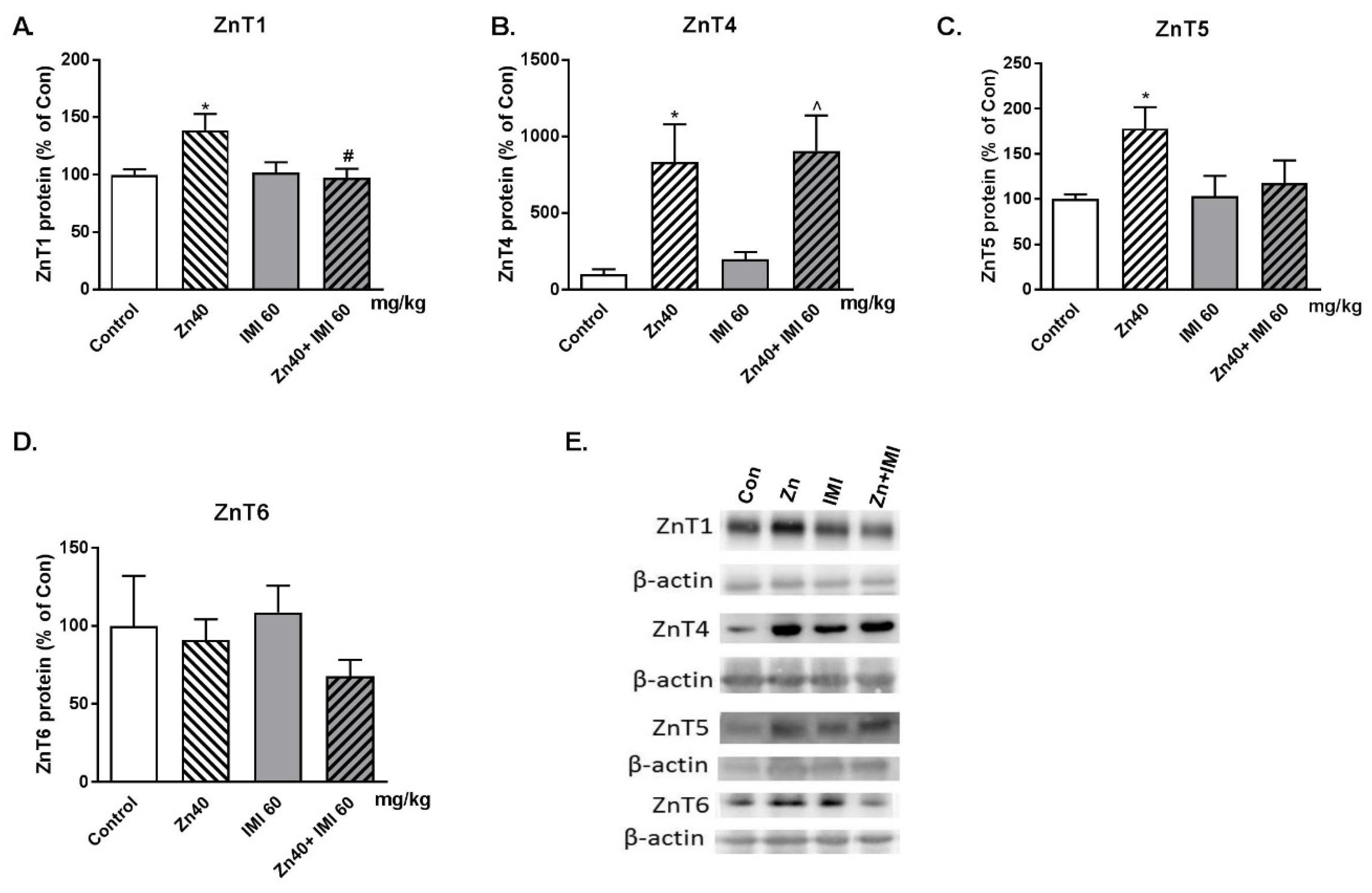
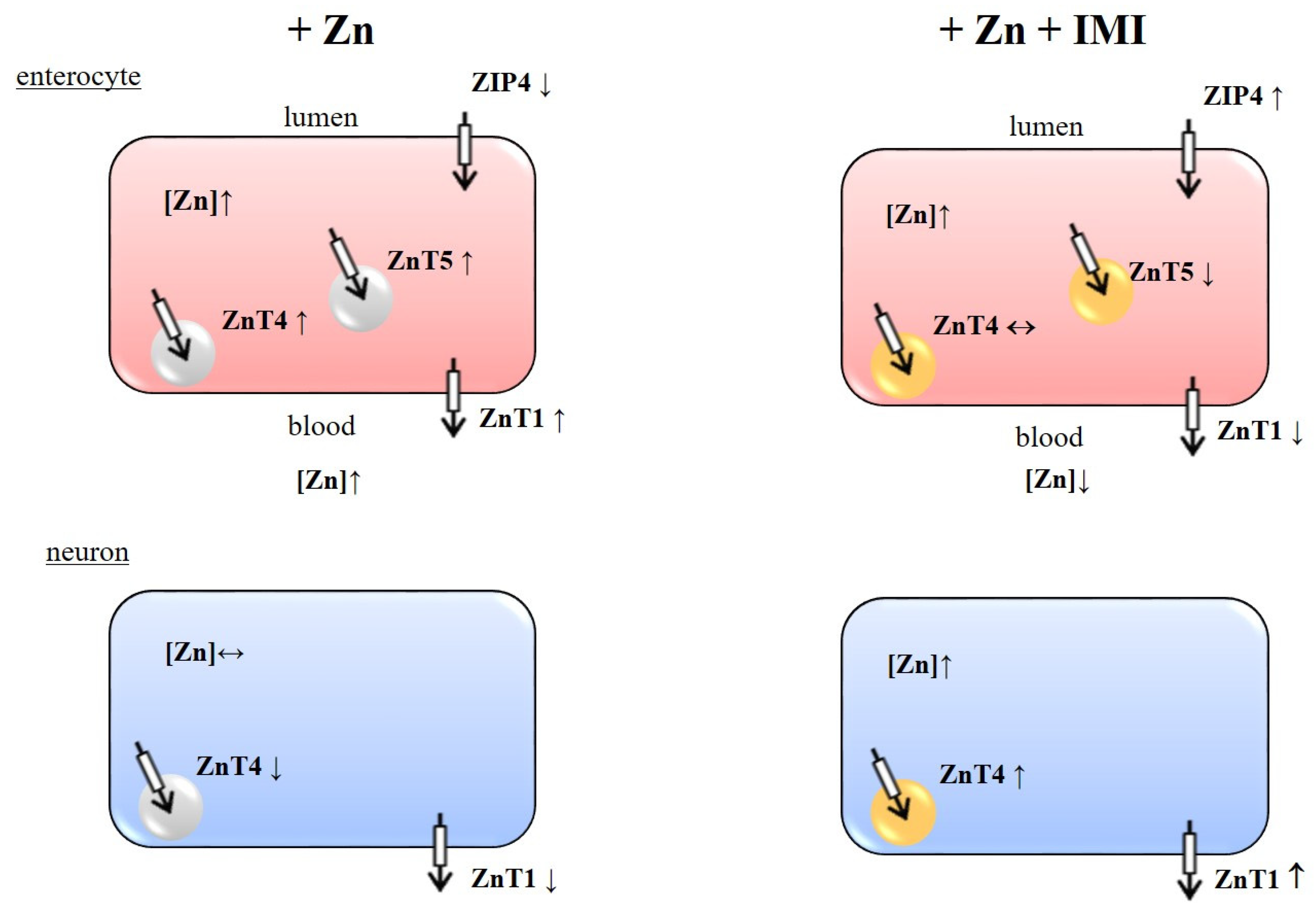
3.6. The Effect of Zn + IMI on the ZnT Transporters Protein Level in the Small Intestine
3.7. The Effect of Zn + IMI on ZIP Transporters and DMT1 Protein Levels in the Small Intestine
3.8. The Effect of Zn and IMI on Zn Concentration in the Mouse Small Intestine
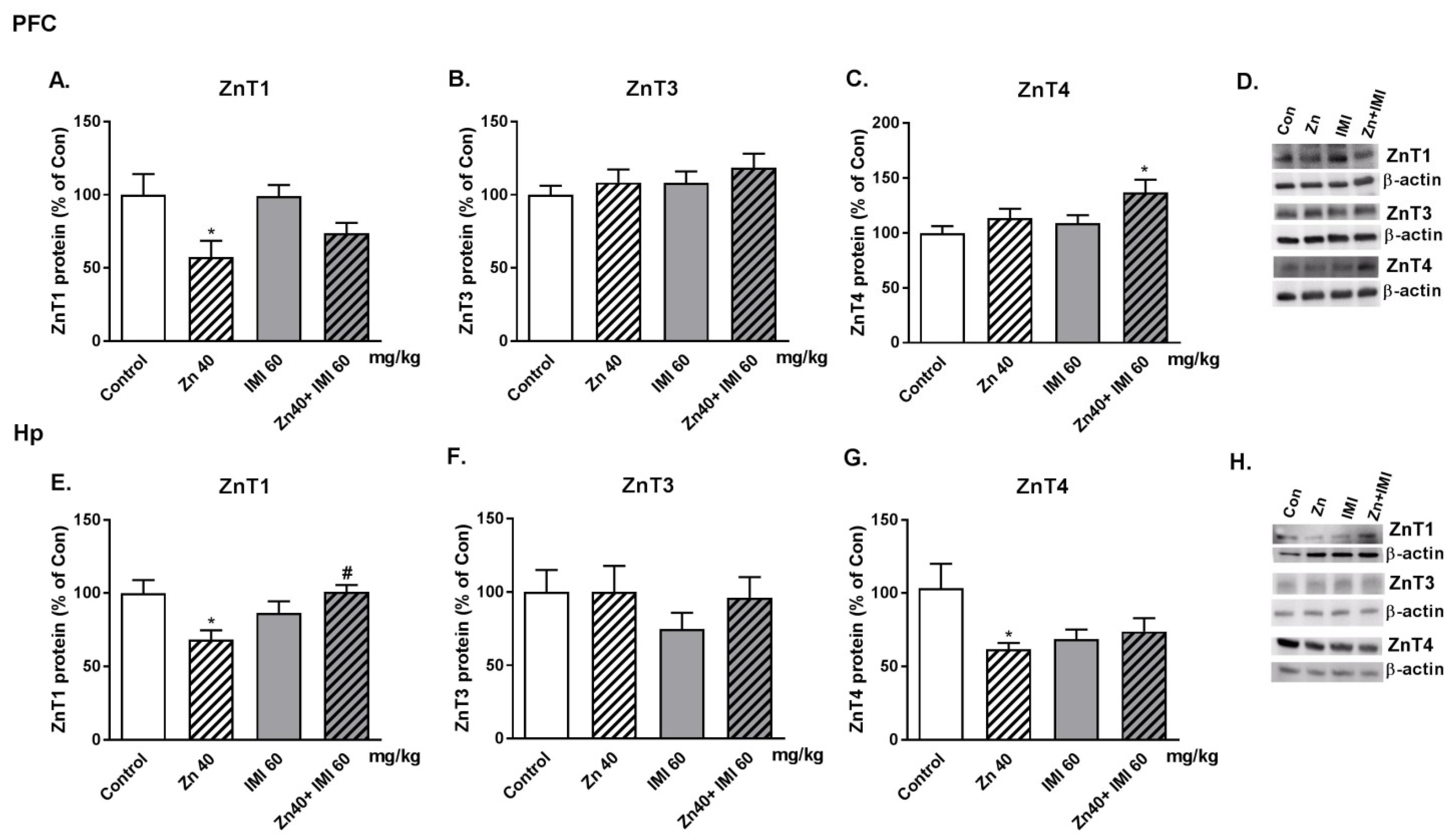
3.9. The Effect of Co-Administration of Zn and IMI on Protein Levels of Zn Transporters (ZnT1, ZnT3, and ZnT4) in the Mouse Brain
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Nowak, G.; Siwek, M.; Dudek, D.; Zieba, A.; Pilc, A. Effect of zinc supplementation on antidepressant therapy in unipolar depression: A preliminary placebo-controlled study. Pol. J. Pharmacol. 2003, 55, 1143–1147. [Google Scholar] [PubMed]
- Siwek, M.; Dudek, D.; Paul, I.A.; Sowa-Kucma, M.; Zieba, A.; Popik, P.; Pilc, A.; Nowak, G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: A double blind, placebo-controlled study. J. Affect. Disord. 2009, 118, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, E.; Shams, J.; Sabetkasaei, M.; M-Shirazi, M.; Rashidkhani, B.; Mostafavi, A.; Bornak, E.; Nasrollahzadeh, J. Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr. Neurosci. 2014, 17, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Solati, Z.; Jazayeri, S.; Tehrani-Doost, M.; Mahmoodianfard, S.; Gohari, M.R. Zinc monotherapy increases serum brain-derived neurotrophic factor (BDNF) levels and decreases depressive symptoms in overweight or obese subjects: A double-blind, randomized, placebo-controlled trial. Nutr. Neurosci. 