Why We Eat Too Much, Have an Easier Time Gaining Than Losing Weight, and Expend Too Little Energy: Suggestions for Counteracting or Mitigating These Problems
Abstract
:1. Introduction
2. How Obesity Generates Serious Health Problems
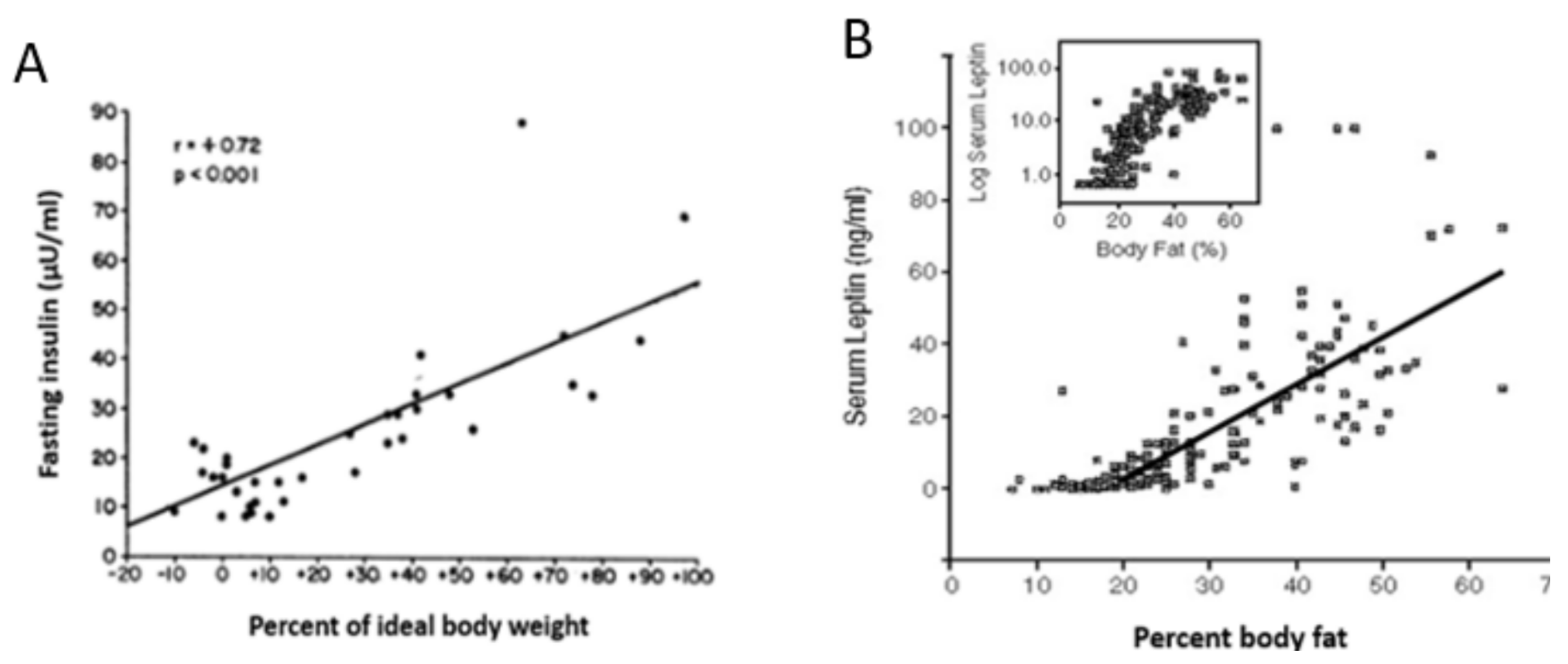
3. Limitations of Human Physiology in Controlling Weight Gain and Loss
Suggestions for How to Deal with Limitations of Human Physiology in Controlling Weight Gain and Loss
4. Limitations of Human Psychology in Controlling Weight Gain and Loss
Using Psychology for Controlling Overeating
5. Features of Developed Societies That Hinder Efforts to Control Our Weight
Suggestions for Counteracting Detrimental Aspects of Features of Developed Societies
6. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. The WHO STEPwise Approach to Noncommunicable Disease Risk Factor Surveillance (STEPS); WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Fryar, C.; Carroll, M.D.; Afful, J. Prevalence of Overweight, Obesity, and Severe Obesity among Adults Aged 20 and Over: United States, 1960–1962 through 2017–2018; NCHS Health E-Stats; U.S. National Center for Health Statistics: Hyattsville, MD, USA, 2020.
