Relationship between Serum 25(OH)D and Depression: Causal Evidence from a Bi-Directional Mendelian Randomization Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Genetic Variants of Serum 25(OH)D and Depression
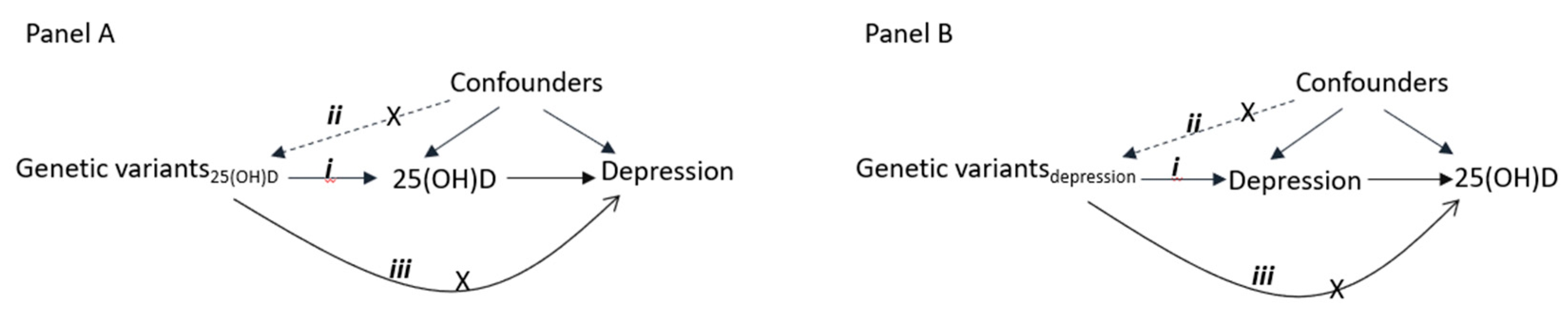
2.3. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berridge, M.J. Vitamin D and Depression: Cellular and Regulatory Mechanisms. Pharmacol. Rev. 2017, 69, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Kar, S.K.; Suthar, N.; Nebhinani, N. Vitamin D and Depression: A Critical Appraisal of the Evidence and Future Directions. Indian J. Psychol. Med. 2020, 42, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Harms, L.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the brain. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Puchacz, E.; Stumpf, W.E.; Stachowiak, E.K.; Stachowiak, M.K. Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells. Mol. Brain Res. 1996, 36, 193–196. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef] [PubMed]
- Okereke, O.I.; Reynolds, C.F.; Mischoulon, D.; Chang, G.; Vyas, C.M.; Cook, N.R.; Weinberg, A.; Bubes, V.; Copeland, T.; Friedenberg, G.; et al. Effect of Long-term Vitamin D3 Supplementation vs Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial. JAMA 2020, 324, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Gowda, U.; Mutowo, M.P.; Smith, B.J.; Wluka, A.E.; Renzaho, A. Vitamin D supplementation to reduce depression in adults: Meta-analysis of randomized controlled trials. Nutrition 2015, 31, 421–429. [Google Scholar] [CrossRef]
- Spedding, S. Vitamin D and Depression: A Systematic Review and Meta-Analysis Comparing Studies with and without Biological Flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.A.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef]
- Jamilian, H.; Amirani, E.; Milajerdi, A.; Kolahdooz, F.; Mirzaei, H.; Zaroudi, M.; Ghaderi, A.; Asemi, Z. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2019, 94, 109651. [Google Scholar] [CrossRef]
- Tønnesen, R.; Hovind, P.H.; Jensen, L.T.; Schwarz, P. Determinants of vitamin D status in young adults: Influence of lifestyle, sociodemographic and anthropometric factors. BMC Public Health 2016, 16, 385. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Peyrot, W.J.; Nivard, M.G.; Mbarek, H.; Boomsma, D.I.; Penninx, B.W. A role for vitamin D and omega-3 fatty acids in major depression? An exploration using genomics. Transl. Psychiatry 2019, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, K.; Melhus, H.; Larsson, S.C. Serum 25-Hydroxyvitamin D Concentrations and Major Depression: A Mendelian Randomization Study. Nutrients 2018, 10, 1987. [Google Scholar] [CrossRef] [PubMed]
- Libuda, L.; Laabs, B.H.; Ludwig, C.; Bühlmeier, J.; Antel, J.; Hinney, A.; Naaresh, R.; Föcker, M.; Hebebrand, J.; König, I.R.; et al. Vitamin D and the Risk of Depression: A Causal Relationship? Findings from a Mendelian Randomization Study. Nutrients 2019, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; Holmes, M.V.; Smith, G.D. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef]
- Tyrrell, J.; Mulugeta, A.; Wood, A.R.; Zhou, A.; Beaumont, R.N.; Tuke, M.A.; Jones, S.E.; Ruth, K.S.; Yaghootkar, H.; Sharp, S.; et al. Using genetics to understand the causal influence of higher BMI on depression. Int. J. Epidemiol. 2019, 48, 834–848. [Google Scholar] [CrossRef]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.-H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D.; et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018, 9, 260. [Google Scholar] [CrossRef]
- Biobank. Available online: http://www.ukbiobank.ac.uk/ (accessed on 10 October 2017).
