Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management
Abstract
:1. Introduction
2. Iron Metabolism
3. Laboratory Tests for the Detection of ID
3.1. Full Blood Count, Blood Film and Red Cell Indices
3.2. Serum Ferritin
3.3. Serum Iron (Fe) and Total Iron Binding Capacity (TIBC)
3.4. Soluble Transferrin Receptor (sTfR)
3.5. Reticulocyte Hemoglobin Content and Reticulocytes
3.6. Red Cell Distribution (RDW)
3.7. Bone Marrow Iron
4. Symptoms
- Symptoms of anemia, which may include weakness, headache, decreased exercise tolerance, fatigue, irritability, or depression. Asthenia, tiredness, and muscle weakness appear even without apparent anemia. IDA may also impair temperature regulation and may make one feel colder than normal.
- Neurodevelopmental delay (children).
- Lack of concentration and lower academic performance (adolescent).
- Worse physical performance (sport competition).
- Pica and pagophagia (ice craving).
- Beeturia (reddish urine after eating beets).
- Restless legs syndrome.
4.1. Pregnancy
4.2. Children
4.3. Productive Working Age
4.4. Elderly
5. Prevalence of Celiac Disease (CD) in Patients with Anemia
5.1. Global Overview
“Since the Oslo Consensus, published in 2013 [33], the scientific community has cknowledged different patterns of clinical presentation. «Classical» CD presents with signs and symptoms of malabsorption. Examples of classical CD are patients with diarrhea and steatorrhea, but also patients with weight loss and anemia or failure to thrive. In non-classical CD (before ‘atypical’ CD), the patient does not suffer from malabsorption (e.g., abdominal pain, diarrhea, or constipation, but without any evidence of malabsorption). This consideration is important because some patients with gastrointestinal symptoms with apparent functional criteria (e.g., irritable bowel syndrome) may ultimately be diagnosed with CD if a clinician with a high index of suspicion decides to investigate the cause of an ID (even without anemia) that any other cause cannot explain. Potential celiac disease (PCD) is defined by the presence of positive serum antibodies, HLA-DQ2/DQ8 haplotypes, and a normal small intestinal mucosa (Marsh grade 0–1) [34]. As will be discussed below, these patients may also present with anemia or ID. Subclinical CD has no signs or symptoms sufficient to trigger CD testing in routine practice. However, an unsuspected ID, without anemia, might be discovered in this subgroup if intentionally sought. Finally, refractory CD (RCD) consists of persistent or recurrent malabsorptive symptoms and signs with villous atrophy (VA) despite a strict GFD for more than 12 months. Again, anemia or ID is part of the spectrum of clinical manifestations in this subgroup”.
5.2. Children
5.3. Index of Suspicion for the Diagnosis of CD in Patients with IDA
5.4. Prevalence of Celiac Disease among Patients with Anemia of Obscure Origin
- The average duration of anemia was 3.6 +/− 1.4 years.
- Most of the GSE patients (73.3%) did not report any gastrointestinal symptoms. Consequently, physicians may fail to consider GSE as a cause of IDA when gastrointestinal symptoms are absent or nonspecific.
- These patients had been treated with oral iron for a mean duration of 1.9 years. Anemia improved in only eight patients (26.8%) treated with oral iron supplementation before GSE diagnosis.
- In GSE patients, the hemoglobin level was inversely correlated with the severity of the histological injury. Patients with Marsh 3 lesions had the most severe anemia, consistent with the role of impaired intestinal absorption in the pathogenesis of IDA. Many authors consider the presence of villous atrophy (e.g., Marsh 3) as one of the major criteria for diagnosing CD [61,62]. To avoid this controversy in the definition of CD, the authors used the term “gluten sensitive enteropathy” rather than CD to describe patients with any degree of intestinal damage together with positive serologic tests.
- In this study, the authors showed a significant objective improvement in hemoglobin levels with GFD alone in patients with positive serology but no villous atrophy (e.g., Marsh 1 or 2). This would be an important point concerning the route of iron administration (oral versus intravenous) in patients with or without villous atrophy (see below). Furthermore, GFD could improve anemia in IDA patients who have positive tTGA/EMA and mild duodenal lesions without villous atrophy.
6. Prevalence of Anemia in CD
6.1. Comprehensive Overview
- Celiac patients with anemia as a prominent symptom showed signs of more severe disease than those presenting with diarrhea, a finding that has also been reported by other authors [69].
- Anemic celiac patients have a longer duration of symptoms and a more severe serological and histologic presentation at diagnosis, as described by Shing et al. [66].
- Finally, anemic patients also showed a slower histologic response, including a worse recovery in the villus/crypt ratio and a significantly lower decrease in IELs count and in the density of ɣδ+ IELs.