- Kelly, T.; Yang, W.; Chen, C.-S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestle, M.; Jacobson, M.F. Halting the obesity epidemic: A public health policy approach. Public Health Rep. 2000, 115, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Hatzenbuehler, M.L.; Keyes, K.M.; Hasin, D.S. Associations Between Perceived Weight Discrimination and the Prevalence of Psychiatric Disorders in the General Population. Obesity 2009, 17, 2033–2039. [Google Scholar] [CrossRef] [Green Version]
- Puhl, R.; Suh, Y. Stigma and Eating and Weight Disorders. Curr. Psychiatry Rep. 2015, 17, 1–10. [Google Scholar] [CrossRef]
- Vartanian, L.R.; Novak, S.A. Internalized Societal Attitudes Moderate the Impact of Weight Stigma on Avoidance of Exercise. Obesity 2011, 19, 757–762. [Google Scholar] [CrossRef]
- Borer, K.T. Physical activity in the prevention and amelioration of osteoporosis n women: Interaction of mechanical, hormonal, and dietary factors. Sports Med. 2005, 35, 779–830. [Google Scholar] [CrossRef]
- Borer, K.T. How effective is exercise in producing fat loss? Kinesiology 2008, 40, 127–138. [Google Scholar]
- Borer, K.T. Nonhomeostatic Control of Human Appetite and Physical Activity in Regulation of Energy Balance. Exerc. Sport Sci. Rev. 2010, 38, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Borer, K.T. Counterregulation of insulin by leptin as key component of autonomic regulation of body weight. World J. Diabetes 2014, 5, 606–629. [Google Scholar] [CrossRef]
- Borer, K.T. Understanding Human Physiological Limitations and Societal Pressures in Favor of Overeating Helps to Avoid Obesity. Nutrients 2019, 11, 227. [Google Scholar] [CrossRef] [Green Version]
- Borer, K.T. How does exercise support dietary approaches to weight loss and better health? Ann. Kinesiol. 2019, 10, 31–58. [Google Scholar] [CrossRef]
- Borer, K.T.; Zheng, Q.; Jafari, A.; Javadi, S.; Kernozek, T. Nutrient Intake Prior to Exercise Is Necessary for Increased Osteogenic Marker Response in Diabetic Postmenopausal Women. Nutrients 2019, 11, 1494. [Google Scholar] [CrossRef] [Green Version]
- Borer, K.T.; Potter, C.D.; Fileccia, N. Basis for the hypoactivity that accompanies rapid weight gain in hamsters. Physiol. Behav. 1983, 30, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Borer, K.T.; Wuorinen, E.; Chao, C.; Burant, C. Exercise energy expenditure is not consciously detected due to oro-gastric, not metabolic, basis of hunger sensation. Appetite 2005, 45, 177–181. [Google Scholar] [CrossRef]
- Borer, K.T.; Wuorinen, E.; Ku, K.; Burant, C. Appetite Responds to Changes in Meal Content, Whereas Ghrelin, Leptin, and Insulin Track Changes in Energy Availability. J. Clin. Endocrinol. Metab. 2009, 94, 2290–2298. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.-J.; Borer, K.T. Third Exposure to a Reduced Carbohydrate Meal Lowers Evening Postprandial Insulin and GIP Responses and HOMA-IR Estimate of Insulin Resistance. PLoS ONE 2016, 11, e0165378. [Google Scholar] [CrossRef]
- Sobhani, I.; Bado, A.; Vissuzaine, C.; Buyse, M.; Kermorgant, S.; Laigneau, J.-P.; Attoub, S.; Lehy, T.; Henin, D.; Mignon, M.; et al. Leptin secretion and leptin receptor in the human stomach. Gut 2000, 47, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-induced insulin resistance: Unravelling the mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef] [Green Version]
- Shulman, G.I. Ectopic Fat in Insulin Resistance, Dyslipidemia, and Cardiometabolic Disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Bagdade, J. Basal Insulin and Obesity. Lancet 1968, 292, 630–631. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, C.; Wagenknecht, L.E.; Rewers, M.J.; Karter, A.J.; Bergman, R.N.; Hanley, A.J.; Haffner, S.M. Disposition Index, Glucose Effectiveness, and Conversion to Type 2 Diabetes: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010, 33, 2098–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendelson, C.R. Mechanisms of hormone action. In Textbook of Endocrine Physiology, 3rd ed.; Oxford University Press: New York, NY, USA, 1996; pp. 29–65. [Google Scholar]
- Olefsky, J.M.; Reaven, G.M. Effects of Age and Obesity on Insulin Binding to Isolated Adipocytes12 12. Endocrinology 1975, 96, 1486–1498. [Google Scholar] [CrossRef]
- Taylor, R. Banting Memorial Lecture 2012 Reversing the twin cycles of Type 2 diabetes. Diabet. Med. 2013, 30, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.S.; Pencina, M.J.; Meigs, J.B.; Vasan, R.S.; Levitzky, Y.S.; D’Agostino, R.B., Sr. Trends in the incidence of type 2 diabetes mellitus from the 1970’s to the 1990’s: The Framingham Heart Study. Circulation 2006, 113, 2914–2918. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, US Department of Health and Human Services. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2014. Available online: http://wwwcdcgov/diabetes/pubs/statsreport14/national-diabetes-reportwebpdf (accessed on 15 September 2021).