- UK Biobank. Biomark Assay Quality Procedures: Approaches Used to Minimise Systematic and Random Errors (and the Wider Epidemiological Implications). Available online: http://biobank.ctsu.ox.ac.uk/showcase/showcase/docs/biomarker_issues.pdf (accessed on 10 April 2019).
- Fry, D.; Almond, R.; Moffat, S.; Gordon, M.; Singh, P. UK Biobank Biomarker Project: Companion Document to Accompany Serum Biomarker Data. 2019. Available online: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf (accessed on 21 May 2020).
- Jameson, J.L.; Longo, D.L. Precision Medicine—Personalized, Problematic, and Promising. N. Engl. J. Med. 2015, 372, 2229–2234. [Google Scholar] [CrossRef]
- WHO. Body Mass Index-BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 16 December 2020).
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Hyde, C.L.; Nagle, M.W.; Tian, C.; Chen, X.; Paciga, S.A.; Wendland, J.R.; Tung, J.Y.; Hinds, D.; Perlis, R.H.; Winslow, A.R. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet. 2016, 48, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Smith, G.D.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.M.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal Relationship between Obesity and Vitamin D Status: Bi-Directional Mendelian Randomization Analysis of Multiple Cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Cizza, G.; Primma, S.; Csako, G. Depression as a risk factor for osteoporosis. Trends Endocrinol. Metab. 2009, 20, 367–373. [Google Scholar] [CrossRef]
- Mulugeta, A.; Zhou, A.; King, C.; Hyppönen, E. Association between major depressive disorder and multiple disease outcomes: A phenome-wide Mendelian randomisation study in the UK Biobank. Mol. Psychiatry 2020, 25, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.C. Vitamin D insufficiency as a cause of hyperparathyroidism. Am. Fam. Physician 2005, 71, 46–49. [Google Scholar] [PubMed]
- Rejnmark, L.; Ejlsmark-Svensson, H. Effects of PTH and PTH Hypersecretion on Bone: A Clinical Perspective. Curr. Osteoporos. Rep. 2020, 18, 103–114. [Google Scholar] [CrossRef]
- Seminog, O.O.; Goldacre, M. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax 2013, 68, 171–176. [Google Scholar] [CrossRef]