2015, 18, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Nowak, G. Zinc, future mono/adjunctive therapy for depression: Mechanisms of antidepressant action. Pharmacol. Rep. 2015, 67, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B.; Szopa, A.; Serefko, A.; Poleszak, E.; Nowak, G. The role of magnesium and zinc in depression: Similarities and differences. Magnes. Res. 2018, 31, 78–89. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, D.; Xiang, H.; Yan, S.; Sun, F.; Wei, Z. Rapid antidepressant actions of imipramine potentiated by zinc through PKA-dependented regulation of mTOR and CREB signaling. Biochem. Biophys. Res. Commun. 2019, 518, 337–343. [Google Scholar] [CrossRef]
- Kroczka, B.; Branski, P.; Palucha, A.; Pilc, A.; Nowak, G. Antidepressant-like properties of zinc in rodent forced swim test. Brain Res. Bull. 2001, 55, 297–300. [Google Scholar] [CrossRef]
- Szewczyk, B.; Branski, P.; Wieronska, J.M.; Palucha, A.; Pilc, A.; Nowak, G. Interaction of zinc with antidepressants in the forced swimming test in mice. Pol. J. Pharmacol. 2002, 54, 681–685. [Google Scholar]
- Cunha, M.P.; Machado, D.G.; Bettio, L.E.; Capra, J.C.; Rodrigues, A.L. Interaction of zinc with antidepressants in the tail suspension test. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1913–1920. [Google Scholar] [CrossRef]
- Wrobel, A.; Serefko, A.; Wlaz, P.; Poleszak, E. The effect of imipramine, ketamine, and zinc in the mouse model of depression. Metab. Brain Dis. 2015, 30, 1379–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Q.; Li, H.; Tian, X.; Shen, Z.; Wang, X.; Mo, F.; Huang, J.; Shen, H. Zinc and imipramine reverse the depression-like behavior in mice induced by chronic restraint stress. J. Affect. Disord. 