- Kaiser, A.B.; Zhang, N.; van der Pluijm, W. Global Prevalence of Type 2 Diabetes over the Next Ten Years (2018–2028). Diabetes 2018, 67, 202. [Google Scholar] [CrossRef]
- Farag, Y.M.; Gaballa, M.R. Diabesity: An overview of a rising epidemic. Nephr. Dial. Transpl. 2011, 26, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, C.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Taira, S.-I.; Shimabukuro, M.; Higa, M.; Yabiku, K.; Kozuka, C.; Ueda, R.; Sunagawa, S.; Ohshiro, Y.; Doi, M.; Nanba, T.; et al. Lipid Deposition in Various Sites of the Skeletal Muscles and Liver Exhibits a Positive Correlation with Visceral Fat Accumulation in Middle-aged Japanese Men with Metabolic Syndrome. Intern. Med. 2013, 52, 1561–1571. [Google Scholar] [CrossRef] [Green Version]
- Van der Zijl, N.J.; Goossens, G.H.; Moors, C.C.; van Raate, D.H.; Muskiet, M.H.; Powels, D.J.; Blaak, E.E.; Diamant, M. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: Impact on ß-cell function in individuals with impaired glucose metabolism. J. Clin. Endocr. Metab. 2011, 96, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Guebre-Egziabher, F.; Alix, P.M.; Koppe, L.; Pelletier, C.C.; Kalbacher, E.; Fouque, D.; Soulage, C.O. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie 2013, 95, 1971–1979. [Google Scholar] [CrossRef]
- Yaziei, D.; Sezer, H. Insulin resistance, obesity and lipotoxicity. Adv. Exp. Biol. Med. 2017, 960, 277–304. [Google Scholar]
- Schenk, S.; Harber, M.P.; Shrivastava, C.R.; Burant, C.F.; Horowitz, J.F. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J. Physiol. 2009, 587, 4949–4961. [Google Scholar] [CrossRef]
- Farias, M.M.; Cuevas, A.M.; Rodriguez, F. Set-Point Theory and Obesity. Metab. Syndr. Relat. Disord. 2011, 9, 85–89. [Google Scholar] [CrossRef]
- Müller, M.J.; Geisler, C.; Heymsfield, S.B.; Bosy-Westphal, A. Recent advances in understanding body weight homeostasis in humans. F1000Research 2018, 7, 1025. [Google Scholar] [CrossRef] [Green Version]
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 2016, 24, 1612–1619. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Farooqi, S.; Keogh, J.M.; Kamath, S.; Jones, S.; Gibson, W.; Trussell, R.; Jebb, S.A.; Lip, G.Y.H.; O’Rahilly, S. Partial leptin deficiency and human adiposity. Nature 2001, 414, 34–35. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Greenberg, A.S.; Fujioka, K.; Dixon, R.M.; Kushner, R.; Hunt, T.; Lubina, J.A.; Patane, J.; Self, B.; Hunt, P.; et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA 1999, 282, 1568–1575. [Google Scholar] [CrossRef]
- Mayer, J.; Roy, P.; Mitra, K.P. Relation between caloric intake, body weight, and physical work: Studies in an industrial male population in West Bengal. Am. J. Clin. Nutr. 1956, 4, 169–175. [Google Scholar] [CrossRef]
- Rising, R.; Harper, I.T.; Fintvielle, A.M.; Ferraro, R.T.; Spraul, M.; Ravussin, E. Determinants of total daily energy expenditure: Variability in physical activity. Am. J. Clin. Nutr. 1994, 59, 800–804. [Google Scholar] [CrossRef]
- Schulz, L.; Schoeller, D.A. A compilation of total daily energy expenditures and body weights in healthy adults. Am. J. Clin. Nutr. 1994, 60, 676–681. [Google Scholar] [CrossRef]
- Cummings, D.E. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol. Behav. 2006, 89, 71–84. [Google Scholar] [CrossRef]
- Borer, K.T.; Lin, P.-J.; Wuorinen, E. Timing of meals and exercise affects hormonal control of glucoregulation, insulin resistance, substrate metabolism, and gastrointestinal hormones but has little effect on appetite in postmenopausal women. Nutrients 2021. (manuscript under review). [Google Scholar]
