Alternative Dietary Patterns for Americans: Low-Carbohydrate Diets
Abstract
:1. Introduction: The Current 2020 Dietary Guidelines Need Greater Flexibility
2. Low-Carbohydrate Diets Defined
3. Unintended Consequences of the DGA: The Obesity and Type 2 Diabetes Epidemic
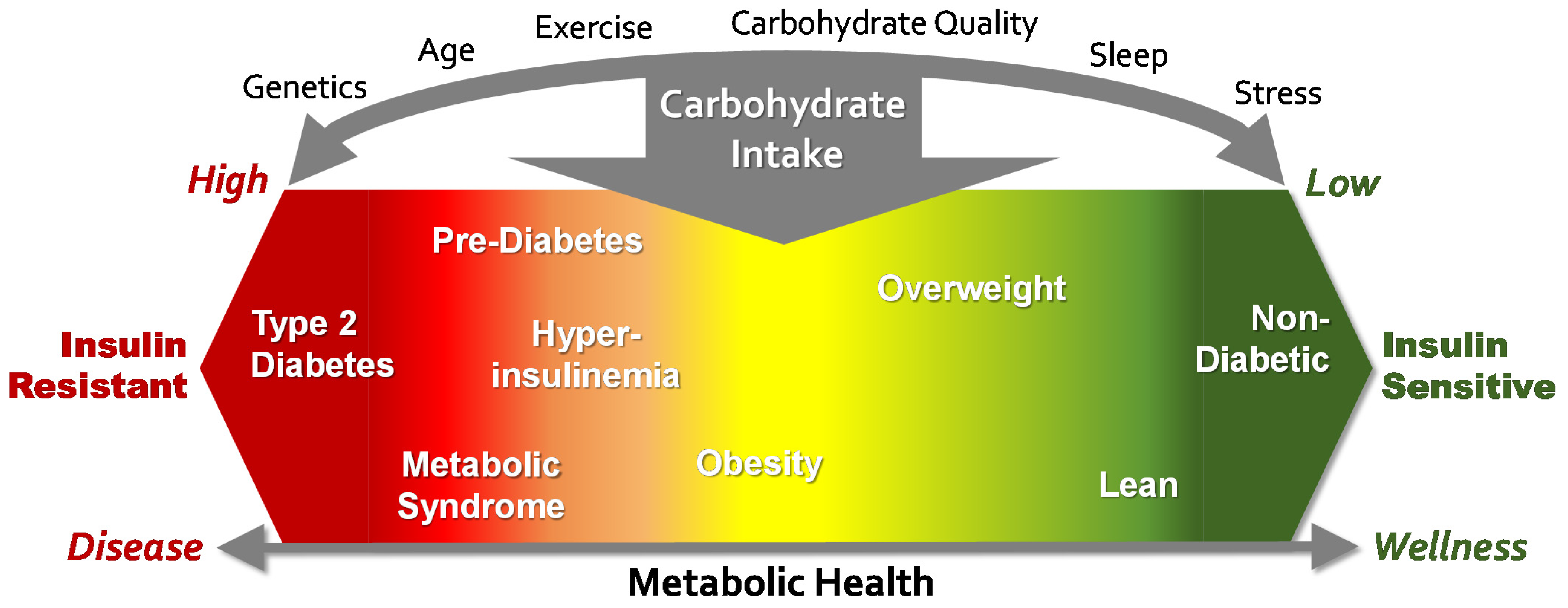
4. The Role of Carbohydrate in the Obesity and T2D Epidemics
5. Obesity and T2D Are Conditions Strongly Associated with Insulin Resistance
6. Scientific Support for a Low-Carbohydrate Diet Option in the DGA
6.1. Obesity
6.2. Metabolic Syndrome
6.3. Type 2 Diabetes (T2D)
6.4. Cardiovascular Disease (CVD)
6.5. Low-Carbohydrate Diets and Mortality Outcomes
6.6. Qualitative Research
7. Principles of Very Low-Carbohydrate (Ketogenic) Diets
8. Macronutrients
8.1. Carbohydrate
- 5–10 g from protein-based foods. Eggs, cheese, and shellfish will carry a few residual grams of carbohydrate from natural sources and added marinades and spices.
- 10–15 g from non-starchy vegetables.
- 5–10 g from nuts/seeds. Most nuts contain 5–6 g carb per ounce.
- 5–10 g from fruits such as berries, olives, tomatoes, avocados.
- 5–10 g from miscellaneous sources such as low-carb desserts, high-fat dressings or drinks with very small amounts of sugar.