6.2. Anemia Outcomes in Celiac Patients after Introduction of a Gluten-Free Diet
7. Mechanisms that Explain the Presence of Anemia in CD
7.1. Micronutrient Deficiencies
7.2. Infection by Helicobacter pylori
7.3. Anemia of Chronic Disease
7.4. Persistence of Anemia in Patients with CD despite Adopting a GFD
7.5. Blood Loss Due to Inflammatory Lesions
7.6. Aplastic Anemia
8. Management of Anemia and Iron Deficiency in Different CD Settings (Algorithms)
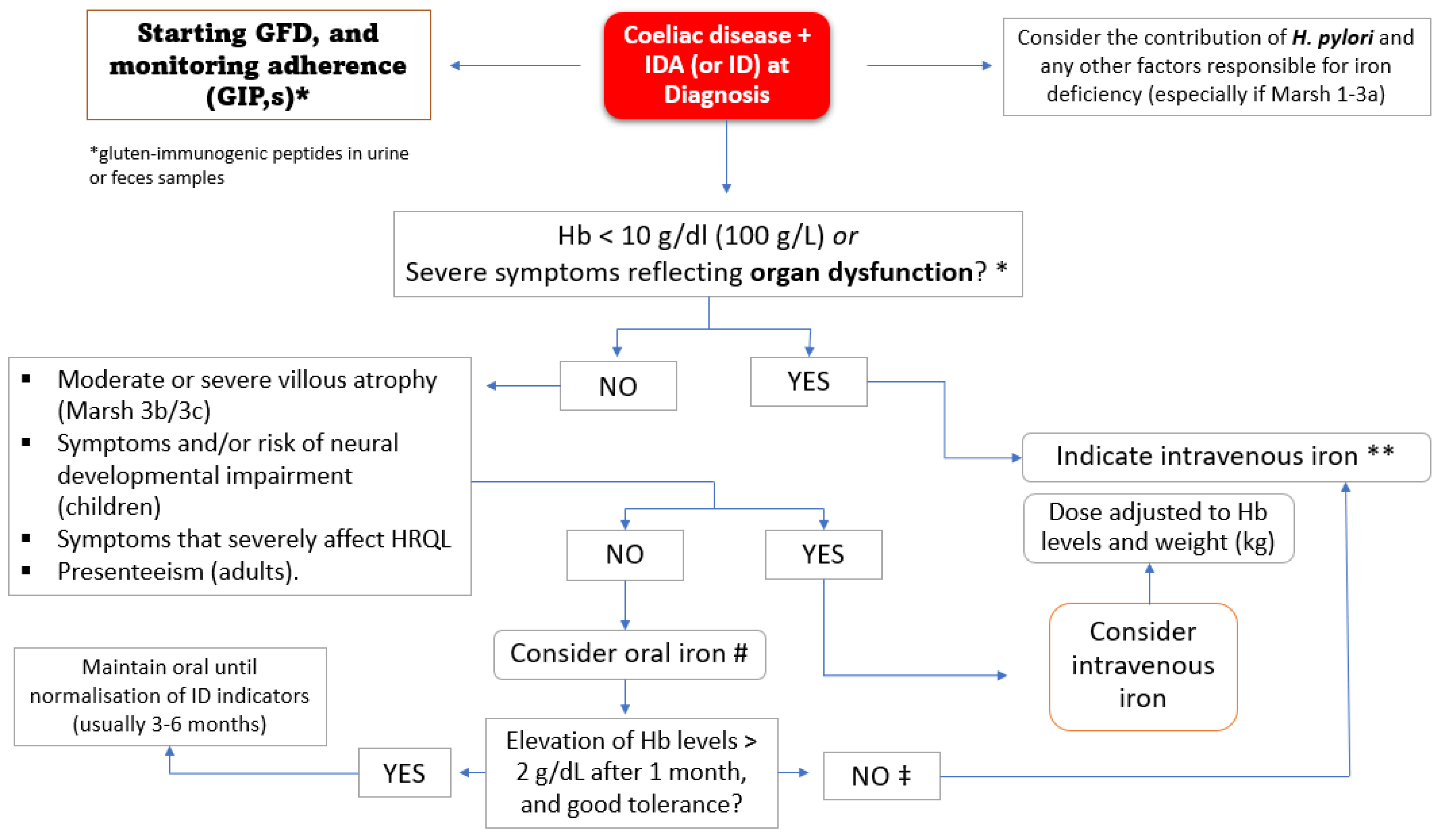
8.1. At the Time of CD Disease Diagnosis
8.2. Allogeneic Red Blood Cell Transfusion (When, How, and to Whom?)
8.3. Replenishment of Iron Storage
8.4. How to Proceed with Iron Replenishment: When, How, and to Whom?
8.5. Oral or Intravenous Iron?
8.6. Oral Iron Considerations
- In children, the recommended iron dose is 2–6 mg/kg/day in terms of elemental iron. In adolescents and adults, it is 100–200 mg daily. Sometimes, these high doses of oral iron cause a paradoxical decrease in iron absorption due to factors such as elevated plasma hepcidin levels [152,153]. In our practice, formulations that provide 40–80 mg of elemental iron, when administered once (80 mg) or twice (40 mg/12 h) daily, are equally effective and better tolerated.
- Toxicity associated with oral iron is higher in elderly patients, and such patients should be treated with lower doses. In fact, doses of 15, 50, or 150 mg of elemental iron may be equally effective in raising hemoglobin and ferritin levels, while adverse effects are significantly less common with lower doses [154].
- Strategies for reducing side effects and improving tolerability include:
- o
- Limiting the dose (≤80–100 mg of elemental iron per day).
- o
- Dividing the total dose and taking it in two daily doses or increasing the time between doses (e.g., every two days) [155].
- o
- Taking iron after dinner (reduces absorption but improves tolerance).
- o
- Changing the formulation (e.g., from ferrous sulphate to ferrous gluconate) or presentation used (e.g., from tablets to oral solution, which makes it easier to titrate doses).
- o
- Some proposed solutions to improve oral iron absorption in CD include the use of probiotics (Lactobacillus plantarum 299v and Bifidobacterium lactis HN019) [156,157] or prebiotics (oligofructose enriched inulin) [158], as well as the use of ferrous bisglycinate chelate (FBC), or the most recent Feralgine®, a compound of FBC and alginic acid that has recently been developed to improve the bioavailability and tolerability profile. Feralgine® and FBC are effective at a dosage of 30–40% compared to FS. Several studies have demonstrated the efficacy and safety of FBC in the treatment of IDA in both adults and children, without showing side effects [159,160,161,162]. In addition, recent studies conducted in adult celiac patients confirmed the good level of absorption and tolerance of Feralgine® in patients with anemia as well as in non-celiac subjects and in those with onset CD [163,164,165].
- o
- Another alternative aimed at reducing the risk of adverse effects associated with iron sulphate is sucrosomial iron (SI). SI a preparation of ferric pyrophosphate covered by a phospholipids and sucrester membrane, can be absorbed across intestinal epithelium by an alternative route, non-mediated by the DMT-1 carrier [166], which may contribute to the reduction of side effects and the prevention of iron instability in the gastrointestinal tract. A study evaluated the efficacy and safety of a new SI formulation (30 mg of iron/day) versus iron sulfate (105 mg of iron/day), in patients with CD. After a follow-up of 90 days both groups showed an increase in Hb levels compared to baseline (+10.1% and +16.2% for sucrosomial and sulfate groups, respectively), and a significant improvement in all iron parameters, with no statistical difference between the two groups. However, patients treated with SI reported a lower severity of abdominal symptoms, such as abdominal and epigastric pain, abdominal bloating, and constipation, and a higher increase in general well-being (+33% vs. +21%) compared to the iron sulfate group [167]. Therefore, SI can be effective in providing iron supplementation in difficult-to-treat populations, such as patients with CD, IDA, and known intolerance to iron sulfate.
- Response to oral iron therapy can be considered satisfactory when an increase in hemoglobin levels of at least 2 g/dL is observed within 3–4 weeks, which is also associated with an improvement in physical well-being and anemia-dependent signs and symptoms, including depapillation of the sides of the tongue, which is a good indicator of recovery. For patients with persistent anemia or ID and doubts about correct adherence to the GFD, it may be important to investigate the presence of gluten immunogenic peptides (GIPs) in fecal or urine samples, as these are present in a significant proportion of patients who declare a correct adherence to the diet. This policy may avoid unnecessary biopsies or limit them to cases where symptoms persist despite good nutritional advice and repeatedly negative GIP results [168].
- If oral iron is not tolerated, or not absorbed due to intestinal inflammation, then intravenous iron should be given.
8.7. Intravenous Iron Replacement Therapy
8.8. Iron Formulations for Intravenous Use
- ▪
- 0.0034: iron content of hemoglobin (0.34%).
- ▪
- 0.07: blood volume 70 mL/kg of body weight = 7% of body weight.
- ▪
- 10,000: conversion factor 1 g/dL = 1000 mg/L.
- ▪
- iron stores (mg): 500 mg if the body weight is greater than 35 kg or 15 mg/kg if the body weight is less than 35 kg.
8.9. Intravenous Iron in Children
- (1)
- Failure to achieve correction of IDA after well-conducted oral iron substitution in the setting of good adherence of at least six months of prescribed supplementation and two formulation attempts.
- (2)
- Confirmed malabsorption or chronic oral iron intolerance, including the category of children with severe neurological/neurodevelopmental impairments leading to feeding limitations.
8.10. Adverse Effects and Contraindications Related to the Use of Intravenous Iron
9. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz, M.; Villar, I.; García-Erce, J.A. An update on iron physiology. World J. Gastroenterol. 2009, 15, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R. Iron Metabolism: From Molecular Mechanisms to Clinical Consequences, 4th ed.; Wiley: Chichester, UK, 2016. [Google Scholar]
- Anderson, G.J.; McLaren, G.D. (Eds.) Iron Physiology and Pathophysiology in Humans; Humana Press: New York, NY, USA, 2012. [Google Scholar]
- Martín-Masot, R.; Nestares, M.T.; Diaz-Castro, J.; López-Aliaga, I.; Alférez, M.J.M.; Moreno-Fernandez, J.; Maldonado, J. Multifactorial Etiology of Anemia in Celiac Disease and Effect of Gluten-Free Diet: A Comprehensive Review. Nutrients 2019, 11, 2557. [Google Scholar] [CrossRef] [Green Version]
- Rockey, D.C.; Altayar, O.; Falck-Ytter, Y.; Kalmaz, D. AGA technical review on gastrointestinal evaluation of iron deficiency anemia. Gastroenterology 2020, 159, 1097–1119. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, G.; Viscido, A.; Longo, S.; Magistroni, M.; Latella, G. Persistent Iron Deficiency Anemia in Patients with Celiac Disease Despite a Gluten-Free Diet. Nutrients 2020, 12, 2176. [Google Scholar] [CrossRef]
- Gargallo-Puyuelo, C.J.; Alfambra, E.; García-Erce, J.A.; Gomollon, F. Iron Treatment May Be Difficult in Inflammatory Diseases: Inflammatory Bowel Disease as a Paradigm. Nutrients 2018, 10, 1959. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; García-Erce, J.A.; Remacha, Á.F. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. J. Clin. Pathol. 2011, 64, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; García-Erce, J.A.; Remacha, A.F. Disorders of iron metabolism. Part 1: Molecular basis of iron homoeostasis. J. Clin. Pathol. 2011, 64, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ripolles-Melchor, J.; Jericó-Alba, C.; Quintana-Díaz, M.; García-Erce, J.A. From blood saving programs to patient blood management and beyond. Med. Clin. 2018, 159, 368–373. [Google Scholar]