2016, 197, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kalappa, B.I.; Anderson, C.T.; Goldberg, J.M.; Lippard, S.J.; Tzounopoulos, T. AMPA receptor inhibition by synaptically released zinc. Proc. Natl. Acad. Sci. USA 2015, 112, 15749–15754. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlegel-Zawadzka, M.; Nowak, G. Alterations in serum and brain trace element levels after antidepressant treatment. Part II. Copper. Biol. Trace Elem. Res. 2000, 73, 37–45. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Cieslik, K.; Klenk-Majewska, B.; Danilczuk, Z.; Wrobel, A.; Lupina, T.; Ossowska, G. Influence of zinc supplementation on imipramine effect in a chronic unpredictable stress (CUS) model in rats. Pharmacol. Rep. 2007, 59, 46–52. [Google Scholar]
- Szewczyk, B.; Poleszak, E.; Wlaz, P.; Wrobel, A.; Blicharska, E.; Cichy, A.; Dybala, M.; Siwek, A.; Pomierny-Chamiolo, L.; Piotrowska, A.; et al. The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 323–329. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Domingo, J.L.; Llobet, J.M.; Paternain, J.L.; Corbella, J. Acute Zinc Intoxication—Comparison of the Antidotal Efficacy of Several Chelating-Agents. Vet. Hum. Toxicol. 1988, 30, 224–228. [Google Scholar] [PubMed]
- Duman, R.S. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol. Med. 2004, 5, 11–25. [Google Scholar] [CrossRef]
- Hara, T.; Takeda, T.A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Aspects Med. 2013, 34, 548–560. [Google Scholar] [CrossRef]
- Kambe, T.; Yamaguchi-Iwai, Y.; Sasaki, R.; Nagao, M. Overview of mammalian zinc transporters. Cell Mol. Life Sci. 2004, 61, 49–68. [Google Scholar] [CrossRef]