- Kendall, A.; Levitsky, D.A.; Strupp, B.J.; Lissner, L. Weight loss on a low-fat diet: Consequence of the imprecision of the control of food intake in humans. Am. J. Clin. Nutr. 1991, 53, 1124–1129. [Google Scholar] [CrossRef]
- Smith, G.P.; Gibbs, J. Satiating effect of cholecystokinin. Ann. N. Y. Acad. Sci. 1985, 448, 417–423. [Google Scholar]
- Cannon, W.B.; Washburn, A.L. An explanation of hunger. Am. J. Physiol. Content 1912, 29, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Berthoud, H.-R. Vagal and hormonal gut-brain communication: From satiation to satisfaction. Neurogastroenterol. Motil. 2008, 20, 64–72. [Google Scholar] [CrossRef]
- Woods, S.C. Gastrointestinal Satiety Signals, I. An overview of gastrointestinal signals that influence food intake. Am. J. Physiol. Liver Physiol. 2004, 286, G7–G13. [Google Scholar] [CrossRef] [Green Version]
- Dethier, V.G.; Solomon, R.L.; Turner, L.H. Sensory input and central excitation and inhibition in the blowfly. J. Comp. Physiol. Psychol. 1965, 60, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.L.; Orr, W.C.; Bollinger, C. Postprandial Sleepiness: Objective Documentation via Polysomnography. Sleep 1983, 6, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarthy, M.V.; Booth, F.W. Eating, exercise, and “thrifty” genotypes: Connecting the dots toward an evolutionary under-standing of modern chronic diseases. J. Appl. Physiol. 2004, 96, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geliebter, A.; Hashim, S.A. Gastric capacity in normal, obese, and bulimic women. Physiol. Behav. 2001, 74, 743–746. [Google Scholar] [CrossRef]
- Smoliga, J.M. Modelling the maximal active consumption rate and its plasticity in humans—Perspectives from hot dog eating competitions. Biol. Lett. 2020, 16, 20200096. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Shott, M.E.; Stoddard, J.; Swindle, S.; Pryor, T.L. Association of Brain Reward Response with Body Mass Index and Ventral Striatal-Hypothalamic Circuitry Among Young Women with Eating Disorders. JAMA Psychiatry 2021, 78, 1123–1133. [Google Scholar] [CrossRef]
- Stewart, W.K.; Fleming, L.W. Features of a successful therapeutic fast of 382 days’ duration. Postgrad. Med. J. 1973, 49, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Janmohammadi, P.; Sajadi, F.; Alizadeh, S.; Daneshzad, E. Comparison of Energy and Food Intake Between Gastric Bypass and Sleeve Gastrectomy: A Meta-analysis and Systematic Review. Obes. Surg. 2019, 29, 1040–1048. [Google Scholar] [CrossRef]
- Mans, E.; Serra-Prat, M.; Palomera, E.; Suñol, X.; Clave, P. Sleeve gastrectomy effects on hunger, satiation, and gastrointestinal hormone and motility responses after a liquid meal test. Am. J. Clin. Nutr. 2015, 102, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Wing, R.R.; Espeland, M.A.; Clark, J.M.; Hazuda, H.P.; Knowler, W.C.; Pownall, H.; Unick, J.; Wadden, T.; Wagenknecht, L.; for the Action for Health in Diabetes (Look AHEAD) Study Group. Association of Weight Loss Maintenance and Weight Regain on 4-Year Changes in CVD Risk Factors: The Action for Health in Diabetes (Look AHEAD) Clinical Trial. Diabetes Care 2016, 39, 1345–1355. [Google Scholar] [CrossRef] [Green Version]
- Knuth, N.D.; Johannsen, D.L.; Tamboli, R.A.; Marks-Shulman, P.A.; Huizenga, R.; Chen, K.; Abumrad, N.N.; Ravussin, E.; Hall, K.D. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity 2014, 22, 2563–2569. [Google Scholar] [CrossRef] [Green Version]
- Pontzer, H. Constrained total energy expenditure and the evolutionary bioogy of energy expenditure. Exerc. Sports Sci. Rev. 2015, 43, 110–116. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Leibel, R.L. 20 years of leptin: Role of leptin in energy homeostasis in humans. J. Endocrinol. 2014, 223, T83–T96. [Google Scholar] [CrossRef]
