Craniopharyngioma, Chronotypes and Metabolic Risk Profile
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.1.1. Patients
2.1.2. Controls
2.2. Anthropometric Parameters
2.3. Assessment of Chronotype
2.4. Sample Size Justification and Power
2.5. Statistical Analysis
3. Results
3.1. Demographic and Anthropometric Parameters
3.2. Metabolic Parameters and Blood Pressure
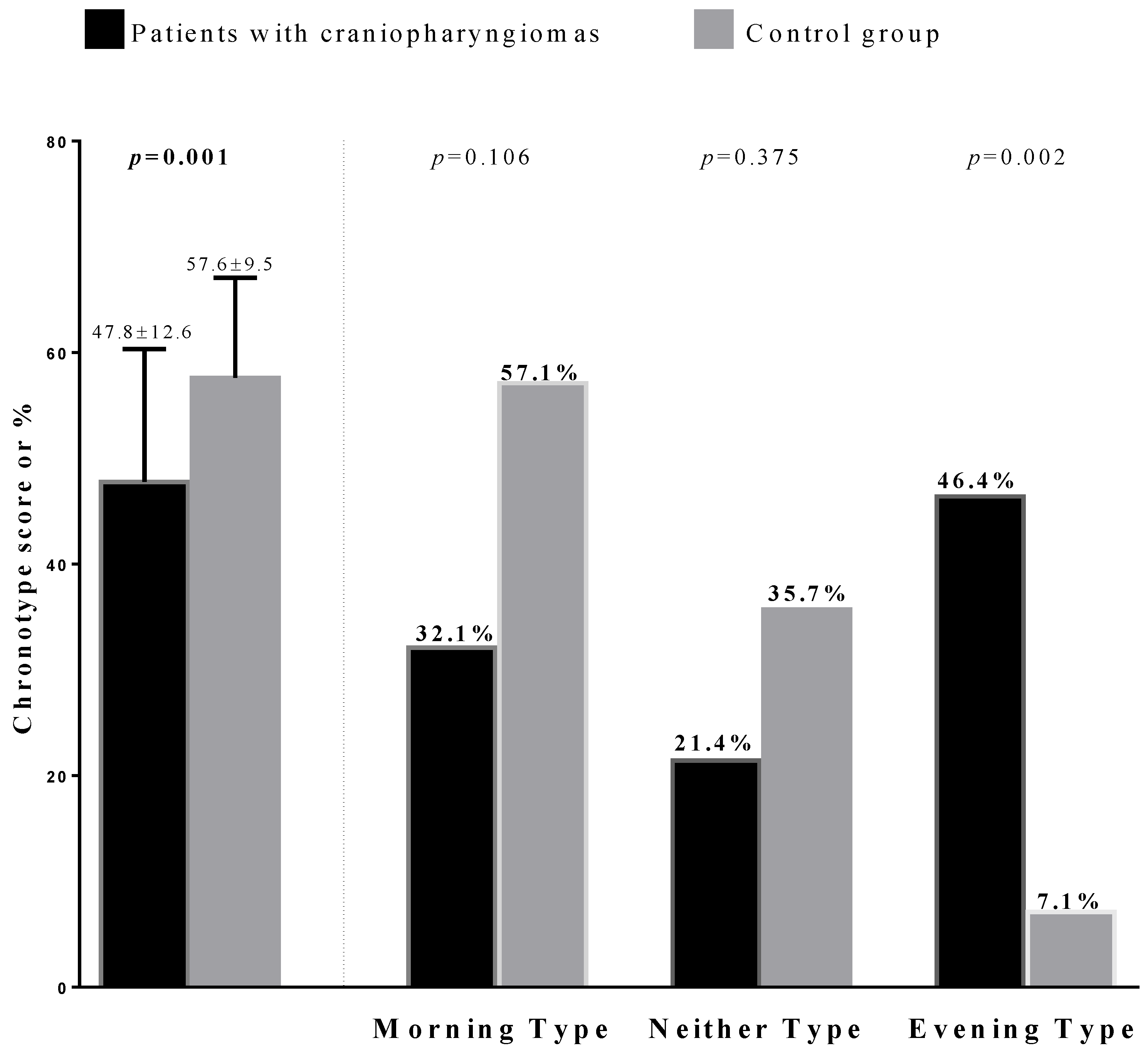
3.3. Chronotype Categories
3.4. Correlation Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | craniopharyngioma |
| BMI | body mass index |
| WC | waist circumference |
References
- Petito, C.K.; DeGirolami, U.; Earle, K.M. Craniopharyngiomas.A clinical and pathological review. Cancer 1976, 37, 1944–1952. [Google Scholar] [CrossRef]
- Nelson, G.A.; Bastian, F.O.; Schlitt, M.; White, R.L. Malignant Transformation in Craniopharyngioma. Neurosurgery 1988, 22, 427–429. [Google Scholar] [CrossRef]
- Karavitaki, N.; Cudlip, S.; Adams, C.B.T.; Wass, J.A.H. Craniopharyngiomas. Endocr. Rev. 2006, 27, 371–397. [Google Scholar] [CrossRef]
- Bunin, G.R.; Surawicz, T.S.; Witman, P.A.; Preston-Martin, S.; Davis, F.; Bruner, J.M. The descriptive epidemiology of craniopharyngioma. J. Neurosurg. 1998, 89, 547–551. [Google Scholar] [CrossRef]
- Mortini, P.; Losa, M.; Pozzobon, G.; Barzaghi, R.; Riva, M.; Acerno, S.; Angius, D.; Weber, G.; Chiumello, G.; Giovanelli, M. Neurosurgical treatment of craniopharyngioma in adults and children: Early and long-term results in a large case series. J. Neurosurg. 2011, 114, 1350–1359. [Google Scholar] [CrossRef]
- Mortini, P.; Gagliardi, F.; Boari, N.; Losa, M. Surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. Crit. Rev. Oncol. Hematol. 2013, 88, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Wijnen, M.; Heuvel-Eibrink, M.M.V.D.; Janssen, J.A.; Catsman-Berrevoets, C.E.; Michiels, E.M.C.; Van Veelen-Vincent, M.-L.C.; Dallenga, A.H.G.; Berge, J.H.V.D.; Van Rij, C.M.; Van Der Lely, A.-J.; et al. Very long-term sequelae of craniopharyngioma. Eur. J. Endocrinol. 2017, 176, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Sterkenburg, A.S.; Hoffmann, A.; Gebhardt, U.; Warmuth-Metz, M.; Daubenbüchel, A.M.; Müller, H.L. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: Newly reported long-term outcomes. Neuro-Oncology 2015, 17, 1029–1038. [Google Scholar] [CrossRef]
- Müller, H.L.; Faldum, A.; Etavard-Gorris, N.; Gebhardt, U.; Oeverink, R.; Kolb, R.; Sörensen, N. Functional Capacity, Obesity and Hypothalamic Involvement: Cross-Sectional Study on 212 Patients with Childhood Craniopharyngioma. Klin. Pädiatr. 2003, 215, 310–314. [Google Scholar] [CrossRef]