8.2. Protein
8.3. Fat
9. Micronutrients
9.1. Sodium and Potassium
9.2. Calcium
9.3. Magnesium
9.4. Vitamin D
9.5. Fiber
10. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2020; p. 25. [Google Scholar]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2020; pp. 54; 64; 70. [Google Scholar]
- Cohen, E.; Cragg, M.; de Fonseka, J.; Hite, A.; Rosenberg, M.; Zhou, B. Statistical review of US macronutrient consumption data, 1965–2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition 2015, 31, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Ströhle, A.; Hahn, A. Diets of modern hunter-gatherers vary substantially in their carbohydrate content depending on ecoenvironments: Results from an ethnographic analysis. Nutr. Res. 2011, 31, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tondt, J.; Yancy, W.S.; Westman, E.C. Application of nutrient essentiality criteria to dietary carbohydrates. Nutr. Res. Rev. 2020, 33, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Ogden, C.L. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA 2018, 319, 1723–1725. [Google Scholar] [CrossRef] [Green Version]
- Menke, A.; Casagrande, S.; Geiss, L.; Cowie, C.C. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015, 314, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- 2020 Dietary Guidelines Advisory Committee and Food Pattern Modeling Team. Added Sugars: Food Pattern Modeling: Ages 2 Years and Older; 2020 Dietary Guidelines Advisory Committee Project; U.S. Department of Agriculture: Washington, DC, USA, 2020; Tables 5.1, 5.10 and 5.11. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-07/FoodPatternModeling_Report_2YearsandOlder.pdf (accessed on 25 February 2020).
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bistrian, B.R. Two Types of Very Low–Carbohydrate Diets. Pediatrics 2018, 142, e20181536A. [Google Scholar] [CrossRef]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [Green Version]
- The Cost of Diabetes. ADA. 2020. Available online: www.diabetes.org/resources/statistics/cost-diabetes (accessed on 26 February 2020).
- Dietary Guidelines Advisory Committee. Scientific Report of the 2000 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2000; p. 36. [Google Scholar]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2015; Part D, Ch 6; p. 13, lines 460–461. [Google Scholar]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.-T.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10 158 incident cases among 262 525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef]
- Zhang, B.; Menzin, J.; Friedman, M.; Korn, J.R.; Burge, R.T. Predicted coronary risk for adults with coronary heart disease and low HDL-C: An analysis from the US National Health and Nutrition Examination Survey. Curr. Med. Res. Opin. 2008, 24, 2711–2717. [Google Scholar] [CrossRef]
- Lichtenstein, A. Proceedings of the Public Meeting of the Dietary Guidelines Advisory Committee, Washington, DC, USA, 15 September 2015. minute 4:35:10 (no longer publicly available).
- Ludwig, D.S.; Willett, W.C.; Volek, J.S.; Neuhouser, M.L. Dietary fat: From foe to friend? Science 2018, 362, 764–770. [Google Scholar] [CrossRef] [Green Version]
- Araújo, J.; Cai, J.; Stevens, J. Prevalence of optimal metabolic health in American adults: National Health and Nutrition Examination Survey 2009–2016. Metab. Syndr. Relat. Disord. 2019, 17, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Coppack, S.W.; Jensen, M.D.; Miles, J.M. In vivo regulation of lipolysis in humans. J. Lipid Res. 1994, 35, 177–193. [Google Scholar] [CrossRef]
- Kelly, C.T.; Mansoor, J.; Dohm, G.L.; Chapman, W.H., III; Pender, J.R., IV; Pories, W.J. Hyperinsulinemic syndrome: The metabolic syndrome is broader than you think. Surgery 2014, 156, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. Fructose and cardiometabolic health: What the evidence from sugar-sweetened beverages tells us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Stenvinkel, P.; Andrews, P.; Sánchez-Lozada, L.; Nakagawa, T.; Gaucher, E.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Jimenez, C.R.; Garcia, G. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J. Intern. Med. 2020, 287, 252–262. [Google Scholar] [CrossRef] [Green Version]
- Lanaspa, M.A.; Ishimoto, T.; Li, N.; Cicerchi, C.; Orlicky, D.J.; Ruzycki, P.; Rivard, C.; Inaba, S.; Roncal-Jimenez, C.A.; Bales, E.S.; et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 2013, 4, 2434. [Google Scholar] [CrossRef]
- Volek, J.S.; Feinman, R.D. Carbohydrate restriction improves the features of Metabolic Syndrome. Metabolic Syndrome may be defined by the response to carbohydrate restriction. Nutr. Metab. 2005, 2, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Volek, J.S.; Fernandez, M.L.; Feinman, R.D.; Phinney, S.D. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res. 2008, 47, 307–318. [Google Scholar] [CrossRef]