- Choi, J.; Im, M.; Pai, S. Serum transferrin receptor concentrations during normal pregnancy. Clin. Chem. 2000, 46, 725–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J. BSH Committee. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020, 188, 819–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekiz, E.; Agaoglu, L.; Karakas, Z.; Gurel, N.; Yalcin, I. The effect of iron deficiency anemia on the function of the immune system. Hematol. J. 2005, 5, 579–583. [Google Scholar] [CrossRef]
- Haas, J.D.; Brownlie, T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef] [Green Version]
- Beard, J.L.; Hendricks, M.K.; Perez, E.M.; Murray-Kolb, L.E.; Berg, A.; Vernon-Feagans, L.; Irlam, J.; Isaacs, W.; Sivem, A.; Tomlinson, M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J. Nutr. 2005, 135, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O.; Hediger, M.L. Anemia and iron-deficiency anemia: Compilation of data on pregnancy outcome. Am. J. Clin. Nutr. 1994, 59, S492–S501. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Parvanta, I.; Ickes, L.; Yip, R.; Brittenham, G.M. Iron supplementation during pregnancy, anemia, and birthweight: A randomised controlled trial. Am. J. Clin. Nutr. 2003, 78, 773–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, D.L.; Williams, M.A.; Miller, R.S.; Qiu, C.; Sorensen, T.K. Maternal iron deficiency anaemia is associated with an increased risk of abruption placentae—A retrospective case control study. J. Obstet. Gynaecol. Res. 2009, 35, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Gambling, L.; Danzeisen, R.; Gair, S.; Lea, R.G.; Charania, Z.; Solanky, N.; Joory, K.D.; Srai, S.K.; McArdle, H.J. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem. J. 2001, 356, 883–889. [Google Scholar] [CrossRef]
- Puolakka, J.; Jänne, O.; Vihko, R. Evaluation by Serum Ferritin Assay of the Influence of Maternal Iron Stores on the Iron Status of Newborns and Infants. Acta Obstet. Gynecol. Scand. 1980, 59, 53–56. [Google Scholar] [CrossRef]
- Colomer, J.; Colomer, C.; Gutierrez, D.; Jubert, A.; Nolasco, A.; Donat, J.; Fernandez-Delgado, R.; Donat, F.; Alvarez-Dardet, C. Anaemia during pregnancy as a risk factor for infant iron deficiency: Report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr. Perinat. Epidemiol. 1990, 4, 196–204. [Google Scholar] [CrossRef]
- Perez, E.M.; Hendricks, M.K.; Beard, J.L.; Murray-Kolb, L.E.; Berg, A.; Tomlinson, M.; Irlam, J.; Isaacs, W.; Njengele, T.; Sive, A.; et al. Mother infant interactions and infant development are altered by maternal iron deficiency anemia. J. Nutr. 2005, 135, 850–855. [Google Scholar] [CrossRef] [Green Version]
- Beard, J.L. Why iron deficiency is important in infant development. J. Nutr. 2008, 138, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Insel, B.J.; Schaefer, C.A.; McKeague, I.W.; Susser, E.S.; Brown, A.S. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch. Gen. Psychiatry 2008, 65, 1136–1144. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, K. Non haematological effects of iron deficiency—A perspective. Indian J. Med. Sci. 2006, 60, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziol, B.J.; Ohira, Y.; Edgerton, V.R.; Simpson, D.R. Changes in work tolerance associated with metabolic and physiological adjustment to moderate and severe iron deficiency anemia. Am. J. Clin. Nutr. 1982, 36, 830–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, C.; Lewis, S.; Barton, J.; Corbett, S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2006, 12, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comín-Colet, J.; Enjuanes, C.; González, G.; Torrens, A.; Cladellas, M.; Meroño, O.; Ribas, N.; Ruiz, S.; Gómez, M.; Verdú, J.M.; et al. Iron deficiency is a key determinant of health related quality of life in patients with chronic heart failure regardless of anaemia status. Eur. J. Heart Fail. 2013, 15, 1164–1172. [Google Scholar] [CrossRef]
- Finkelstein, F.O.; Story, K.; Firanek, C.; Mendelssohn, D.; Barre, P.; Takano, T.; Soroka, S.; Mujais, S. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2008, 4, 33–38. [Google Scholar] [CrossRef]
- Mirza, F.G.; Abdul-Kadir, R.; Breymann, C.; Fraser, I.S.; Taher, A. Impact and management of iron deficiency and iron deficiency anemia in women’s health. Expert Rev. Hematol. 2018, 11, 727–736. [Google Scholar] [CrossRef]
- Dahlerup, J.; Lindgren, S.; Moum, B. Iron deficiency and iron deficiency anemia are global health problems. Lakartidningen 2015, 10, 112. [Google Scholar]
- Strauss, W.E.; Auerbach, M. Health-related quality of life in patients with iron deficiency anemia: Impact of treatment with intravenous iron. Patient Relat. Outcome Meas. 2018, 27, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2012, 62, 43–52. [Google Scholar] [CrossRef]
- Trovato, C.M.; Montuori, M.; Valitutti, F.; Leter, B.; Cucchiara, S.; Oliva, S. The Challenge of Treatment in Potential Celiac Disease. Gastroenterol. Res. Pract. 2019, 2019, 8974751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, Z.; Eliakim, R.; Stalnikowicz, R.; Rachmilewitz, D. Role of small bowel biopsy in the endoscopic evaluation of adults with iron deficiency anemia. Am. J. Gastroenterol. 1996, 91, 2099–2102. [Google Scholar] [PubMed]
- Annibale, B.; Capurso, G.; Chistolini, A.; D’Ambra, G.; DiGiulio, E.; Monarca, B.; Delle Fave, G. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am. J. Med. 2001, 111, 439–445. [Google Scholar] [CrossRef]
- Corazza, G.R.; Valentini, R.A.; Andreani, M.L.; D’Anchino, M.; Leva, M.T.; Ginaldi, L.; De Feudis, L.; Quaglino, D.; Gasbarrini, G. Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scand. J. Gastroenterol. 1995, 30, 153–156. [Google Scholar] [CrossRef]
- Dickey, W.; Kenny, B.D.; McMillan, S.A.; Porter, K.G.; McConnell, J.B. Gastric as well as duodenal biopsies may be useful in the investigation of iron deficiency anaemia. Scand. J. Gastroenterol. 1997, 32, 469–472. [Google Scholar] [CrossRef]
- Howard, M.R.; Turnbull, A.J.; Morley, P.; Hollier, P.; Webb RClarke, A. A prospective study of the prevalence of undiagnosed coeliac disease in laboratory defined iron and folate de-ficiency. J. Clin. Pathol. 2002, 55, 754–757. [Google Scholar] [CrossRef] [Green Version]
- Kepczyk, T.; Kadakia, S.C. Prospective evaluation of gastrointestinal tract in patients with iron-deficiency anemia. Dig. Dis. Sci. 1995, 40, 1283–1289. [Google Scholar] [CrossRef]
- McIntyre, A.S.; Long, R.G. Prospective survey of investigations in outpatients referred with iron deficiency anaemia. Gut 1993, 34, 1102–1107. [Google Scholar] [CrossRef] [Green Version]
- Oxentenko, A.S.; Grisolano, S.W.; Murray, J.A.; Burgart, L.J.; Dierkhising, R.A.; Alexander, J.A. The insensitivity of endoscopic markers in celiac disease. Am. J. Gastroenterol. 2002, 97, 933–938. [Google Scholar] [CrossRef]
- Ransford Rupert, A.J.; Hayes, M.; Palmer, M.; Hall, M.J. A controlled, prospective screening study of celiac disease presenting as iron deficiency anemia. J. Clin. Gastroenterol. 2002, 35, 228–233. [Google Scholar] [CrossRef]
- Unsworth, D.J.; Lock, R.J.; Harvey, R.F. Improving the diagnosis of coeliac disease in anaemic women. Br. J. Haematol. 2000, 111, 898–901. [Google Scholar]
- Annibale, B.; Lahner, E.; Chistolini, A.; Gallucci, C.; Di Giulio, E.; Capurso, G.; Luana, O.; Monarca, B.; Delle, F.G. Endoscopic evaluation of the upper gastrointestinal tract is worthwhile in premenopausal women with iron-deficiency anaemia irrespective of menstrual flow. Scand. J. Gastroenterol. 2003, 38, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Van Mook, W.N.K.A.; Bourass-Bremer, I.H.D.N.; Bos, L.P.; Verhoeven, H.M.J.M.; Engels, L.G.J.B. The outcome of esophagogastroduodenoscopy (EGD) in asymptomatic outpatients with iron de-ficiency anemia after a negative colonoscopy. Eur. J. Intern. Med. 2001, 12, 122–126. [Google Scholar] [CrossRef]