- Müller, H.L.; Heinrich, M.; Bueb, K.; Etavard-Gorris, N.; Gebhardt, U.; Oeverink, R.; Kolb, R.; Sörensen, N. Perioperative Dexamethasone Treatment in Childhood Craniopharyngioma: Influence on short-term and long-term weight development. Exp. Clin. Endocrinol. Diabetes 2003, 111, 330–334. [Google Scholar] [CrossRef]
- Nogueira, M.C.; Júnior, A.S.B.; Koenigkam-Santos, M.; Moreira, A.C.; Nonino, C.B.; de Castro, M. Nutritional and endocrinologic evaluation of patients with craniopharyngioma. Clin. Nutr. Espen 2015, 10, e213–e218. [Google Scholar] [CrossRef] [PubMed]
- De Vile, C.J.; Grant, D.B.; Hayward, R.D.; E Kendall, B.; Neville, B.G.; Stanhope, R. Obesity in childhood craniopharyngioma: Relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J. Clin. Endocrinol. Metab. 1996, 81, 2734–2737. [Google Scholar] [CrossRef] [Green Version]
- Daousi, C.; Dunn, A.J.; Foy, P.M.; Macfarlane, I.A.; Pinkney, J.H. Endocrine and neuroanatomic features associated with weight gain and obesity in adult patients with hypothalamic damage. Am. J. Med. 2005, 118, 45–50. [Google Scholar] [CrossRef]
- Müller, H.L.; Emser, A.; Faldum, A.; Bruhnken, G.; Etavard-Gorris, N.; Gebhardt, U.; Oeverink, R.; Kolb, R.; Sörensen, N. Longitudinal Study on Growth and Body Mass Index before and after Diagnosis of Childhood Craniopharyngioma. J. Clin. Endocrinol. Metab. 2004, 89, 3298–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, H.L. Craniopharyngioma. Endocr. Rev. 2014, 35, 513–543. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Schmid, E.M.; Schutte, P.J.; Voormolen, J.; Biermasz, N.R.; Van Thiel, S.W.; Corssmit, E.P.M.; Smit, J.W.A.; Roelfsema, F.; Romijn, J.A. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin. Endocrinol. 2004, 62, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Crowley, R.K.; Hamnvik, O.-P.; O’Sullivan, E.P.; Behan, L.A.; Smith, D.; Agha, A.; Thompson, C.J. Morbidity and Mortality in patients with Craniopharyngioma after Surgery. Clin. Endocrinol. 2010, 73, 516–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Amicis, R.; Galasso, L.; Leone, A.; Vignati, L.; De Carlo, G.; Foppiani, A.; Montaruli, A.; Roveda, E.; Cè, E.; Esposito, F.; et al. Is Abdominal Fat Distribution Associated with Chronotype in Adults Independently of Lifestyle Factors? Nutrients 2020, 12, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Makarem, N.; Paul, J.; Giardina, E.-G.V.; Liao, M.; Aggarwal, B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiol. Int. 2020, 37, 673–685. [Google Scholar] [CrossRef]
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health. Adv. Nutr. 2019, 10, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Altieri, B.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A. Chronotype and cardio metabolic health in obesity: Does nutrition matter? Int. J. Food Sci. Nutr. 2021, 67, 1–9. [Google Scholar] [CrossRef]