- Prakash, A.; Bharti, K.; Majeed, A.B. Zinc: Indications in brain disorders. Fundam. Clin. Pharmacol. 2015, 29, 131–149. [Google Scholar] [CrossRef]
- Rafalo, A.; Zadrozna, M.; Nowak, B.; Kotarska, K.; Wiatrowska, K.; Pochwat, B.; Sowa-Kucma, M.; Misztak, P.; Nowak, G.; Szewczyk, B. The level of the zinc homeostasis regulating proteins in the brain of rats subjected to olfactory bulbectomy model of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 72, 36–48. [Google Scholar] [CrossRef]
- Rafalo-Ulinska, A.; Piotrowska, J.; Kryczyk, A.; Opoka, W.; Sowa-Kucma, M.; Misztak, P.; Rajkowska, G.; Stockmeier, C.A.; Datka, W.; Nowak, G.; et al. Zinc transporters protein level in postmortem brain of depressed subjects and suicide victims. J. Psychiatr. Res. 2016, 83, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, D.R.; Vallortigara, J.; Alghamdi, A.; Hortobagyi, T.; Ballard, C.; Thomas, A.J.; O’Brien, J.T.; Aarsland, D.; Francis, P.T. Depression and synaptic zinc regulation in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia. Am. J. Geriatr. Psychiatry 2015, 23, 141–148. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, B. Dietary zinc absorption: A play of Zips and ZnTs in the gut. IUBMB Life 2010, 62, 176–182. [Google Scholar] [CrossRef]
- McMahon, R.J.; Cousins, R.J. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc. Natl. Acad. Sci. USA 1998, 95, 4841–4846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opoka, W.; Kryczyk, A.; Krakowska, A.; Piotrowska, J.; Gdula-Argasinska, J.; Kala, K.; Linek, M.; Muszynska, B. The evaluation of effect of selected metal ions on the efficiency of passive and active transport of imipramine. Psychiatr. Pol. 2019, 53, 1169–1179. [Google Scholar] [CrossRef] [PubMed]