- Mahadev, S.; Laszkowska, M.; Sundström, J.; Björkholm, M.; Lebwohl, B.; Green, P.H.R.; Ludvigsson, J.F. Prevalence of celiac disease in patients with iron deficiency anemia. A Systematic review with meta-analysis. Gastroenterology 2018, 155, 374–382. [Google Scholar] [CrossRef]
- Abdalla, A.; Saifullah, S.M.; Osman, M.; Baniya, R.; Sidahmed, S.; LaChance, J.; Bachuwa, G. Prevalence of occult celiac disease in females with iron deficiency in the United States: An NHANES analysis. J. Community Hosp. Intern. Med. Perspect. 2017, 14, 347–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.A.; McLachlan, S.; Adams, P.C.; Eckfeldt, J.H.; Garner, C.P.; Vulpe, C.; Gordeuk, V.R.; Brantner, T.; Leiendecker–Foster, C.; Killeen, A.A.; et al. Association between Celiac disease and iron deficiency in caucasians, but not non-caucasians. Clin. Gastroenterol. Hepatol. 2013, 11, 808–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikkakoski, S.; Savilahti, E.; Kolho, K.-L. Undiagnosed coeliac disease and nutritional deficiencies in adults screened in primary health care. Scand. J. Gastroenterol. 2007, 42, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Baghbanian, M.; Farahat, A.; Vahedian, H.A.; Sheyda, E.; Zare-Khormizi, M.R. The prevalence of Celiac disease in patients with iron-deficiency anemia in center and South Area of Iran. Arq. Gastroenterol. 2015, 52, 278–282. [Google Scholar] [CrossRef] [Green Version]
- Javid, G.; Lone, S.N.; Shoukat, A.; Khan, B.A.; Yattoo, G.N.; Shah, A.; Sodi, J.S.; Khan, M.A.; Zarger, S.A. Prevalence of celiac disease in adult patients with iron-deficiency anemia of obscure origin in Kashmir (India). Indian J. Gastroenterol. 2015, 34, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Kavimandan, A.; Sharma, M.; Verma, A.K.; Das, P.; Mishra, P.; Sinha, S.; Anant Mohan, A.; Sreenivas, V.; Gupta, S.D.; Makharia, G.K. Prevalence of celiac disease in nutritional anemia at a tertiary care center. Indian J. Gastroenterol. 2014, 33, 114–118. [Google Scholar] [CrossRef]
- Zamani, F.; Mohamadnejad, M.; Shakeri, R.; Amiri, A.; Najafi, S.; Alimohamadi, S.M.; Tavangar, S.M.; Ghavamzadeh, A.; Malekzadeh, R. Gluten sensitive enteropathy in patients with iron deficiency anemia of unknown origin. World J. Gastroenterol. 2008, 14, 7381–7385. [Google Scholar] [CrossRef]
- Narang, M.; Natarajan, R.; Shah, D.; Puri, A.S.; Manchanda, V.; Kotru, M. Celiac disease in children with moderate-to-severe iron-deficiency anemia. Indian Pediatr. 2018, 15, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Shahriari, M.; Honar, N.; Yousefi, A.; Javaherizadeh, H. Association of potential celiac disease and refractory iron deficiency anemia in children and adolescents. Arq. Gastroenterol. 2018, 55, 78–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volta, U.; Caio, G.; Giancola, F.; Rhoden, K.J.; Ruggeri, E.; Boschetti, E.; Sthamghellini, V.; De Giorgio, E. Features and progression of potential celiac disease in adults. Clin. Gastroenterol. Hepatol. 2016, 14, 686–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, M.; Lenhart, A.; Baker, J.; Dickens, J.; Weissman, A.; Read, A.J.; Saini, S.; Saini, S.D. Primary care physicians are under-testing for celiac disease in patients with iron deficiency anemia: Results of a national survey. PLoS ONE 2017, 20, e0184754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smukalla, S.; Lebwohl, B.; Mears, J.G.; Leslie, L.A.; Green, P.H. How often do hematologists consider celiac disease in iron-deficiency anemia? Results of a national survey. Clin. Adv. Hematol. Oncol. 2014, 12, 100–105. [Google Scholar]
- Feighery, C.; Conlon, N.; Jackson, J. Adult population screening for coeliac disease: Comparison of tissue-transglutaminase antibody and anti-endomysial antibody tests. Eur. J. Gastroenterol. Hepatol. 2006, 18, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.A.; Brar, P.; Diamond, B.; Rotterdam, H.; Green, P.H. Utility in clinical practice of immunoglobulin an anti-tissue transglutaminase antibody for the diagnosis of celiac disease. Clin. Gastroenterol. Hepatol. 2006, 4, 726–730. [Google Scholar] [CrossRef]
- Bode, S.; Gudmand-Hoyer, E. Symptoms and haematologic features in consecutive adult coeliac patients. Scand. J. Gastroenterol. 1996, 31, 54–60. [Google Scholar] [CrossRef]
- Harper, J.; Holleran, S.; Ramakrishnan, R.; Bhagat, G.; Peter, H.R. Green PH. Anemia in celiac disease is multifactorial in etiology. Am. J. Hematol. 2007, 82, 996–1000. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Litzow, M.R.; Murray, J.A. Hematologic manifestations of celiac disease. Blood 2007, 109, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Arora, S.; Makharia, G.K. Presence of anemia in patients with celiac disease suggests more severe disease. Indian J. Gastroenterol. 2014, 33, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, G.; Cataldo, F.; Rotolo, N.; Spina, M.; Corazza, G.R. The clinical pattern of subclinical/silent celiac disease: An analysis on 1026 consecutive cases. Am. J. Gastroenterol. 1999, 94, 691–696. [Google Scholar] [CrossRef]
- Saukkonen, J.; Kaukinen, K.; Koivisto, A.M.; Mäki, M.; Laurila, K.; Sievänen, H.; Collin, P.; Kurppa, K. Clinical characteristics and the dietary response in celiac disease patients presenting with or without anemia. J. Clin. Gastroenterol. 2017, 51, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Abu Daya, H.; Lebwohl, B.; Lewis, S.; Green, P.H. Celiac disease patients presenting with anemia have more severe disease than those presenting with diarrhea. Clin. Gastroenterol. Hepatol. 2013, 11, 1472–1477. [Google Scholar] [CrossRef]
- Leffler, D.A.; Green, P.H.; Fasano, A. Extraintestinal manifestations of coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Rampertab, S.D.; Pooran, N.; Brar, P.; Singh, P.; Green, P.H. Trends in the presentation of celiac disease. Am. J. Med. 2006, 119, 355.e9–355.e14. [Google Scholar] [CrossRef]
- Annibale, B.; Severi, C.; Chistolini, A.; Antonelli, G.; Lahner, E.; Marcheggiano, A.; Iannoni, C.; Monarca, B.; Fave, G.D. Efficacy of gluten-free diet alone on recovery from iron deficiency anemia in adult celiac patients. Am. J. Gastroenterol. 2001, 96, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Sansotta, N.; Amirikian, K.; Guandalini, S.; Jericho, H. Celiac Disease Symptom Resolution: Efectiveness of the Gluten-free Diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 48–52. [Google Scholar] [CrossRef]
- De Falco, L.; Tortora, R.; Imperatore, N.; Bruno, M.; Capasso, M.; Girelli, D.; Castagna, A.; Caporaso, N.; Iolascon, A.; Rispo, A. The role of TMPRSS6 and HFE variants in iron deficiency anemia in celiac disease. Am. J. Hematol. 2018, 93, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Sari, R.; Yildirim, B.; Sevinc, A.; Buyukberber, S. Gluten-free diet improves iron-deficiency anaemia in patients with coeliac disease. J. Health Popul Nutr. 2000, 18, 54–56. [Google Scholar]
- Verbeek, W.H.; Von Blomberg, B.M.E.; Scholten, P.E.; Kuik, D.J.; Mulder, C.J.; Schreurs, M.W. The presence of small intestinal intraepithelial gamma/delta T-lymphocytes is inversely correlated with lymphoma development in refractory celiac disease. Am. J. Gastroenterol. 2008, 103, 3152–3158. [Google Scholar] [CrossRef]
- Bledsoe, A.C.; King, K.S.; Larson, J.J.; Snyder, M.; Absah, I.; Murray, J.A. Micronutrient deficiencies are common in contemporary celiac disease despite lack of overt malabsorption symptoms. Mayo Clin. Proc. 2019, 94, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Dahele, A.; Ghosh, S. Vitamin B12 deficiency in untreated celiac disease. Am. J. Gastroenterol. 2001, 96, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Berry, N.; Basha, J.; Varma, N.; Varma, S.; Prasad, K.K.; Vaiphei, K.; Dhaka, N.; Sinha, S.K.; Kochhar, R. Anemia in celiac disease is multifactorial in etiology: A prospective study from India. JGH Open 2018, 2, 196–200. [Google Scholar] [CrossRef]