- Çolak, Y.; Nordestgaard, B.G.; Afzal, S. Low vitamin D and risk of bacterial pneumonias: Mendelian randomisation studies in two population-based cohorts. Thorax 2020. [Google Scholar] [CrossRef]
- Greiller, C.L.; Martineau, A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; Goodall, E.C.; et al. Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis. Health Technol. Assess. 2019, 23, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Vimaleswaran, K.S.; Whittaker, J.C.; Hingorani, A.D.; Hyppönen, E. Evaluation of Genetic Markers as Instruments for Mendelian Randomization Studies on Vitamin D. PLoS ONE 2012, 7, e37465. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Alves, A.C.; Heerspink, H.J.L.; Tikkanen, E.; Eriksson, J.; et al. Association of vitamin D status with arterial blood pressure and hypertension risk: A mendelian randomisation study. Lancet Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef]
- Maddock, J.; Zhou, A.; Cavadino, A.; Kuźma, E.; Bao, Y.; Smart, M.C.; Saum, K.U.; Schöttker, B.; Engmann, J.; Kjærgaard, M.; et al. Vitamin D and cognitive function: A Mendelian randomisation study. Sci. Rep. 2017, 7, 13230. [Google Scholar] [CrossRef]
- Scragg, R. Limitations of vitamin D supplementation trials: Why observational studies will continue to help determine the role of vitamin D in Health. J. Steroid Biochem. Mol. Biol. 2018, 177, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Nicholl, B.I.; Cullen, B.; Martin, D.; Ul-Haq, Z.; Evans, J.; Gill, J.M.R.; Roberts, B.; Gallacher, J.; Mackay, D.; et al. Prevalence and Characteristics of Probable Major Depression and Bipolar Disorder within UK Biobank: Cross-Sectional Study of 172,751 Participants. PLoS ONE 2013, 8, e75362. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef] [PubMed]

| n (%) | Depression | Serum 25(OH)D in nmol/L | |||
|---|---|---|---|---|---|
| n (%) | p-Value 1 | Median (IQR) | p-Value 2 | ||
| Sex | <1.0 × 10−300 | 0.02 | |||
| Male | 116,698 (50.8) | 11,292 (9.7) | 48.8 (34.4, 64.0) | ||
| Female | 113,134 (49.2) | 18,855 (16.7) | 48.9 (34.3, 63.9) | ||
| Age | 7.7 × 10−87 | <1.0 × 10−300 | |||