- Wong, P.M.; Hasler, B.P.; Kamarck, T.W.; Muldoon, M.F.; Manuck, S.B. Social Jetlag, Chronotype, and Cardiometabolic Risk. J. Clin. Endocrinol. Metab. 2015, 100, 4612–4620. [Google Scholar] [CrossRef] [PubMed]
- Culnan, E.; Kloss, J.D.; Grandner, M. A prospective study of weight gain associated with chronotype among college freshmen. Chronobiol. Int. 2013, 30, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chartrand, T.L. Morningness–Eveningness and Risk Taking. J. Psychol. 2015, 149, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Aprano, S.; Framondi, L.; Di Matteo, R.; Riccio, P.A.; Savastano, S.; Colao, A. The OPERA Prevention Project. Int. J. Food Sci. Nutr. 2021, 72, 1–3. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A.; The Opera Prevention Project. Chronotype and Adherence to the Mediterranean Diet in Obesity: Results from the Opera Prevention Project. Nutrients 2020, 12, 1354. [Google Scholar] [CrossRef]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist–Hip Ratio. Eur. J. Clin. Nutr. 2009, 64, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef]
- Yu, J.H.; Yun, C.-H.; Ahn, J.H.; Suh, S.; Cho, H.J.; Lee, S.K.; Yoo, H.J.; A Seo, J.; Kim, S.G.; Choi, K.M.; et al. Evening Chronotype Is Associated With Metabolic Disorders and Body Composition in Middle-Aged Adults. J. Clin. Endocrinol. Metab. 2015, 100, 1494–1502. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Annunziata, G.; Megna, M.; Falco, A.; Balato, A.; Colao, A.; Savastano, S. Coffee consumption, metabolic syndrome and clinical severity of psoriasis: Good or bad stuff? Arch. Toxicol. 2018, 92, 1831–1845. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A.; Opera Prevention Project. Sleep Quality in Obesity: Does Adherence to the Mediterranean Diet Matter? Nutrients 2020, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.L. Childhood craniopharyngioma—current concepts in diagnosis, therapy and follow-up. Nat. Rev. Endocrinol. 2010, 6, 609–618. [Google Scholar] [CrossRef]
- Erfurth, E.M.; Holmer, H.; Fjalldal, S.B. Mortality and morbidity in adult craniopharyngioma. Pituitary 2012, 16, 46–55. [Google Scholar] [CrossRef]
- Elowe-Gruau, E.; Beltrand, J.; Brauner, R.; Pinto, G.; Samara-Boustani, D.; Thalassinos, C.; Busiah, K.; Laborde, K.; Boddaert, N.; Zerah, M.; et al. Childhood Craniopharyngioma: Hypothalamus-Sparing Surgery Decreases the Risk of Obesity. J. Clin. Endocrinol. Metab. 2013, 98, 2376–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, C.L. Hypothalamic Obesity in Craniopharyngioma Patients: Disturbed Energy Homeostasis Related to Extent of Hypothalamic Damage and Its Implication for Obesity Intervention. J. Clin. Med. 2015, 4, 1774–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickering, L.; Klose, M.; Feldt-Rasmussen, U.; Jennum, P. Polysomnographic findings in craniopharyngioma patients. Sleep Breath 2017, 21, 975–982. [Google Scholar] [CrossRef]