- Andrews, N.C. The iron transporter DMT1. Int. J. Biochem. Cell Biol. 1999, 31, 991–994. [Google Scholar] [CrossRef]
- Canonne-Hergaux, F.; Gruenheid, S.; Ponka, P.; Gros, P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood 1999, 93, 4406–4417. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; De Falco, L. Mutations in the gene encoding DMT1: Clinical presentation and treatment. Semin. Hematol. 2009, 46, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Barisani, D.; Parafioriti, A.; Bardella, M.T.; Zoller, H.; Conte, D.; Armiraglio, E.; Trovato, C.; Koch, R.O.; Weiss, G. Adaptive changes of duodenal iron transport proteins in celiac disease. Physiol. Genom. 2004, 17, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Tolone, C.; Bellini, G.; Punzo, F.; Papparella, A.; Miele, E.; Vitale, A.; Nobili, B.; Strisciuglio, C.; Rossi, F. The DMT1 IVS4 + 44C >A polymorphism and the risk of iron deficiency anemia in children with celiac disease. PLoS ONE 2017, 12, e0185822. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [Green Version]
- Guevara Pacheco, G.; Chávez Cortés, E.; Castillo-Durán, C. Deficiencia de micronutrientes y enfermedad celíaca. Arch. Argent Pediatr. 2014, 112, 457–463. [Google Scholar]
- Freeman, H.J. Neurological disorders in adult celiac disease. Can. J. Gastroenterol. 2008, 22, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarion, T.R.; Kumar, N.; Hogan, W.J.; Murray, J.A. Copper deficiency in celiac disease. J. Clin. Gastroenterol. 2009, 43, 162–164. [Google Scholar] [CrossRef]
- Cavallieri, F.; Fin, N.; Contardi, S.; Fiorini, M.; Corradini, E.; Valzania, F. Subacute copper-deficiency myelopathy in as patient with occult celiac disease. J. Spinal Cord Med. 2017, 40, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; Katsikeros, R.; Manton, N.; Krebs, N.F.; Hambidge, K.M.; Butler, R.N.; Davidson, G.P. Zinc homeostasis and gut function in children with celiac disease. Am. J. Clin. Nutr. 2011, 94, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Baydoun, A.; Maakaron, J.E.; Halawi, H.; Abou Rahal, J.; Taher, A.T. Hematological manifestations of celiac disease. Scand. J. Gastroenterol. 2012, 47, 1401–1411. [Google Scholar] [CrossRef]
- Tsay, F.W.; Hsu, P.I. H. pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santambrogio, E.; Orsucci, L. Helicobacter pylori and hematological disorders. Minerva Gastroenterol. Dietol. 2019, 65, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Hudak, L.; Jaraisy, A.; Haj, S.; Muhsen, K. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter 2017, 22, e12330. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Capurso, G.; Delle Fave, G. The stomach and iron deficiency anaemia: A forgotten link. Dig. Liver Dis. 2003, 35, 288–295. [Google Scholar] [CrossRef]
- Hershko, C.; Ronson, A. Iron deficiency, Helicobacter infection and gastritis. Acta Haematol. 2009, 122, 97–102. [Google Scholar] [CrossRef]
- Sapmaz, F.; Başyiğit, S.; Kalkan, İ.H.; Kısa, Ü.; Kavak, E.E.; Güliter, S. The impact of Helicobacter pylori eradication on serum hepcidin-25 level and iron parameters in patients with iron deficiency anemia. Wien Klin Wochenschr. 2016, 128, 335–340. [Google Scholar] [CrossRef]
- Simondi, D.; Ribaldone, D.G.; Bonagura, G.A.; Foi, S.; Sapone, N.; Garavagno, M.; Villanacci, V.; Bernardi, D.; Pellicano, R.; Rizzetto, M.; et al. Helicobacter pylori in celiac disease and in duodenal intraepithelial lymphocytosis: Active protagonist or innocent bystander? Clin. Res. Hepatol Gastroenterol. 2015, 39, 740–745. [Google Scholar] [CrossRef]
- Azab, S.F.; Esh, A.M. Serum hepcidin levels in Helicobacter pylori-infected children with iron-deficiency anemia: A case-control study. Ann. Hematol. 2013, 92, 1477–1483. [Google Scholar] [CrossRef]
- Cuoco, L.; Cammarota, G.; Jorizzo, R.A.; Santarelli, L.; Cianci, R.; Montalto, M.; Gasbarrini, A.; Gasbarrini, G. Link between Helicobacter pylori infection and iron-deficiency anaemia in patients with coeliac disease. Scand. J. Gastroenterol. 2001, 36, 1284–1288. [Google Scholar]
- Rostami-Nejad, M.; Aldulaimi, D.; Livett, H.; Rostami, K. H. pylori associated with iron deficiency anemia even in celiac disease patients; strongly evidence based but weakly reflected in practice. Gastroenterol. Hepatol. Bed Bench. 2015, 8, 178–182. [Google Scholar]
- Samasca, G.; Deleanu, D.; Sur, G.; Lupan, I.; Giulia, A.; Carpa, R. Is it necessary to screen Helicobacter pylori infection in patients with celiac disease and iron deficiency? Gastroenterol. Hepatol. 2016, 9, 345. [Google Scholar]
- Hershko, C.; Skikne, B. Pathogenesis and management of iron deficiency anemia: Emerging role of celiac disease, helicobacter pylori, and autoimmune gastritis. Semin. Hematol. 2009, 46, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, G.; Markopoulos, K.; Albertini, R.; Di Sabatino, A.; Biagi, F.; Ciccocioppo, R.; Arbustini, E.; Corazza, G.R. Anemia of chronic disease and defective erythropoietin production in patients with celiac disease. Haematologica 2008, 93, 1785–1791. [Google Scholar] [CrossRef] [Green Version]
- Talarico, V.; Giancotti, L.; Mazza, G.A.; Miniero, R.; Bertini, M. Iron deficiency anemia in celiac disease. Nutrients 2021, 13, 1695. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bañares, F.; Beltrán, B.; Salas, A.; Comino, I.; Ballester-Clau, R.; Ferrer, C.; Molina-Infante, J.; Rosinach, M.; Modolell, I.; Rodríguez-Moranta, F.; et al. CADER study group. Persistent villous atrophy in de novo adult patients with celiac disease and strict control of gluten-free diet adherence: A Multicenter Prospective Study (CADER Study). Am. J. Gastroenterol. 2021, 116, 1036–1043. [Google Scholar] [CrossRef]
- Stenling, R.; Fredrikzon, B.; Engberg, S.; Falkmer, S. Surface infrastructure of the small intestine mucosa in children with celiac disease. I. Untreated disease and effects of long-term gluten elimination and challenge. Ultrastruct. Pathol. 1984, 6, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Dyduch, A.; Karczewska, K.; Grzybek, H.; Kaminski, M. Transmission electron microscopy of microvilli of intestinal epithelial cells in celiac disease in remission and transient gluten enteropathy in children after a gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 269–272. [Google Scholar] [CrossRef]
- Magliocca, F.M.; Bonamico, M.; Petrozza, V.; Correr, S.; Montuori, M.; Triglione, P.; Carpino, F. Scanning electron microscopy of the small intestine during gluten-challenge in celiac disease. Arch. Histol. Cytol. 1992, 55, 125–130. [Google Scholar] [CrossRef]
- Capannolo, A.; Viscido, A.; Barkad, M.A.; Valerii, G.; Ciccone, F.; Melideo, D.; Frieri, G.; Latella, G. Non-Celiac Gluten Sensitivity among Patients Perceiving Gluten-Related Symptoms. Digestion 2015, 92, 8–13. [Google Scholar] [CrossRef]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R. The Study Group for Non-Celiac Gluten Sensitivity. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [Green Version]
- Sbarbati, A.; Valletta, E.; Bertini, M.; Cipolli, M.; Morroni, M.; Pinelli, L.; Tatò, L. Gluten sensitivity and ‘normal’ histology: Is the intestinal mucosa really normal? Dig. Liver Dis. 2003, 35, 768–773. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Seiler, C.L.; Santesso, N.; Alaedini, A.; Semrad, C.; Lee, A.R.; Bercik, P.; Lebwohl, B.; Leffler, D.A.; Kelly, C.P.; et al. Association Between Inflammatory Bowel Diseases and Celiac Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 159, 884–903. [Google Scholar] [CrossRef] [PubMed]