- Moller, D.E.; Flier, J.S. Insulin resistance—mechanisms, syndromes, and implications. N. Engl. J. Med. 1991, 325, 938–948. [Google Scholar] [PubMed]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, e128308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.F.; Dufour, S.; Savage, D.B.; Bilz, S.; Solomon, G.; Yonemitsu, S.; Cline, G.W.; Befroy, D.; Zemany, L.; Kahn, B.B.; et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 12587–12594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarsland, A.; Wolfe, R.R. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J. Lipid Res. 1998, 39, 1280–1286. [Google Scholar] [CrossRef]
- Krauss, R.M. All low-density lipoprotein particles are not created equal. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 959–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westman, E.C.; Yancy, W.S., Jr.; Humphreys, M. Dietary treatment of diabetes mellitus in the pre-insulin era (1914-1922). Perspect. Biol. Med. 2006, 49, 77–83. [Google Scholar] [CrossRef]
- Creighton, B.C.; Hyde, P.N.; Maresh, C.M.; Kraemer, W.J.; Phinney, S.D.; Volek, J.S. Paradox of hypercholesterolaemia in highly trained, keto-adapted athletes. BMJ Open Sport Exerc. Med. 2018, 4, e000429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFountain, R.A.; Miller, V.J.; Barnhart, E.C.; Hyde, P.N.; Crabtree, C.D.; McSwiney, F.T.; Beeler, M.K.; Buga, A.; Sapper, T.N.; Short, J.A.; et al. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019, 184, e538–e547. [Google Scholar] [CrossRef]
- Sharman, M.J.; Gómez, A.L.; Kraemer, W.J.; Volek, J.S. Very low-carbohydrate and low-fat diets affect fasting lipids and postprandial lipemia differently in overweight men. J. Nutr. 2004, 134, 880–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volek, J.S.; Sharman, M.J.; Gomez, A.L.; Scheett, T.P.; Kraemer, W.J. An isoenergetic very low carbohydrate diet improves serum HDL cholesterol and triacylglycerol concentrations, the total cholesterol to HDL cholesterol ratio and postprandial lipemic responses compared with a low fat diet in normal weight, normolipidemic women. J. Nutr. 2003, 133, 2756–2761. [Google Scholar] [PubMed]
- Catlin, G. Letters and Notes on the Manners, Customs, and Conditions of the North American Indians; Willis P. Hazard: Whitefish MT, USA, 1844; Volume 1 & 2. [Google Scholar]
- McClellan, W.S.; Du Bois, E.F. Clinical calorimetry XLV. Prolonged meat diets with a study of kidney function and ketosis. J. Biol. Chem. 1930, 87, 651–668. [Google Scholar] [CrossRef]
- Orr, J.B.; Gilks, J.L. Studies of Nutrition. The Physique and Health of Two African Tribes; His Majesty’s Stationery Office: London, UK, 1931. [Google Scholar]
- Kossoff, E.H.; Zupec-Kania, B.A.; Amark, P.E.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Helen Cross, J.; Dahlin, M.G.; et al. Optimal clinical management of children receiving the ketogenic diet: Recommendations of the International Ketogenic Diet Study Group. Epilepsia 2009, 50, 304–317. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Rho, J.M. Epilepsy and the Ketogenic Diet; Springer Science & Business Media: Berlin, Germany, 2004. [Google Scholar]
- Wilder, R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin. Proc. 1921, 2, 307–308. [Google Scholar]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Koutnik, A.P.; Goldberg, E.L.; Upadhyay, V.; Turnbaugh, P.J.; Verdin, E.; Newman, J.C. Investigating ketone bodies as immunometabolic countermeasures against respiratory viral infections. Med 2020, 1, 43–65. [Google Scholar] [CrossRef]
- Yurista, S.R.; Chong, C.R.; Badimon, J.J.; Kelly, D.P.; de Boer, R.A.; Westenbrink, B.D. Therapeutic Potential of Ketone Bodies for Patients With Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1660–1669. [Google Scholar] [CrossRef]
- Hamdy, O.; Tasabehji, M.W.; Elseaidy, T.; Tomah, S.; Ashrafzadeh, S.; Mottalib, A. Fat Versus Carbohydrate-Based Energy-Restricted Diets for Weight Loss in Patients With Type 2 Diabetes. Curr. Diab. Rep. 2018, 18, 128. [Google Scholar] [CrossRef] [Green Version]
- Palgi, A.; Read, J.L.; Greenberg, I.; Hoefer, M.A.; Bistrian, B.R.; Blackburn, G.L. Multidisciplinary treatment of obesity with a protein-sparing modified fast: Results in 668 outpatients. Am. J. Public Health 1985, 75, 1190–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vertes, V. Very low calorie diets--history, safety and recent developments. Postgrad. Med. 1984, 60, 56–58. [Google Scholar]
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Mills, K.T.; Yao, L.; Demanelis, K.; Eloustaz, M.; Yancy, W.S., Jr.; Kelly, T.N.; He, J.; Bazzano, L.A. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: A meta-analysis of randomized controlled clinical trials. Am. J. Epidemiol. 2012, 176 (Suppl. 7), S44–S54. [Google Scholar] [CrossRef] [Green Version]
- Sackner-Bernstein, J.; Kanter, D.; Kaul, S. Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis. PLoS One 2015, 10, e0139817. [Google Scholar] [CrossRef]
- Santos, F.L.; Esteves, S.S.; da Costa Pereira, A.; Yancy, W.S., Jr.; Nunes, J.P. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes. Rev. 2012, 13, 1048–1066. [Google Scholar] [CrossRef]
- Hjorth, M.F.; Zohar, Y.; Hill, J.O.; Astrup, A. Personalized Dietary Management of Overweight and Obesity Based on Measures of Insulin and Glucose. Annu. Rev. Nutr. 2018, 38, 245–272. [Google Scholar] [CrossRef]