- Bereket, A.; Kiess, W.; Lustig, R.H.; Müller, H.L.; Goldstone, A.; Weiss, R.; Yavuz, Y.; Hochberg, Z. Hypothalamic obesity in children. Obes. Rev. 2012, 13, 780–798. [Google Scholar] [CrossRef]
- Nieste, I.; Franssen, W.M.; Spaas, J.; Bruckers, L.; Savelberg, H.H.; Eijnde, B.O. Lifestyle interventions to reduce sedentary behaviour in clinical populations: A systematic review and meta-analysis of different strategies and effects on cardiometabolic health. Prev. Med. 2021, 148, 106593. [Google Scholar] [CrossRef]
- Canuto, R.; Garcez, A.; de Souza, R.V.; Kac, G.; Olinto, M.T.A. Nutritional intervention strategies for the management of overweight and obesity in primary health care: A systematic review with meta-analysis. Obes. Rev. 2021, 22–34. [Google Scholar] [CrossRef]
- Bray, G.A.; Gallagher, T.F. Manifestations of hypothalamic obesity in man: A comprehensive investigation of eight patients and a eeview of the literature. Medicine 1975, 54, 301–330. [Google Scholar] [CrossRef]
- Rakhshani, N.; Jeffery, A.S.; Schulte, F.; Barrera, M.; Atenafu, E.; Hamilton, J.K. Evaluation of a Comprehensive Care Clinic Model for Children With Brain Tumor and Risk for Hypothalamic Obesity. Obesity 2010, 18, 1768–1774. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Faggiano, F.; Maiorino, M.I.; Parrillo, M.; Pugliese, G.; Ruggeri, R.M.; Scarano, E.; Savastano, S.; Colao, A.; et al. Obesity in Prader–Willi syndrome: Physiopathological mechanisms, nutritional and pharmacological approaches. J. Endocrinol. Investig. 2021, 44, 2057–2070. [Google Scholar] [CrossRef]
- Montaruli, A.; Galasso, L.; Caumo, A.; Cè, E.; Pesenti, C.; Roveda, E.; Esposito, F. The circadian typology: The role of physical activity and melatonin. Sport Sci. Health 2017, 13, 469–476. [Google Scholar] [CrossRef]
- Adan, A.; Archer, S.N.; Hidalgo, M.P.; Di Milia, L.; Natale, V.; Randler, C. Circadian Typology: A Comprehensive Review. Chronobiol. Int. 2012, 29, 1153–1175. [Google Scholar] [CrossRef] [Green Version]
- Randler, C.; Faßl, C.; Kalb, N. From Lark to Owl: Developmental changes in morningness-eveningness from new-borns to early adulthood. Sci. Rep. 2017, 7, 45874. [Google Scholar] [CrossRef]
- Randler, C.; Engelke, J. Gender differences in chronotype diminish with age: A meta-analysis based on morningness/chronotype questionnaires. Chronobiol. Int. 2019, 36, 888–905. [Google Scholar] [CrossRef]