- Eigner, W.; Bashir, K.; Primas, C.; Kazemi-Shirazi, L.; Wrba, F.; Trauner, M.; Vogelsang, H. Dynamics of occurrence of refractory coeliac disease and associated complications over 25 years. Aliment. Pharmacol. Ther. 2017, 45, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hujoel, I.A.; Murray, J.A. Refractory Celiac Disease. Curr. Gastroenterol. Rep. 2020, 22, 18. [Google Scholar] [CrossRef]
- Rokkas, T.; Niv, Y. The role of video capsule endoscopy in the diagnosis of celiac disease. Eur. J. Gastroenterol. Hepatol. 2012, 24, 303–308. [Google Scholar] [CrossRef]
- Zammit, S.C.; Elli, L.; Scaramella, L.; Sanders, D.S.; Tontini, G.E. Sidhu R Small bowel capsule endoscopy in refractory celiac disease: A luxury or a necessity? Ann. Gastroenterol. 2021, 34, 188–195. [Google Scholar]
- Branchi, F.; Locatelli, M.; Tomba, C.; Conte, D.; Ferretti, F.; Elli, L. Enteroscopy and radiology for the management of celiac disease complications: Time for a pragmatic roadmap. Dig. Liver Dis. 2016, 48, 578–586. [Google Scholar] [CrossRef]
- Chetcuti Zammit, S.; Sanders, D.S.; Cross, S.S.; Sidhu, R. Capsule endoscopy in the management of refractory coeliac disease. J. Gastrointest. Liver Dis. 2019, 28, 15–22. [Google Scholar] [CrossRef]
- Chetcuti Zammit, S.; Sanders, D.S.; Sidhu, R. Refractory coeliac disease: What should we be doing different? Curr. Opin. Gastroenterol. 2020, 36, 215–222. [Google Scholar] [CrossRef]
- Rejeski, J.; Conway, J.; Zhou, Y. Collagenous Sprue. Am. J. Med. Sci. 2020, 359, 310–311. [Google Scholar] [CrossRef]
- Irfan, O.; Mahmood, S.; Nand, H.; Billoo, G. Celiac disease associated with aplastic anemia in a 6-year-old girl: A case report and review of the literature. J. Med. Case Rep. 2018, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grey-Davies, E.; Hows, J.M.; Marsh, J.C.W. Aplastic anaemia in association with coeliac disease: A series of three cases. Br. J. Haematol. 2008, 143, 258–260. [Google Scholar] [CrossRef]
- Maheshwari, A.; Nirupam, N.; Aneja, S.; Meena, R.; Chandra, J.; Kumar, P. Association of Celiac Disease with Aplastic Anemia. Indian J. Pediatr. 2012, 79, 1372–1373. [Google Scholar] [CrossRef]
- Salmeron, G.; Patey, N.; De Latour, R.P.; Raoux, E.; Gluckman, E.; Brousse, N.; Socié, G.; Robin, M. Coeliac disease and aplastic anaemia: A specific entity? Br. J. Haematol. 2009, 146, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Ray, Y.; Bowmik, P.; Rahman, M.; Dikshit, N.; Goswami, R.P. Rare association of coeliac disease with aplastic anaemia: Report of a case from India. Indian J. Hematol. Blood Transfus. 2014, 30, 208–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badyal, R.K.; Sachdeva, M.U.S.; Varma, N.; Thapa, B.R. A Rare Association of Celiac Disease and Aplastic Anemia: Case Report of a Child and Review of Literature. Pediatr. Dev. Pathol. 2014, 17, 470–473. [Google Scholar] [CrossRef]
- Gornatti, M.; Aguirre, M.A. Gastrointestinal bleeding and complicated celiac disease in anticoagulated patient. Medicina 2020, 80, 718–721. [Google Scholar]
- Yang, D.H.; Myung, S.J.; Chang, H.S.; Song, J.W.; Yang, S.K.; Lee, G.H.; Jung, H.Y.; Hong, W.S.; Kim, J.H.; Min, Y.I.; et al. A case of enteropathy-associated T-cell lymphoma presenting with recurrent hematochezia. Korean J. Gastroenterol. 2003, 42, 527–532. [Google Scholar]
- Pun, A.H.; Kasmeridis, H.; Rieger, N.; Loganathan, A. Enteropathy associated T-cell lymphoma presenting with multiple episodes of small bowel haemorrhage and perforation. J. Surg. Case Rep. 2014, 20, rju013. [Google Scholar] [CrossRef] [Green Version]
- Delabie, J.; Holte, H.; Vose, J.M.; Ullrich, F.; Jae, E.S.; Savage, K.J.; Connors, J.M.; Rimsza, L.; Harris, N.L.; Müller-Hermelink, K.; et al. Enteropathy-associated T-cell lymphoma: Clinical and histological findings from the international peripheral T-cell lymphoma project. Blood 2011, 118, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Gombotz, H. Patient blood management: A patient-orientated approach to blood replacement with the goal of reducing anemia, blood loss and the need for blood transfusion in elective surgery. Transfus. Med. Hemother. 2012, 39, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Jericó, C.; Osorio, J.; García-Erce, J.A.; Pera, M. Patient Blood Management strategies for iron deficiency anemia management in gastric cancer. Eur. J. Gastroenterol. Hepatol. 2019, 31, 547–548. [Google Scholar] [CrossRef]
- Althoff, F.C.; Neb, H.; Herrmann, E.; Trentino, K.M.; Vernich, L.; Füllenbach, C.; Freedman, J.; Waters, J.H.; Farmer, S.; Leahy, M.F.; et al. Multimodal patient blood management program based on a three-pillar strategy: A systematic review and meta-analysis. Ann. Surg. 2019, 269, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Stanworth, S.J.; Alexander, J.H.; Roubinian, N.; Fergusson, D.A.; Triulzi, D.J.; Goodman, S.G.; Rao, S.V.; Doree, C.; Hebert, P.C. Clinical trials evaluating red blood cell transfusion thresholds: An up-dated systematic review and with additional focus on patients with cardiovascular disease. Am. Heart J. 2018, 200, 96. [Google Scholar] [CrossRef]
- Vilppula, A.; Kaukinen, K.; Luostarinen, L.; Krekelä, I.; Patrikainen, H.; Valve, R.; Mäki, M.; Collin, P. Increasing prevalence and high incidence of celiac disease in elderly people: A population-based study. BMC Gastroenterol. 2009, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.W.; Ellis, H.J.; Asante, M.A.; Ciclitira, P.J. Celiac disease in the elderly. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Rashtak, S.; Murray, J.A. Celiac disease in the elderly. Gastroenterol. Clin. N. Am. 2009, 38, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Cappello, M.; Gaetano Morreale, G.C.; Licata, A. Elderly Onset Celiac Disease: A Narrative Review. Clin. Med. Insights Gastroenterol. 2016, 9, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, H.J. Adult celiac disease in the elderly. World J. Gastroenterol. 2008, 14, 6911–6914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hankey, G.L.; Holmes, G.K. Coeliac disease in the elderly. Gut 1994, 35, 65–67. [Google Scholar] [CrossRef] [Green Version]
- De Franceschi, L.; Iolascon, A.; Taherd, A.; Cappelini, M.D. Clinical management of iron deficiency anemia in adults: Sistemic review on advances in diagnosis and treatment. Eur. J. Intern. Med. 2017, 42, 16–23. [Google Scholar] [CrossRef]
- Lerner, N.B.; Sills, R. Iron deficiency anemia. In Nelson Text of Pediatrics, 20th ed.; Kliegman, R.M., Stanton, B.F., Schor, N.F., St. Gemelli, G.V., Behrman, R.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 2322–2326. [Google Scholar]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Elli, L.; Poggiali, E.; Tomba, C.; Andreozzi, F.; Nava, I.; Bardella, M.T.; Campostrini, N.; Girelli, D.; Conte, D.; Cappellini, M.D. Does TMPRSS6RS855791 polymorphism contribute to iron deficiency in treated celiac disease? Am. J. Gastroenterol. 2015, 110, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Singh, P.; Agnihotri, A.; Das, P.; Mishra, A.; Verma, A.K.; Ahuja, A.; Sreenivas, V.; Khadgawat, R.; Gupta, S.D.; et al. Celiac disease: A disease with varied manifestations in adults and adolescents. J. Dig. Dis. 2013, 14, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Valitutti, F.; Trovato, C.M.; Montuori, M.; Cucchiara, S. Pediatric Celiac Disease: Follow-Up in the Spotlight. Adv. Nutr. 2017, 8, 356–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar]
- Montoro, M.; Bujanda, L.; Calvet, X.; Canelles, P.; García-Erce, J.A.; García-López, S.; Gisbert, J.P.; Gomollón, F.; Hervás, A.J.; Lanas, A.; et al. Management of anaemia and iron deficiency in gastrointestinal bleeding. PRODIGGEST Project: Healthcare Protocols to improve interdisciplinary management of gastrointestinal diseases in hospital settings. Span. Assoc. Gastroenterol. 2017, 1–48. Available online: https://www.aegastro.es/documents/prodiggest/Prodiggest-Management-of-anaemia-and-iron-deficiency-in-gastrointestinal-bleeding.pdf (accessed on 23 September 2021).