- McClain, A.D.; Otten, J.J.; Hekler, E.B.; Gardner, C.D. Adherence to a low-fat vs. low-carbohydrate diet differs by insulin resistance status. Diabetes Obes. Metab. 2013, 15, 87–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; McKenzie, A.L.; Bhanpuri, N.H.; Campbell, W.W.; Volek, J.S.; Phinney, S.D.; McCarter, J.P. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-randomized Clinical Trial. Front. Endocrinol. (Lausanne) 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.; Ayre, J.; Franklin, J.; Markovic, T.; Caterson, I.; Sainsbury, A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 64–76. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, A.L.; Athinarayanan, S.J.; McCue, J.J.; Adams, R.N.; Keyes, M.; McCarter, J.P.; Volek, J.S.; Phinney, S.D.; Hallberg, S.J. Type 2 Diabetes Prevention Focused on Normalization of Glycemia: A Two-Year Pilot Study. Nutrients 2021, 13, 749. [Google Scholar] [CrossRef]
- Myette-Côté, É.; Durrer, C.; Neudorf, H.; Bammert, T.D.; Botezelli, J.D.; Johnson, J.D.; DeSouza, C.A.; Little, J.P. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: A randomized trial. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1210–R1219. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.U.; Van Itallie, T.B. Composition of weight lost during short-term weight reduction. Metabolic responses of obese subjects to starvation and low-calorie ketogenic and nonketogenic diets. J. Clin. Invest. 1976, 58, 722–730. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia 1981, 21, 165–171. [Google Scholar] [CrossRef]
- Phinney, S.D.; Horton, E.S.; Sims, E.A.; Hanson, J.S.; Danforth, E., Jr.; LaGrange, B.M. Capacity for moderate exercise in obese subjects after adaptation to a hypocaloric, ketogenic diet. J. Clin. Invest. 1980, 66, 1152–1161. [Google Scholar] [CrossRef]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Appendicular lean mass and mortality among prefrail and frail older adults. J. Nutr. Health Aging 2017, 21, 342–345. [Google Scholar] [CrossRef]
- Coleman, J.L.; Carrigan, C.T.; Margolis, L.M. Body composition changes in physically active individuals consuming ketogenic diets: A systematic review. J. Int. Soc. Sports Nutr. 2021, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, C.; Lattanzi, G.; Taylor, S.F.; Manfrini, S.; Khazrai, Y.M. Very low calorie ketogenic diets in overweight and obesity treatment: Effects on anthropometric parameters, body composition, satiety, lipid profile and microbiota. Obes. Res. Clin. Pract. 2020, 14, 491–503. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Fukuda, T.; Oyabu, C.; Tanaka, M.; Asano, M.; Yamazaki, M.; Fukui, M. Impact of low-carbohydrate diet on body composition: Meta-analysis of randomized controlled studies. Obes. Rev. 2016, 17, 499–509. [Google Scholar] [CrossRef]
- Krieger, J.W.; Sitren, H.S.; Daniels, M.J.; Langkamp-Henken, B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression 1. Am. J. Clin. Nutr. 2006, 83, 260–274. [Google Scholar] [CrossRef]
- Romano, L.; Marchetti, M.; Gualtieri, P.; Di Renzo, L.; Belcastro, M.; De Santis, G.L.; Perrone, M.A.; De Lorenzo, A. Effects of a Personalized VLCKD on Body Composition and Resting Energy Expenditure in the Reversal of Diabetes to Prevent Complications. Nutrients 2019, 11, 1526. [Google Scholar] [CrossRef] [Green Version]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef]
- DeHaven, J.; Sherwin, R.; Hendler, R.; Felig, P. Nitrogen and sodium balance and sympathetic-nervous-system activity in obese subjects treated with a low-calorie protein or mixed diet. N. Engl. J. Med. 1980, 302, 477–482. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Adibi, S.A. Protein sparing during treatment of obesity: Ketogenic versus nonketogenic very low calorie diet. Metabolism 1992, 41, 406–414. [Google Scholar] [CrossRef]
- Phinney, S.D. Low-calorie protein versus mixed diet. N. Engl. J. Med. 1980, 303, 158. [Google Scholar] [PubMed]
- Hoffer, L.J.; Bistrian, B.R.; Young, V.; Blackburn, G.; Matthews, D. Metabolic effects of very low calorie weight reduction diets. J. Clin. Investig. 1984, 73, 750–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phinney, S.D.; LaGrange, B.M.; O’Connell, M.; Danforth, E., Jr. Effects of aerobic exercise on energy expenditure and nitrogen balance during very low calorie dieting. Metabolism 1988, 37, 758–765. [Google Scholar] [CrossRef]
- Phinney, S.D.; Bistrian, B.R.; Wolfe, R.; Blackburn, G. The human metabolic response to chronic ketosis without caloric restriction: Physical and biochemical adaptation. Metabolism 1983, 32, 757–768. [Google Scholar] [CrossRef]
- Howard, B.V.; Manson, J.E.; Stefanick, M.L.; Beresford, S.A.; Frank, G.; Jones, B.; Rodabough, R.J.; Snetselaar, L.; Thomson, C.; Tinker, L.; et al. Low-fat dietary pattern and weight change over 7 years: The Women’s Health Initiative Dietary Modification Trial. JAMA 2006, 295, 39–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The 2020 Dietary Guidelines Advisory Committee. Afternoon Session. Minute 29:16. In Proceedings of the 2020 Dietary Guidelines Advisory Committee Public Meeting, Online, 12 March 2020. [Google Scholar]
- Minute 29:16. In Proceedings of the Public meeting of the Dietary Guidelines Advisory Committee, Little Elm, TX, USA, 23 March 2020.