- Heldstab, S.A.; van Schaik, C.P.; Isler, K. Being fat and smart: A comparative analysis of the fat-brain trade-off in mammals. J. Hum. Evol. 2016, 100, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.C.K. The evolution of human fatness and susceptibility to obesity: An ethological approach. Biol. Rev. 2006, 81, 183–205. [Google Scholar] [CrossRef]
- Crook, C. Taste perception in the newborn infant. Infant Behav. Dev. 1978, 1, 52–69. [Google Scholar] [CrossRef]
- Berridge, K.C. ‘Liking’ and ‘wanting’ food rewards: Brain substrates and roles in eating disorders. Physiol. Behav. 2009, 97, 537–550. [Google Scholar] [CrossRef] [Green Version]
- Raynor, H.A.; Vadiveloo, M. Understanding the Relationship Between Food Variety, Food Intake, and Energy Balance. Curr. Obes. Rep. 2018, 7, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [Green Version]
- De Castro, J.M. Social facilitation of duration and size but not rate of the spontaneous meal intake of humans. Physiol. Behav. 1990, 47, 1129–1135. [Google Scholar] [CrossRef]
- Ross, S.; Ross, J.G. Social Facilitation of Feeding Behavior in Dogs: I. Group and Solitary Feeding. Pedagog. Semin. J. Genet. Psychol. 1949, 74, 97–108. [Google Scholar] [CrossRef]
- Strobel, M.G.; Macdonald, G.E. Induction of eating in newly hatched chicks. J. Comp. Physiol. Psychol. 1974, 86, 493–502. [Google Scholar] [CrossRef]
- Nguyen, B.P.; Powell, L.M. The impact of restaurant consumption among US adults: Effects on energy and nutrient intakes. Public Health Nutr. 2014, 17, 2445–2452. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.L.; Bargh, J.A.; Brownell, K.D. Priming effects of television food advertising on eating behavior. Health Psychol. 2009, 28, 404–413. [Google Scholar] [CrossRef]
- Dongen, M.V.-V.; Kok, F.J.; de Graaf, C. Eating rate of commonly consumed foods promotes food and energy intake. Appetite 2011, 56, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Rolls, B.J.; Morris, E.L.; Roe, L.S. Portion size of food affects energy intake in normal-wight and overweight men and women. Am. J. Clin. Nutr. 2002, 76, 1207–1213. [Google Scholar] [CrossRef]
- Rolls, B.J.; Roe, L.S.; Kral, T.V.; Meengs, J.S.; Wall, D.E. Increasing the portion size of a packaged snack increases energy intake in men and women. Appetite 2004, 42, 63–69. [Google Scholar] [CrossRef]
- Wansink, B.; Kim, J. Bad Popcorn in Big Buckets: Portion Size Can Influence Intake as Much as Taste. J. Nutr. Educ. Behav. 2005, 37, 242–245. [Google Scholar] [CrossRef]
- Vercammen, K.A.; Frelier, J.M.; Moran, A.J.; Dunn, C.G.; Musicus, A.A.; Wolfson, J.; Ullah, O.S.; Bleich, S.N. Understanding price incentives to upsize combination meals at large US fast-food restaurants. Public Health Nutr. 2020, 23, 348–355. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic inter-vention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omotola, O.; Legan, S.; Slade, E.; Adekunle, A.; Pendergast, J.S. Estradiol regulates daily rhythms underlying diet-induced obesity in female mice. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1172–E1181. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syn-drome. Cell Metab. 2020, 31, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Lin, S.; Varady, K.A. Changes in body weight and metabolic risk during time restricted feeding in premenopausal versus postmenopausal women. Exp. Gerontol. 2021, 154, 111545. [Google Scholar] [CrossRef]
- Bassett, D.R.; Schneider, P.L.; Huntington, G.E. Physical activity in an Old Order Amish community. Med. Sci. Sports Exerc. 2004, 36, 79–85. [Google Scholar] [CrossRef]
- Swanson, K.C.; Cormack, G.R. The relations between driving behavior, physical activity, and weight status among Canadian adults. J. Phys. Act. Health 2012, 9, 352–359. [Google Scholar] [CrossRef]