- Kanerva, N.; Kronholm, E.; Partonen, T.; Ovaskainen, M.-L.; Kaartinen, N.E.; Konttinen, H.; Broms, U.; Männistö, S. Tendency Toward Eveningness Is Associated With Unhealthy Dietary Habits. Chronobiol. Int. 2012, 29, 920–927. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Tuccinardi, D.; Nicastro, V.; Barrea, L.; Colao, A.; Savastano, S. Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Sleep disturbances: One of the culprits of obesity-related cardiovascular risk? Int. J. Obes. Suppl. 2020, 10, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Przepiórka, A.; Błachnio, A.; Siu, N.Y.-F. The relationships between self-efficacy, self-control, chronotype, procrastination and sleep problems in young adults. Chronobiol. Int. 2019, 36, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Borel, A.-L. Sleep Apnea and Sleep Habits: Relationships with Metabolic Syndrome. Nutrients 2019, 11, 2628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraù, F.; Spagnolo, F.; Cotta, O.R.; Cannavò, L.; Alibrandi, A.; Russo, G.T.; Aversa, T.; Trimarchi, F.; Cannavò, S. Visceral adiposity index as an indicator of cardiometabolic risk in patients treated for craniopharyngioma. Endocrine 2017, 58, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [Green Version]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social Jetlag and Obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef]
- Montaruli, A.; Castelli, L.; Mulè, A.; Scurati, R.; Esposito, F.; Galasso, L.; Roveda, E. Biological Rhythm and Chronotype: New Perspectives in Health. Biomology 2021, 11, 487. [Google Scholar] [CrossRef]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Ludin, A.F.M. The Association between Chronotype and Dietary Pattern among Adults: A Scoping Review. Int. J. Environ. Res. Public Health 2019, 17, 68. [Google Scholar] [CrossRef] [Green Version]
- Després, J.-P. Abdominal obesity: The most prevalent cause of the metabolic syndrome and related cardiometabolic risk. Eur. Hear. J. Suppl. 2006, 8, B4–B12. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Craniopharyngioma Patients n = 28 |
|---|---|
| Craniopharyngioma treatment | |
| Neurosurgery only | 16 |
| Neurosurgery + Radiotherapy | 12 |
| Pituitary hormone deficiency At least one deficit | |
| GH | 20 |
| Gonadotropins | 18 |
| TSH | 20 |
| ACTH | 25 |
| Central diabetes insipidus | 23 |
| Panhypopituitarism | 18 |
| rGH replacement therapy | 16 |
| Parameters | Craniopharyngioma Patients n = 28 | Control Group n = 28 | p-Value |
|---|---|---|---|
| Demographic characteristics | |||
| Gender (Males) | 13 (46.4%) | 13 (46.4%) | 0.205 |
| Age (Years) | 42.6 ± 15.8 | 46.5 ± 12.9 | 0.355 |
| Anthropometric measurements | |||
| Weight (kg) | 95.4 ± 15.5 | 94.7 ± 17.8 | 0.859 |
| Height (m) | 1.7 ± 0.08 | 1.6 ± 0.11 | 0.568 |
| BMI (kg/m2) | 34.3 ± 3.5 | 34.7 ± 2.6 | 0.574 |
| Grade I obesity (n, %) | 15 (53.6%) | 15 (53.6%) | 0.560 |