- USDA. What is a Healthy Diet? Available online: https://ask.usda.gov/s/article/What-is-a-healthy-diet (accessed on 26 February 2020).
- US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 7th ed.; US Government Printing Office: Washington, DC, USA, 2010. [Google Scholar]
- Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Keogh, J.B.; Clifton, P.M. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am. J. Clin. Nutr. 2009, 90, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; Makris, A.P.; Rosenbaum, D.L.; Brill, C.; Stein, R.I.; Mohammed, B.S.; Miller, B.; Rader, D.J.; et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Ann. Intern. Med. 2010, 153, 147–157. [Google Scholar] [CrossRef]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Stern, L.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, M.; Gracely, E.J.; Samaha, F.F. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann. Intern. Med. 2004, 140, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Brewer Jr, H.B.; Cleeman, J.I.; Smith Jr, S.C.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Peer reviewed: Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [Green Version]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Wycherley, T.P.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 858–871. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lv, L.; Yang, Y.; Chen, D.; Liu, G.; Chen, L.; Song, Y.; He, L.; Li, X.; Tian, H.; et al. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly diagnosed type 2 diabetes mellitus. Clin. Endocrinol. (Oxf) 2012, 76, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Glycemic variability and oxidative stress: A link between diabetes and cardiovascular disease? Int. J. Mol. Sci. 2014, 15, 18381–18406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorst, C.; Kwok, C.S.; Aslam, S.; Buchan, I.; Kontopantelis, E.; Myint, P.K.; Heatlie, G.; Loke, Y.; Rutter, M.K.; Mamas, M.A. Long-term Glycemic Variability and Risk of Adverse Outcomes: A Systematic Review and Meta-analysis. Diabetes Care 2015, 38, 2354–2369. [Google Scholar] [CrossRef] [Green Version]
- Ahola, A.J.; Harjutsalo, V.; Forsblom, C.; Saraheimo, M.; Groop, P.H. Associations of dietary macronutrient and fibre intake with glycaemia in individuals with Type 1 diabetes. Diabet Med. 2019, 36, 1391–1398. [Google Scholar] [CrossRef]
- Ranjan, A.; Schmidt, S.; Damm-Frydenberg, C.; Holst, J.J.; Madsbad, S.; Norgaard, K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: A randomized open-label crossover trial. Diabetes Obes. Metab. 2017, 19, 1479–1484. [Google Scholar] [CrossRef]
- Chang, C.R.; Francois, M.E.; Little, J.P. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am. J. Clin. Nutr. 2019, 109, 1302–1309. [Google Scholar] [CrossRef]
- Bistrian, B.R.; Blackburn, G.L.; Flatt, J.P.; Sizer, J.; Scrimshaw, N.S.; Sherman, M. Nitrogen metabolism and insulin requirements in obese diabetic adults on a protein-sparing modified fast. Diabetes 1976, 25, 494–504. [Google Scholar] [CrossRef]
- Dashti, H.M.; Al-Zaid, N.S.; Mathew, T.C.; Al-Mousawi, M.; Talib, H.; Asfar, S.K.; Behbahani, A.I. Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol. Cell Biochem. 2006, 286, 1–9. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: An open-label, non-randomized, controlled study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.A.; Mathew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar] [CrossRef]
- Saslow, L.R.; Daubenmier, J.J.; Moskowitz, J.T.; Kim, S.; Murphy, E.J.; Phinney, S.D.; Ploutz-Snyder, R.; Goldman, V.; Cox, R.M.; Mason, A.E. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr. Diabetes 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucuzzella, M.T.; Tondt, J.; Dockter, N.E.; Saslow, L.; Wood, T.R. A low-carbohydrate survey: Evidence for sustainable metabolic syndrome reversal. J. Insul. Resist. 2017, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Gann, D.; Gann, E. Type 2 Diabetic Patients on Insulin Can Reduce or Eliminate Insulin with Lifestyle Change and Carbohydrate Restriction. Curr. Res. Diabetes Obes. J. 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Unwin, D.; Livesey, G.; Haslam, D. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: The glycaemic index revisited. J. Insul. Resist. 2016, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar]
- Hamdy, O.; Mottalib, A.; Morsi, A.; El-Sayed, N.; Goebel-Fabbri, A.; Arathuzik, G.; Shahar, J.; Kirpitch, A.; Zrebiec, J. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: A 5-year longitudinal study. BMJ Open Diabetes Res. Care 2017, 5, e000259. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Hu, T.; Reynolds, K.; Yao, L.; Bunol, C.; Liu, Y.; Chen, C.S.; Klag, M.J.; Whelton, P.K.; He, J. Effects of low-carbohydrate and low-fat diets: A randomized trial. Ann. Intern. Med. 2014, 161, 309–318. [Google Scholar] [CrossRef]