- Fernández-Bañares, F.; Monzón, H.; Forné, M. A short review of malabsorption and anemia. World J. Gastroenterol. 2009, 15, 4644–4652. [Google Scholar] [CrossRef]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef]
- Schrier, S.L. So, you know how to treat iron deficiency anemia. Blood 2015, 126, 1971. [Google Scholar] [CrossRef]
- Rimon, E.; Kagansky, N.; Kagansky, M.; Mechnick, L.; Mashiah, T.; Namir, M.; Levy, S. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am. J. Med. 2005, 118, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, N.U.; Zeder, C.; Brittenham, G.M.; Moretti, D.; Zimmermann, M.B. Iron absorption from supplements is greater with alter nate day than with consecutive day dosing in iron-deficient anemic women. Haematologica 2020, 105, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppe, M.; Onning, G.; Berggren, A.; Hulthen, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
- Rosen, G.M.; Morrissette, S.; Larson, A.; Stading, P.; Griffin, K.H.; Barnes, T.L. Use of a Probiotic to Enhance Iron Absorption in a Randomized Trial of Pediatric Patients Presenting with Iron Deficiency. J. Pediatr. 2019, 207, 192–197. [Google Scholar] [CrossRef]
- Ferus, K.; Drabinska, N.; Krupa-Kozak, U.; Jarocka-Cyrta, E. Randomized, Placebo-Controlled, Pilot Clinical Trial to Evaluate the Effect of Supplementation with Prebiotic Synergy 1 on Iron Homeostasis in Children and Adolescents with Celiac Disease Treated with a Gluten-Free Diet. Nutrients 2018, 10, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagna, R.; Spada, E.; Mazzone, R.; Saracco, P.; Boetti, T.; Cester, E.A.; Bertino, E.; Coscia, A. Efficacy of Supplementation with Iron Sulfate Compared to Iron Bisglycinate Chelate in Preterm Infants. Curr. Pediatr. Rev. 2018, 14, 123–129. [Google Scholar] [CrossRef] [PubMed]
- João Name, J.; Rodrigues Vasconcelos, A.; Valzachi Rocha Maluf, M.C. Iron Bisglycinate Chelate and Polymaltose Iron for the Treatment of Iron Deficiency Anemia: A Pilot Randomized Trial. Curr. Pediatr. Rev. 2018, 14, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.A.; Pedrelli, L.; Battaglia, E.; Giancotti, L.; Miniero, R. Oral iron absorption test with ferrous bisglycinate chelate in hildren with celiac disease: Preliminary results. Minerva Pediatr. 2019, 10, 139–143. [Google Scholar]
- Rondinelli, M.B.; Di Bartolomei, A.; De Rosa, A.; Pirelli, L. Oral Iron Absorption Test (OIAT): A forgotten screening test for iron absorption from the gastrointestinal tract. A casa series of Iron Deficiency Anemia (IDA) patients treated with FERALGINE®. J. Blood Disord. Med. 2017, 2, 1. [Google Scholar]
- Vernero, M.; Boano, V.; Ribaldone, D.G.; Pellicano, R.; Astegiano, M. Oral iron supplementation with Feralgine® in inflammatory bowel disease: A retrospective observational study. Minerva Gastroenterol. Dietol. 2019, 65, 2–203. [Google Scholar] [CrossRef]
- Giancotti, L.; Talarico, V.; Mazza, G.A.; Marrazzo, S.; Gangemi, G.; Miniero, R.; Bertini, M. FERALGINE™ a new approach for Iron Deficiency Anemia in Celiac Patients. Nutrients 2019, 11, 887. [Google Scholar] [CrossRef] [Green Version]
- Talarico, V.; Giancotti, L.; Miniero, R.; Bertini, M. Iron Deficiency Anemia Refractory to Conventional Therapy but Responsive to Feralgine® in a Young Woman with Celiac Disease. Int. Med. Case Rep. J. 2021, 14, 89–93. [Google Scholar] [CrossRef]
- Fabiano, A.; Brilli, E.; Fogli, S.; Beconcini, D.; Carpi, S.; Tarantino, G.; Zambito, Y. Sucrosomial® iron absorption studied by in vitro and ex-vivo models. Eur. J. Pharm. Sci. 2018, 111, 425–431. [Google Scholar] [CrossRef]
- Elli, L.; Ferretti, F.; Branchi, F.; Tomba, C.; Lombardo, V.; Scricciolo, A.; Doneda, L.; Roncoroni, L. Sucrosomial Iron Supplementation in anemic patients with celiac Disease not tolerating oral ferrous sulfate: A prospective study. Nutrients 2018, 10, 330. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Carnicer, Á.; Garzón-Benavides, M.; Fombuena, B.; Segura, V.; García-Fernández, F.; Sobrino Rodríguez, S.; Gómez-Izquierdo, L.; Montes-Cano, M.A.; Rodríguez-Herrera, A.; Millán, R.; et al. Negative predictive value of the repeated absence of gluten immunogenic peptides in the urine of treated celiac patients in predicting mucosal healing: New proposals for follow-up in celiac disease. Am. J. Clin. Nutr. 2020, 112, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Pérez-Edo, L. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Norsa, L.; Zullo, A.; Carroccio, A.; Girelli, C.; Oliva, S.; Romano, C.; Leandro, G.; Bellini, M.; Marmo, R.; et al. Diagnosis of chronic anaemia in gastrointestinal disorders: A guideline by the Italian Association of Hospital Gastroenterologist and Endoscopist (AIGO) and the Italian Society of Paediatric Gastroenterology Hepatology and Nutrition (SIGENP). Dig. Liver Dis. 2019, 51, 471–474. [Google Scholar] [CrossRef]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B. British Society of Gastroenterology Guidelines for the management of iron deficiency anaemia. Br. Soc. Gastroenterol. 2011, 60, 1309–1316. [Google Scholar]
- Koduru, P.; Abraham, B.P. The role of ferric carboxymaltose in the treatment of iron deficiency anemia in patients with gastrointestinal disease. Ther. Adv. Gastroenterol. 2016, 9, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Klip, I.T.; Jankowska, E.A.; Enjuanes, C.; Voors, A.A.; Banasiak, W.; Bruguera, J.; Rozentryt, P.; Polonski, L.; van Veldhuisen, D.J.; Ponikowski, P.; et al. The additive burden of iron deficiency in the cardiorenal-anaemia axis: Scope of a problem and its consequences Eur. J. Heart Fail. 2014, 16, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Ballard, H. Clinical use of intravenous iron: Administration, efficacy, and safety. Hematol. Am. Soc. Hematol. Educ. Program 2010, 2010, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Carman, N.; Muir, R.; Lewindon, P. Ferric carboxymaltose in the treatment of iron deficiency in pediatric inflammatory bowel disease. Transl. Pediatr. 2019, 8, 28–34. [Google Scholar] [CrossRef]
- Papadopoulos, M.; Patel, D.; Korologou-Linden, R.; Goto, E.; Soondrum, K.; Fell, J.M.E.; Epstein, J. Safety, and efficacy of parenteral iron in children with inflammatory bowel disease. Br. J. Clin. Pharmacol. 2018, 84, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Peyrin-Biroulet, L.; Lopez, A.; Cummings, J.R.; Dignass, A.; Detlie, T.E.; Danese, S. Review article: Treating–to –target for inflammatory bowel disease-associated anaemia. Aliment. Pharmacol. Ther. 2018, 48, 610. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Shamoun, M.; McCavit, T.L.; Adix, L.; Buchanan, G.R. Intravenous Ferric Carboxymaltose in Children with Iron Deficiency Anemia Who Respond Poorly to Oral Iron. J. Pediatr. 2017, 180, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Mantadakis, E.; Roganovic, J. Safety and efficacy of ferric carboxymaltose in children and adolescents with iron deficiency anemia. J. Pediatr. 2017, 184, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akin, M.; Sarbay, H.; Guler, S.; Balci, Y.I.; Polat, A. Response to parenteral iron therapy distinguish unexplained refractory iron deficiency anemia from iron-refractory iron deficiency anemia. Int. J. Lab. Hematol. 2016, 38, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Mantadakis, E.; Tsouvala, E.; Xanthopoulou, V.; Chatzimichael, A. Intravenous iron sucrose for children with iron deficiency anemia: A single institution study. World J. Pediatr. 2016, 12, 109–113. [Google Scholar] [CrossRef]