| 39–49 years | 51,191 (22.3) | 7201 (14.1) | 46.1 (32.1, 62.0) | ||
| 50–59 years | 73,641 (32.0) | 10,548 (14.3) | 47.5 (33.2, 62.8) | ||
| 60–73 years | 105,000 (45.7) | 12,398 (11.8) | 51.0 (36.6, 65.4) | ||
| BMI | 4.5 × 10−154 | <1.0 × 10−300 | |||
| Underweight, <18.5 kg/m2 | 1083 (0.5) | 167 (15.4) | 48.1 (31.2, 67.2) | ||
| Normal, (≥18.5 and <25) kg/m2 | 75,087 (32.7) | 9031 (12.0) | 52.3 (36.7, 67.6) | ||
| Overweight, (≥25 and <30) kg/m2 | 98,778 (43.0) | 11,961 (12.1) | 49.8 (35.6, 64.3) | ||
| Obese, ≥30 kg/m2 | 54,151 (23.6) | 8821 (16.3) | 42.9 (30.2, 57.2) | ||
| Missing | 733 (0.3) | 167 (22.8) | 39.7 (25.9, 56.0) | ||
| Education | 3.4 × 10−13 | 6.0 × 10−66 | |||
| None | 38,458 (16.7) | 5030 (13.1) | 49.7 (34.8, 65.0) | ||
| NVQ/CSE/A levels | 81,147 (35.3) | 11,044 (13.6) | 49.7 (35.0, 64.9) | ||
| Degree/professional | 108,287 (47.1) | 13,881 (12.8) | 47.9 (33.8, 62.8) | ||
| Missing | 1940 (0.8) | 192 (9.9) | 50.0 (34.7, 65.0) | ||
| Physical activity | 2.0 × 10−200 | <1.0 × 10−300 | |||
| None | 12,000 (5.2) | 2542 (21.2) | 35.4 (23.9, 51.7) | ||
| Light/moderate | 191,144 (83.2) | 25,075 (13.1) | 48.9 (34.7, 63.7) | ||
| Strenuous sports | 25,932 (11.3) | 2337 (9.0) | 54.2 (39.3, 69.2) | ||
| Missing | 756 (0.3) | 193 (25.5) | 38.6 (24.7, 56.1) | ||
| Oily fish consumption | 4.8 × 10−36 | <1.0 × 10−300 | |||
| Never | 24,100 (10.5) | 3775 (15.7) | 44.2 (29.7, 60.6) | ||
| <Once a week | 76,738 (33.4) | 10,090 (13.2) | 47.1 (32.6, 62.6) | ||
| Once a week | 87,870 (38.2) | 10,737 (12.2) | 49.9 (35.7, 64.6) | ||
| >Once a week | 40,130 (17.5) | 5400 (13.5) | 52.2 (38.2, 66.4) | ||
| Missing | 994 (0.4) | 145 (14.6) | 45.9 (30.6, 61.9) | ||
| Sun protection use | 1.8 × 10−60 | <1.0 × 10−300 | |||
| Do not go in sunshine | 1048 (0.5) | 251 (24.0) | 31.7 (21.1, 45.7) | ||
| Never/rarely | 18,794 (8.2) | 2506 (13.3) | 43.9 (29.8, 59.8) | ||
| Sometimes | 77,559 (33.7) | 9443 (12.2) | 48.6 (34.3, 63.5) | ||
| Most of the time | 84,409 (36.7) | 11,164 (13.2) | 49.7 (35.4, 64.6) | ||
| Always | 47,902 (20.8) | 6764 (14.1) | 49.7 (34.9, 65.0) | ||
| Missing | 120 (0.1) | 19 (15.8) | 33.2 (22.4, 48.6) | ||
| Long standing illness | <1.0 × 10−300 | 5.9 × 10−280 | |||