| Treatment (mg/kg) | Distance Travelled (cm) |
|---|---|
| Control Zn 40 IMI 60 Zn 40 + IMI 60 | 618.0 ± 72.29 487.2 ± 108.3 466.7 ± 93.61 348.5 ± 58.32 |
| Treatment (mg/kg) | Zn Conc. in Serum (µg/mL) | Zn Conc. in Brain (µg/g) |
|---|---|---|
| A. | ||
| Control | 0.4 ± 0.04 | 12.0 ± 0.19 |
| Zn 40 | 6.0 ± 0.85 **** | 11.6 ± 0.08 |
| Zn 40 + IMI 60 | 3.8 ± 0.48 **** # | 13.1 ± 0.14 **** #### |
| B. | ||
| Control | 0.6 ± 0.1 | 16.3 ± 0.35 |
| IMI 60 | 1.5 ± 0.15 * | 16.5 ± 0.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafało-Ulińska, A.; Poleszak, E.; Szopa, A.; Serefko, A.; Rogowska, M.; Sowa, I.; Wójciak, M.; Muszyńska, B.; Krakowska, A.; Gdula-Argasińska, J.; et al. Imipramine Influences Body Distribution of Supplemental Zinc Which May Enhance Antidepressant Action. Nutrients 2020, 12, 2529. https://doi.org/10.3390/nu12092529
Rafało-Ulińska A, Poleszak E, Szopa A, Serefko A, Rogowska M, Sowa I, Wójciak M, Muszyńska B, Krakowska A, Gdula-Argasińska J, et al. Imipramine Influences Body Distribution of Supplemental Zinc Which May Enhance Antidepressant Action. Nutrients. 2020; 12(9):2529. https://doi.org/10.3390/nu12092529
Chicago/Turabian StyleRafało-Ulińska, Anna, Ewa Poleszak, Aleksandra Szopa, Anna Serefko, Magdalena Rogowska, Ireneusz Sowa, Magdalena Wójciak, Bożena Muszyńska, Agata Krakowska, Joanna Gdula-Argasińska, and et al. 2020. "Imipramine Influences Body Distribution of Supplemental Zinc Which May Enhance Antidepressant Action" Nutrients 12, no. 9: 2529. https://doi.org/10.3390/nu12092529