| Grade II obesity (n, %) | 13 (46.4%) | 13 (46.4%) | |
| WC (cm) | 106.1 ± 10.1 | 99.2 ± 10.6 | 0.011 |
| Blood pressure | |||
| SBP (mmHg) | 121.9 ± 12.8 | 114.8 ± 11.2 | 0.022 |
| DBP (mmHg) | 77.8 ± 9.0 | 73.5 ± 10.5 | 0.003 |
| Metabolic profile | |||
| Plasma glucose (mg/dL) | 101.8 ± 13.1 | 95.2 ± 7.5 | 0.015 |
| Total cholesterol (mg/dL) | 207.3 ± 47.0 | 182.9 ± 33.7 | 0.006 |
| LDL cholesterol (mg/dL) | 135.7 ± 44.6 | 111.7 ± 38.4 | 0.007 |
| HDL cholesterol (mg/dL) | 40.7 ± 9.4 | 46.2 ± 9.6 | 0.023 |
| Triglycerides (mg/dL) | 154.3 ± 48.9 | 124.9 ± 35.1 | 0.003 |
| Parameters | Morning Type n = 9, 32.1% | Neither Type n = 6, 21.4% | Evening Type n = 13, 46.4% | F-Value | p-Value |
|---|---|---|---|---|---|
| Gender | |||||
| Males (n, %) | 5 (55.6%) | 3 (50.0%) | 5 (38.5%) | 0.885 | |
| Females (n, %) | 4 (44.4%) | 3 (50.0%) | 8 (61.5%) | ||
| Age (years) | 41.7 ± 12.3 | 55.3 ± 15.8 | 37.5 ± 15.7 | 3.06 | 0.063 |
| Anthropometric measurement | |||||
| BMI (kg/m2) | 31.2 ± 1.2 | 32.0 ± 1.7 | 37.5 ± 1.9 a,b | 42.75 | 0.000 |
| Grade I obesity (n, %) | 9 (100.0%) | 5 (83.3%) | 1 (7.7%) | 0.000 | |
| Grade II obesity (n, %) | 0 (0.0%) | 1 (16.7%) | 12 (92.3%) | ||
| WC (cm) | 100.9 ± 8.4 | 102.3 ± 6.5 | 111.5 ± 10.3 a | 4.29 | 0.037 |
| Blood pressure | |||||
| SBP (mmHg) | 114.4 ± 8.8 | 116.2 ± 15.2 | 129.6 ± 9.9 a,b | 6.22 | <0.05 |
| DBP (mmHg) | 73.9 ± 5.5 | 73.0 ± 8.4 | 82.7 ± 9.2 a,b | 5.43 | <0.05 |
| Metabolic profile | |||||
| Glycemia levels (mg/dL) | 95.2 ± 8.2 | 95.8 ± 12.3 | 109.2 ± 12.9 a,b | 4.94 | <0.05 |
| Total cholesterol (mg/dL) | 193.3 ± 24.0 | 174.3 ± 47.5 | 232.1 ± 47.8 b | 4.68 | 0.029 |
| Triglycerides (mg/dL) | 142.3 ± 35.9 | 117.2 ± 52.4 | 179.6 ± 43.4 b | 4.80 | 0.021 |
| LDL cholesterol (mg/dL) | 117.4 ± 18.5 | 108.9 ± 41.6 | 160.8 ± 47.3 a,b | 5.06 | <0.05 |
| HDL cholesterol (mg/dL) | 47.4 ± 9.1 | 42.0 ± 10.7 | 35.4 ± 5.4 b | 6.23 | 0.005 |
| Parameters | Morning Type n = 9, 32.1% | Neither Type n = 6, 21.4% | Evening Type n = 13, 46.4% | F-Value | p-Value |
|---|---|---|---|---|---|
| Gender | |||||
| Males (n, %) | 11, 68.8% | 2, 20.0% | 0, 0% | 0.885 | |
| Females (n, %) | 5, 31.3% | 8, 80.0% | 2, 100% | ||
| Age (years) | 47.31 ± 16.44 | 44.40 ± 17.01 | 50.50 ± 6.36 | 0.16 | 1.000 |
| Anthropometric measurement | |||||
| BMI (kg/m2) | 33.42 ± 1.95 | 35.93 ± 2.69 | 38.70 ± 0.72 a | 7.46 | 0.012 |
| Grade I obesity (n, %) | 12, 75.0% | 3, 30.0% | 0, 0% | 0.000 | |
| Grade II obesity (n, %) | 4, 25.0% | 7, 70.0% | 2, 100% | ||
| WC (cm) | 96.38 ± 9.82 | 99.60 ± 7.94 | 120.00 ± 0.00 a,b | 6.17 | <0.05 |
| Blood pressure | |||||
| SBP (mmHg) | 115.31 ± 11.61 | 114.70 ± 12.23 | 112.50 ± 3.53 | 0.05 | 1.000 |
| DBP (mmHg) | 72.19 ± 10.33 | 73.80 ± 11.54 | 82.50 ± 3.53 | 0.08 | 0.620 |