- Hu, T.; Yao, L.; Reynolds, K.; Whelton, P.K.; Niu, T.; Li, S.; He, J.; Bazzano, L.A. The Effects of a Low-Carbohydrate Diet vs. a Low-Fat Diet on Novel Cardiovascular Risk Factors: A Randomized Controlled Trial. Nutrients 2015, 7, 7978–7994. [Google Scholar] [CrossRef] [Green Version]
- Volek, J.S.; Ballard, K.D.; Silvestre, R.; Judelson, D.A.; Quann, E.E.; Forsythe, C.E.; Fernandez, M.L.; Kraemer, W.J. Effects of dietary carbohydrate restriction versus low-fat diet on flow-mediated dilation. Metabolism 2009, 58, 1769–1777. [Google Scholar] [CrossRef]
- Feinman, R.D.; Volek, J.S. Low carbohydrate diets improve atherogenic dyslipidemia even in the absence of weight loss. Nutr. Metab. (Lond) 2006, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Sharman, M.J.; Kraemer, W.J.; Love, D.M.; Avery, N.G.; Gomez, A.L.; Scheett, T.P.; Volek, J.S. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J. Nutr. 2002, 132, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, C.; Zhang, B.; Wang, Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem. Biophys. 2014, 69, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.L.; Abbasi, F.A.; Blasey, C.; Reaven, G.M.; Kim, S.H. Beyond fasting plasma glucose: The association between coronary heart disease risk and postprandial glucose, postprandial insulin and insulin resistance in healthy, nondiabetic adults. Metabolism 2013, 62, 1223–1226. [Google Scholar] [CrossRef]
- Gast, K.B.; Tjeerdema, N.; Stijnen, T.; Smit, J.W.; Dekkers, O.M. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS One 2012, 7, e52036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, J.; Zheng, S.; Luo, Q.; Zhou, C.; Wang, C. Fasting insulin, insulin resistance, and risk of cardiovascular or all-cause mortality in non-diabetic adults: A meta-analysis. Biosci. Rep. 2017, 37, BSR20170947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volek, J.S.; Sharman, M.J.; Forsythe, C.E. Modification of lipoproteins by very low-carbohydrate diets. J. Nutr. 2005, 135, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Samaha, F.F.; Foster, G.D.; Makris, A.P. Low-carbohydrate diets, obesity, and metabolic risk factors for cardiovascular disease. Curr. Atheroscler. Rep. 2007, 9, 441–447. [Google Scholar] [CrossRef]
- Volk, B.M.; Kunces, L.J.; Freidenreich, D.J.; Kupchak, B.R.; Saenz, C.; Artistizabal, J.C.; Fernandez, M.L.; Bruno, R.S.; Maresh, C.M.; Kraemer, W.J.; et al. Effects of step-wise increases in dietary carbohydrate on circulating saturated Fatty acids and palmitoleic Acid in adults with metabolic syndrome. PLoS ONE 2014, 9, e113605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef]
- Diamond, D.M.; O’Neill, B.J.; Volek, J.S. Low carbohydrate diet: Are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, C.E.; Phinney, S.D.; Feinman, R.D.; Volk, B.M.; Freidenreich, D.; Quann, E.; Ballard, K.; Puglisi, M.J.; Maresh, C.M.; Kraemer, W.J.; et al. Limited effect of dietary saturated fat on plasma saturated fat in the context of a low carbohydrate diet. Lipids 2010, 45, 947–962. [Google Scholar] [CrossRef] [Green Version]
- King, I.B.; Lemaitre, R.N.; Kestin, M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: Investigation of a biomarker of total fat intake. Am. J. Clin. Nutr. 2006, 83, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Raatz, S.K.; Bibus, D.; Thomas, W.; Kris-Etherton, P. Total fat intake modifies plasma fatty acid composition in humans. J. Nutr. 2001, 131, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Phinney, S.D.; Bistrian, B.R.; Evans, W.; Gervino, E.; Blackburn, G. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776. [Google Scholar] [CrossRef]
- Klein, S.; Wolfe, R.R. Carbohydrate restriction regulates the adaptive response to fasting. Am. J. Physiol. 1992, 262, E631–E636. [Google Scholar] [CrossRef]
- Warensjö, E.; Risérus, U.; Vessby, B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia 2005, 48, 1999–2005. [Google Scholar] [CrossRef] [Green Version]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.S.; Sharp, S.J.; Jansen, E.; Luben, R.N.; Khaw, K.-T.; Wareham, N.J.; Forouhi, N.G. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: A pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1214–1222. [Google Scholar]
- Wang, L.; Folsom, A.R.; Zheng, Z.-J.; Pankow, J.S.; Eckfeldt, J. Investigators AS. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003, 78, 91–98. [Google Scholar]