| No | 156,658 (68.2) | 15,275 (9.8) | 49.7 (35.3, 64.5) | ||
| Yes | 68,590 (29.8) | 14,113 (20.6) | 46.8 (32.2, 62.6) | ||
| Missing | 4584 (2.0) | 759 (16.6) | 46.9 (32.9, 61.9) | ||
| n (%) | Depression n (%) | Odds of Depression (n = 202,413) 6 | |||
|---|---|---|---|---|---|
| Basic 1 OR (95%CI) | Socioeconomic 2 OR (95%CI) | Lifestyle 3 OR (95%CI) | |||
| Serum 25(OH)D level 4 | |||||
| <25 | 21,688 (10.7) | 3209 (14.8) | Reference | Reference | Reference |
| ≥25 and <50 | 82,389 (40.7) | 10,548 (12.8) | 0.72 (0.68, 0.75) | 0.76 (0.73, 0.80) | 0.85 (0.81, 0.89) |
| ≥50 and <75 | 72,843 (36.0) | 9206 (12.6) | 0.64 (0.61, 0.67) | 0.70 (0.66, 0.73) | 0.83 (0.79, 0.87) |
| >75 | 25,493 (12.6) | 3312 (13.0) | 0.62 (0.58, 0.66) | 0.67 (0.63, 0.71) | 0.83 (0.78, 0.88) |
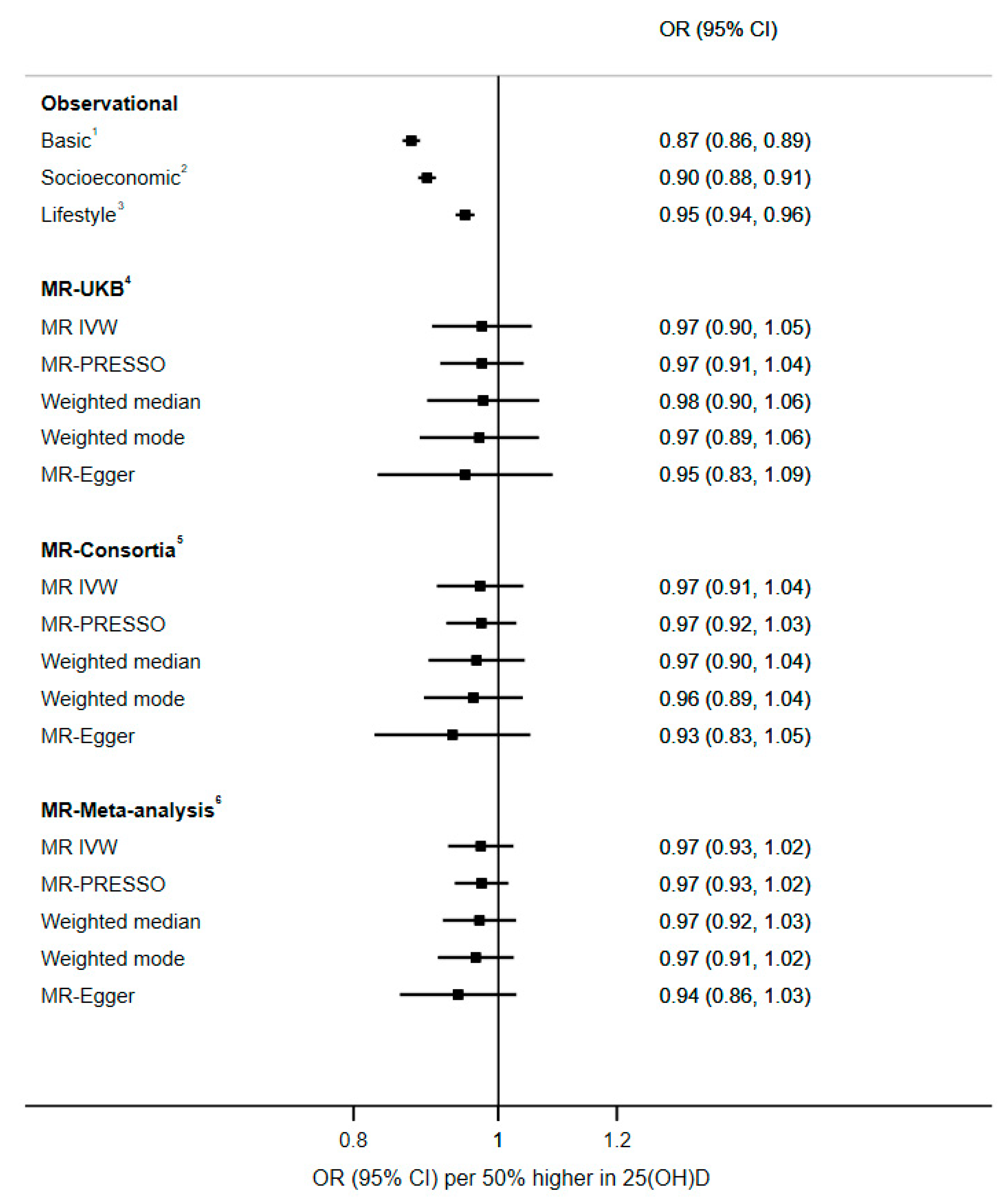
| Per 50% higher serum 25(OH)D 5 | 202,413 | 26,270 (13.0) | 0.87 (0.86, 0.89) | 0.90 (0.88, 0.91) | 0.95 (0.94, 0.96) |
| Ptrend | 2.1 × 10−72 | 4.9 × 10−50 | 4.0 × 10−12 | ||
| Pcurvature | 8.0 × 10−12 | 2.4 × 10−6 | 2.8 × 10−4 | ||
| Psex-interaction | 3.8 × 10−6 | 7.9 × 10−4 | 9.4 × 10−4 | ||
| Page-interaction | 0.03 | 0.04 | 0.07 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulugeta, A.; Lumsden, A.; Hyppönen, E. Relationship between Serum 25(OH)D and Depression: Causal Evidence from a Bi-Directional Mendelian Randomization Study. Nutrients 2021, 13, 109. https://doi.org/10.3390/nu13010109
Mulugeta A, Lumsden A, Hyppönen E. Relationship between Serum 25(OH)D and Depression: Causal Evidence from a Bi-Directional Mendelian Randomization Study. Nutrients. 2021; 13(1):109. https://doi.org/10.3390/nu13010109
Chicago/Turabian StyleMulugeta, Anwar, Amanda Lumsden, and Elina Hyppönen. 2021. "Relationship between Serum 25(OH)D and Depression: Causal Evidence from a Bi-Directional Mendelian Randomization Study" Nutrients 13, no. 1: 109. https://doi.org/10.3390/nu13010109
APA StyleMulugeta, A., Lumsden, A., & Hyppönen, E. (2021). Relationship between Serum 25(OH)D and Depression: Causal Evidence from a Bi-Directional Mendelian Randomization Study. Nutrients, 13(1), 109. https://doi.org/10.3390/nu13010109