| Metabolic profile | |||||
| Glycemia levels (mg/dL) | 92.38 ± 7.11 | 97.10 ± 4.41 | 108.50 ± 7.77 a | 6.51 | 0.007 |
| Total cholesterol (mg/dL) | 169.00 ± 22.29 | 194.70 ± 38.34 | 234.50 ± 14.84 a | 5.86 | 0.017 |
| Triglycerides (mg/dL) | 120.06 ± 27.19 | 119.80 ± 38.31 | 189.00 ± 12.72 a,b | 4.52 | <0.05 |
| LDL cholesterol (mg/dL) | 96.55 ± 32.16 | 127.34 ± 40.13 | 154.20 ± 13.01 | 4.03 | 0.109 |
| HDL cholesterol (mg/dL) | 48.44 ± 9.70 | 43.40 ± 9.81 | 42.50 ± 0.70 | 1.02 | 1.000 |
| Simple Correlations | ||
|---|---|---|
| Parameters | r | p-Value |
| Age (years) | 0.023 | 0.909 |
| Anthropometric measurement | ||
| BMI (kg/m2) | −0.836 | 0.000 |
| WC (cm) | −0.676 | 0.000 |
| Blood pressure | ||
| SBP (mmHg) | −0.490 | 0.010 |
| DBP (mmHg) | −0.394 | 0.042 |
| Metabolic profile | ||
| Glycemia levels (mg/dL) | −0.502 | 0.008 |
| Total cholesterol (mg/dL) | −0.378 | 0.050 |
| LDL cholesterol (mg/dL) | −0.432 | 0.024 |
| HDL cholesterol (mg/dL) | 0.551 | 0.003 |
| Triglycerides (mg/dL) | −0.398 | 0.040 |
| Simple Correlations | ||
|---|---|---|
| Parameters | r | p-Value |
| Age (years) | 0.075 | 0.710 |
| Anthropometric measurement | ||
| BMI (kg/m2) | −0.654 | 0.000 |
| WC (cm) | −0.563 | 0.002 |
| Blood pressure | ||
| SBP (mmHg) | 0.039 | 0.846 |
| DBP (mmHg) | −0.108 | 0.592 |
| Metabolic profile | ||
| Glycemia levels (mg/dL) | −0.745 | 0.000 |
| Total cholesterol (mg/dL) | −0.551 | 0.003 |
| LDL cholesterol (mg/dL) | −0.501 | 0.008 |
| HDL cholesterol (mg/dL) | 0.291 | 0.141 |
| Triglycerides (mg/dL) | −0.302 | 0.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Somma, C.; Scarano, E.; Barrea, L.; Solari, D.; Riccio, E.; Arianna, R.; Cavallo, L.M.; Romano, F.; Di Benedetto, E.; Rodriguez, A.; et al. Craniopharyngioma, Chronotypes and Metabolic Risk Profile. Nutrients 2021, 13, 3444. https://doi.org/10.3390/nu13103444
Di Somma C, Scarano E, Barrea L, Solari D, Riccio E, Arianna R, Cavallo LM, Romano F, Di Benedetto E, Rodriguez A, et al. Craniopharyngioma, Chronotypes and Metabolic Risk Profile. Nutrients. 2021; 13(10):3444. https://doi.org/10.3390/nu13103444
Chicago/Turabian StyleDi Somma, Carolina, Elisabetta Scarano, Luigi Barrea, Domenico Solari, Enrico Riccio, Rossana Arianna, Luigi Maria Cavallo, Fiammetta Romano, Elea Di Benedetto, Alice Rodriguez, and et al. 2021. "Craniopharyngioma, Chronotypes and Metabolic Risk Profile" Nutrients 13, no. 10: 3444. https://doi.org/10.3390/nu13103444
APA StyleDi Somma, C., Scarano, E., Barrea, L., Solari, D., Riccio, E., Arianna, R., Cavallo, L. M., Romano, F., Di Benedetto, E., Rodriguez, A., de Alteriis, G., & Colao, A. (2021). Craniopharyngioma, Chronotypes and Metabolic Risk Profile. Nutrients, 13(10), 3444. https://doi.org/10.3390/nu13103444