- Yamagishi, K.; Nettleton, J.A.; Folsom, A.R.; Investigators, A.S. Plasma fatty acid composition and incident heart failure in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2008, 156, 965–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warensjö, E.; Sundström, J.; Vessby, B.; Cederholm, T.; Risérus, U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: A population-based prospective study. Am. J. Clin. Nutr. 2008, 88, 203–209. [Google Scholar] [CrossRef]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Mitri, J.; Tomah, S.; Mottalib, A.; Salsberg, V.; Ashrafzadeh, S.; Pober, D.M.; Eldib, A.H.; Tasabehji, M.W.; Hamdy, O. Effect of dairy consumption and its fat content on glycemic control and cardiovascular disease risk factors in patients with type 2 diabetes: A randomized controlled study. Am. J. Clin. Nutr. 2020, 112, 293–302. [Google Scholar] [CrossRef]
- Paillard, F.; Catheline, D.; Le Duff, F.; Bouriel, M.; Deugnier, Y.; Pouchard, M.; Daubert, J.-C.; Legrand, P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, Y.; Agren, J.; Uusitupa, M.; Cederberg, H.; Vangipurapu, J.; Stancakova, A.; Schwab, U.; Kuusisto, J.; Laakso, M. Association of erythrocyte membrane fatty acids with changes in glycemia and risk of type 2 diabetes. Am. J. Clin. Nutr. 2014, 99, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Vessby, B.; Aro, A.; Skarfors, E.; Berglund, L.; Salminen, I.; Lithell, H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes 1994, 43, 1353–1357. [Google Scholar] [CrossRef]
- Djoussé, L.; Weir, N.L.; Hanson, N.Q.; Tsai, M.Y.; Gaziano, J.M. Plasma phospholipid concentration of cis-palmitoleic acid and risk of heart failure. Circ. Heart Fail. 2012, 5, 703–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djoussé, L.; Matthan, N.R.; Lichtenstein, A.H.; Gaziano, J.M. Red blood cell membrane concentration of cis-palmitoleic and cis-vaccenic acids and risk of coronary heart disease. Am. J. Cardiol. 2012, 110, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Fisher, N.D.; Curfman, G. Hypertension—a public health challenge of global proportions. JAMA 2018, 320, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Lagiou, P.; Sandin, S.; Lof, M.; Trichopoulos, D.; Adami, H.O.; Weiderpass, E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: Prospective cohort study. BMJ 2012, 344, e4026. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, L.M.; Winkvist, A.; Eliasson, M.; Jansson, J.H.; Hallmans, G.; Johansson, I.; Lindahl, B.; Lenner, P.; Van Guelpen, B. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur. J. Clin. Nutr. 2012, 66, 694–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A.; Psaltopoulou, T.; Orfanos, P.; Hsieh, C.; Trichopoulos, D. Low-carbohydrate–high-protein diet and long-term survival in a general population cohort. Eur. J. Clin. Nutr. 2007, 61, 575–581. [Google Scholar] [CrossRef]
- Akter, S.; Mizoue, T.; Nanri, A.; Goto, A.; Noda, M.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Tsugane, S. Low carbohydrate diet and all cause and cause-specific mortality. Clin. Nutr. 2021, 40, 2016–2024. [Google Scholar] [CrossRef]
- Halton, T.L.; Willett, W.C.; Liu, S.; Manson, J.E.; Albert, C.M.; Rexrode, K.; Hu, F.B. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N. Engl. J. Med. 2006, 355, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLoS ONE 2013, 8, e55030. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Okuda, N.; Okamura, T.; Kadota, A.; Miyagawa, N.; Hayakawa, T.; Kita, Y.; Fujiyoshi, A.; Nagai, M.; Takashima, N.; et al. Low-carbohydrate diets and cardiovascular and total mortality in Japanese: A 29-year follow-up of NIPPON DATA80. Br. J. Nutr. 2014, 112, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, P.; Becker, W.; Warensjö, E.; Olsson, E.; Byberg, L.; Gustafsson, I.-B.; Karlström, B.; Cederholm, T. Mediterranean and carbohydrate-restricted diets and mortality among elderly men: A cohort study in Sweden. Am. J. Clin. Nutr. 2010, 92, 967–974. [Google Scholar] [CrossRef]
- Ho, F.K.; Gray, S.R.; Welsh, P.; Petermann-Rocha, F.; Foster, H.; Waddell, H.; Anderson, J.; Lyall, D.; Sattar, N.; Gill, J.M.R.; et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: Prospective cohort study of UK Biobank participants. BMJ 2020, 368, m688. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.T.; van Dam, R.M.; Hankinson, S.E.; Stampfer, M.; Willett, W.C.; Hu, F.B. Low-carbohydrate diets and all-cause and cause-specific mortality: Two cohort studies. Ann. Intern. Med. 2010, 153, 289–298. [Google Scholar] [CrossRef]
- Lee, C.-L.; Liu, W.-J.; Wang, J.-S. Associations of low-carbohydrate and low-fat intakes with all-cause mortality in subjects with prediabetes with and without insulin resistance. Am. J. Clin. Nutr. 2021, 40, 3601–3607. [Google Scholar] [CrossRef]
- Li, S.; Flint, A.; Pai, J.K.; Forman, J.P.; Hu, F.B.; Willett, W.C.; Rexrode, K.M.; Mukamal, K.J.; Rimm, E.B. Low carbohydrate diet from plant or animal sources and mortality among myocardial infarction survivors. J. Am. Heart Assoc. 2014, 3, e001169. [Google Scholar] [CrossRef] [Green Version]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Sattar, N.; Banach, M. Lower carbohydrate diets and all-cause and cause-specific mortality: A population-based cohort study and pooling of prospective studies. Eur. Heart J. 2019, 40, 2870–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [Green Version]