- Laass, M.W.; Straub, S.; Chainey, S.; Virgin, G.; Cushway, T. Effectiveness, and safety of ferric carboxymaltose treatment in children and adolescents with inflammatory bowel disease and other gastrointestinal diseases. BMC Gastroenterol. 2014, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.S.; Crary, S.E.; McCavit, T.L.; Buchanan, G.R. Intravenous low molecular weight iron dextran in children with iron deficiency anemia unresponsive to oral iron. Pediatr. Blood Cancer 2013, 60, 1747–1752. [Google Scholar] [CrossRef]
- Mattiello, V.; Schmugge, M.; Hengartner, H.; von der Weid, N.; Renella, R.; SPOG Pediatric Hematology Working Group. Diag-nosis and management of iron deficiency in children with or without anemia: Consensus recommendations of the SPOG Pediatric Hematology Working Group. Eur. J. Pediatr. 2020, 179, 527–545. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Comment |
|---|---|
| * These values vary according to age, sex, elevation, smoking habit, and physiological conditions such as pregnancy. |
| Hemoglobin Thresholds for RBC Transfusion # ‡ | |
|---|---|
| Acute anemia | Chronic anemia 1 |
| Hb < 7 g/dL In those patients without cardiovascular or pulmonary comorbidities [A] or signs of organ dysfunction [B]. | Hb < 5 g/dL In those patients without cardiovascular or pulmonary comorbidities [A] or signs of organ dysfunction [B]. |
| Hb < 8 g/dL In those patients with cardiovascular or pulmonary comorbidities [A]. | Hb < 6 g/dL Only in those patients with cardiovascular or pulmonary comorbidities [A]. |
| Hb < 9–10 g/dL In those patients with signs of organ dysfunction [B]. | Hb < 7 g/dL Only in those patients with signs of organ dysfunction [B]. |
[A] Cardiovascular risk factors influencing the decision to transfuse RBC concentrates in patients with acute anemia:
| [B] Signs of organ dysfunction They are indicative of severe tissue hypoxia (e.g., in cases of massive hemorrhage, where hemoglobin levels remain “elevated” due to hemoconcentration).
|
| |
| Guidance and Considerations in Relation to Oral Iron Replacement |
|---|
| The dose of oral iron depends on patient age, the estimated iron deficit, how quickly it needs to be corrected, and side effects. |
| Absorption improves when iron is taken in a moderately acidic medium; therefore, it is recommended that iron be taken with ascorbic acid (250–300 mg) or half a glass of orange juice. Some ferric gluconate formulations contain ascorbic acid with 80 mg of elemental iron. |
| Some food components, such as phosphates, phytates, and tannates (which are found in coffee, tea, cocoa, and red wine), inhibit iron absorption. Other foodstuffs that impair iron absorption are cereals, dietary fiber, eggs, milk, and generally any foods with a high calcium content. Many of these items regularly form part of patients’ breakfasts. The summary of product characteristics for most oral iron products therefore recommend taking oral iron at least 1 h before or 2 h after eating. However, although the administration of oral iron together with food decreases absorption, it improves tolerance and is one of the strategies used by many doctors in the event of side effects (see above). |
| Iron is best absorbed as the ferrous (Fe++) salt in a mildly acidic medium. Gastric acidity is helpful and medications that reduce gastric acid (e.g., antacids, histamine receptor blockers, proton pump inhibitors) may impair iron absorption. Other medications that impair oral iron absorption are calcium supplements and certain antibiotics (quinolones and tetracyclines), and, therefore, oral iron should be taken at least 2 h before or after these medications. |
| Enteric-coated or sustained-release capsules are less efficient for oral absorption because iron is released too far distally in the intestinal tract (or not at all). |
| Gastrointestinal symptoms associated with taking oral iron are common and include metallic taste, dyspepsia, nausea, vomiting, flatulence, diarrhea, and constipation. Some patients may also be bothered by the dark green or tarry stools (they should be warned if they are to undergo a colonoscopy). As a result of this, compliance with oral iron administration may be low. The severity and impact of these effects has been demonstrated in various systematic reviews and meta-analyses of randomized studies [149,166], and they are estimated to affect 30–43% of patients, depending on the formulation used. Supplements containing smaller amounts of elemental iron are associated with less gastrointestinal toxicity, especially in elderly patients [154]. Taking iron after dinner reduces absorption but improves tolerance. The reader is referred to the recommended doses in Section 8.6 of the text. |
| Brand Name | Venofer®, Feriv® (Iron Sucrose) | Ferlixit®, Ferrlecit® (Fe-Gluconate) 1 | CosmoFer® (Iron Dextran) | Ferinject® (Ferric Carboxymaltose) |
|---|---|---|---|---|
| Indication | Iron deficiency | Iron deficiency | Iron deficiency | Iron deficiency |
| Max. iron dose in one infusion | 200 mg | 125 mg (12.5 mg/mL) 2,3 | 20 mg/kg of body weight | 1000 mg |
| Duration of the dose by injection | 30 min | 1 h | 4–6 h | 15 min |
| Max. iron dose by injection | 200 mg (3 times/week) | 125 mg (12.5 mg/mL) 2,3 | 200 mg (3 times/week) | 1000 mg (once/week) |
| No. of hosp. visits for adm. 1000 mg | 5 | 8 | 1 by infusion 5 by injection | 1 |
| Oral vs. Intravenous Iron Replacement | Advantages | Limitations |
|---|---|---|
| Oral iron |
|
|
| Intravenous iron |
|
|
| Recommendations for the Administration of Intravenous Iron |
|---|
|
| Actions to Be Taken in the Event of Adverse Effects |
|---|
MILD
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoro-Huguet, M.A.; Santolaria-Piedrafita, S.; Cañamares-Orbis, P.; García-Erce, J.A. Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management. Nutrients 2021, 13, 3437. https://doi.org/10.3390/nu13103437
Montoro-Huguet MA, Santolaria-Piedrafita S, Cañamares-Orbis P, García-Erce JA. Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management. Nutrients. 2021; 13(10):3437. https://doi.org/10.3390/nu13103437
Chicago/Turabian StyleMontoro-Huguet, Miguel A., Santos Santolaria-Piedrafita, Pablo Cañamares-Orbis, and José Antonio García-Erce. 2021. "Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management" Nutrients 13, no. 10: 3437. https://doi.org/10.3390/nu13103437
APA StyleMontoro-Huguet, M. A., Santolaria-Piedrafita, S., Cañamares-Orbis, P., & García-Erce, J. A. (2021). Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management. Nutrients, 13(10), 3437. https://doi.org/10.3390/nu13103437