- Shan, Z.; Guo, Y.; Hu, F.B.; Liu, L.; Qi, Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern. Med. 2020, 180, 513–523. [Google Scholar] [CrossRef]
- Pujol-Busquets, G.; Smith, J.; Larmuth, K.; Fàbregues, S.; Bach-Faig, A. Exploring the Perceptions of Women from Under-Resourced South African Communities about Participating in a Low-Carbohydrate High-Fat Nutrition and Health Education Program: A Qualitative Focus Group Study. Nutrients 2020, 12, 894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.; Raffray, M.; Roy-Fleming, A.; Blunden, S.; Brazeau, A.-S. Ketogenic diet as a normal way of eating in adults with type 1 and type 2 diabetes: A qualitative study. Can. J. Diabetes 2021, 45, 137–143.e131. [Google Scholar] [CrossRef] [PubMed]
- Cupit, C.; Redman, E. Supporting people to implement a reduced carbohydrate diet: A qualitative study in family practice. BMJ Nutr. Prev. Health 2021, 4, 226–234. [Google Scholar] [CrossRef]
- Hamdy, O.; Barakatun-Nisak, M.Y. Nutrition in Diabetes. Endocrinol. Metab. Clin. N. Am. 2016, 45, 799–817. [Google Scholar] [CrossRef]
- Hamdy, O.; Horton, E.S. Protein content in diabetes nutrition plan. Curr. Diab. Rep. 2011, 11, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; de Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef] [PubMed]
- Zinn, C.; Rush, A.; Johnson, R. Assessing the nutrient intake of a low-carbohydrate, high-fat (LCHF) diet: A hypothetical case study design. BMJ Open 2018, 8, e018846. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; Wang, X.; Liu, L.; Yan, H.; Lee, S.F.; Mony, P.; Devanath, A.; et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N. Engl. J. Med. 2014, 371, 612–623. [Google Scholar] [CrossRef] [Green Version]
- Lanaspa, M.A.; Kuwabara, M.; Andres-Hernando, A.; Li, N.; Cicerchi, C.; Jensen, T.; Orlicky, D.J.; Roncal-Jimenez, C.A.; Ishimoto, T.; Nakagawa, T.; et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 3138–3143. [Google Scholar] [CrossRef] [Green Version]
- Andres-Hernando, A.; Jensen, T.J.; Kuwabara, M.; Orlicky, D.J.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Garcia, G.E.; Ishimoto, T.; Maclean, P.S.; et al. Vasopressin mediates fructose-induced metabolic syndrome by activating the V1b receptor. JCI Insight 2021, 6, e140848. [Google Scholar] [CrossRef]
- Kanbay, M.; Aslan, G.; Afsar, B.; Dagel, T.; Siriopol, D.; Kuwabara, M.; Incir, S.; Camkiran, V.; Rodriguez-Iturbe, B.; Lanaspa, M.A.; et al. Acute effects of salt on blood pressure are mediated by serum osmolality. J. Clin. Hypertens. (Greenwich) 2018, 20, 1447–1454. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium and type 2 diabetes. World J. Diabetes 2015, 6, 1152–1157. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Morán, M.; Guerrero-Romero, F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: A randomized double-blind controlled trial. Diabetes Care 2003, 26, 1147–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Féry, F.; Balasse, E.O. Ketone body production and disposal in diabetic ketosis: A comparison with fasting ketosis. Diabetes 1985, 34, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Sholl, J.; Mailing, L.J.; Wood, T.R. Reframing Nutritional Microbiota Studies To Reflect an Inherent Metabolic Flexibility of the Human Gut: A Narrative Review Focusing on High-Fat Diets. mBio 2021, 12, e00579-00521. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volek, J.S.; Phinney, S.D.; Krauss, R.M.; Johnson, R.J.; Saslow, L.R.; Gower, B.; Yancy, W.S., Jr.; King, J.C.; Hecht, F.M.; Teicholz, N.; et al. Alternative Dietary Patterns for Americans: Low-Carbohydrate Diets. Nutrients 2021, 13, 3299. https://doi.org/10.3390/nu13103299
Volek JS, Phinney SD, Krauss RM, Johnson RJ, Saslow LR, Gower B, Yancy WS Jr., King JC, Hecht FM, Teicholz N, et al. Alternative Dietary Patterns for Americans: Low-Carbohydrate Diets. Nutrients. 2021; 13(10):3299. https://doi.org/10.3390/nu13103299
Chicago/Turabian StyleVolek, Jeff S., Stephen D. Phinney, Ronald M. Krauss, Richard J. Johnson, Laura R. Saslow, Barbara Gower, William S. Yancy, Jr., Janet C. King, Frederick M. Hecht, Nina Teicholz, and et al. 2021. "Alternative Dietary Patterns for Americans: Low-Carbohydrate Diets" Nutrients 13, no. 10: 3299. https://doi.org/10.3390/nu13103299
APA StyleVolek, J. S., Phinney, S. D., Krauss, R. M., Johnson, R. J., Saslow, L. R., Gower, B., Yancy, W. S., Jr., King, J. C., Hecht, F. M., Teicholz, N., Bistrian, B. R., & Hamdy, O. (2021). Alternative Dietary Patterns for Americans: Low-Carbohydrate Diets. Nutrients, 13(10), 3299. https://doi.org/10.3390/nu13103299