- Bell, A.C.; Ge, K.; Popkin, B.M. The Road to Obesity or the Path to Prevention: Motorized Transportation and Obesity in China. Obes. Res. 2002, 10, 277–283. [Google Scholar] [CrossRef]
- Lindström, M. Means of transportation to work and overweight and obesity: A population-based study in southern Sweden. Prev. Med. 2008, 46, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Frank, L.D.; Andresen, M.; Schmid, T.L. Obesity relationships with community design, physical activity, and time spent in cars. Am. J. Prev. Med. 2004, 27, 87–96. [Google Scholar] [CrossRef]
- Van Dijk, J.W.; Manders, R.J.; Hartgens, F.; Stehouwer, C.D.; Praet, S.F.; van Loon, L.J. Postprandial hyperglycemia is highly prevalent throughout the day in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2011, 93, 31–37. [Google Scholar] [CrossRef] [Green Version]
- DECODE Study Group; European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef]
- Després, J.-P.; Lamarche, B.; Mauriège, P.; Cantin, B.; Dagenais, G.R.; Moorjani, S.; Lupien, P.-J. Hyperinsulinemia as an Independent Risk Factor for Ischemic Heart Disease. N. Engl. J. Med. 1996, 334, 952–958. [Google Scholar] [CrossRef]
- Bhat, S.L.; Abbasi, F.A.; Blasey, C.; Reaven, G.M.; Kim, S. Beyond fasting plasma glucose: The association between coronary heart disease risk and postprandial glucose, postprandial insulin and insulin resistance in healthy, nondiabetic adults. Metabolism 2013, 62, 1223–1226. [Google Scholar] [CrossRef]
- Pyörälä, M.; Miettinen, H.; Laakso, M.; Pyörälä, K. Hyperinsulinemia predicts coronary heart disease in healthy middle-aged men: The 22-year follow-up results of the Helsinki Policemen Study. Circulation 1998, 98, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Zavaroni, I.; Bonini, L.; Gasparini, P.; Barilli, A.; Zuccarelli, A.; Dall’Aglio, E.; Delsignore, R.; Reaven, G. Hyperinsulinemia in a normal population as a predictor of non—insulin-dependent diabetes mellitus, hypertension, and coronary heart disease: The barilla factory revisited. Metabolism 1999, 48, 989–994. [Google Scholar] [CrossRef]
- Steven, S.; Carey, P.E.; Small, P.K.; Taylor, R. Reversal of Type 2 diabetes after bariatric surgery is determined by the degree of achieved weight loss in both short- and long-duration diabetes. Diabet. Med. 2014, 32, 47–53. [Google Scholar] [CrossRef]
- Cohen, E.; Cragg, M.; Defonseka, J.; Hite, A.; Rosenberg, M.; Zhou, B. Statistical review of US macronutrient consumption data, 1965–2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrients 2015, 31, 727–732. [Google Scholar] [CrossRef]
- US Department of Agriculture; US Department of Health and Human Services. Dietary Guidelines for Americans, 7th ed.; Government Printing Office: Washington, DC, USA, 2010.
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Vandevijvere, S.; Chow, C.C.; Hall, K.D.; Umali, E.; Swinburn, B.A. Increased food energy supply as a major driver of the obesity epidemic: A global analysis. Bull. World Health Organ. 2015, 93, 446–456. [Google Scholar] [CrossRef]
- Zobel, E.H.; Hansen, T.; Rossing, P.; von Scholten, B.J. Global Changes in Food Supply and the Obesity Epidemic. Curr. Obes. Rep. 2016, 5, 449–455. [Google Scholar] [CrossRef]
- Lustig, R. Ultraprocessed Food: Addictive, Toxic, and Ready for Regulation. Nutrients 2020, 12, 3401. [Google Scholar] [CrossRef]
- Rauber, F.; Chang, K.; Vamos, E.P.; da Costa Louzada, M.L.; Monteiro, C.A.; Millett, C.; Bertazzi Levy, R. Ultra-processed food consumption and risk of obesity: A prospective cohort study of UK Biobank. Eur. J. Nutr. 2021, 60, 2169–2180. [Google Scholar] [CrossRef]
- WHO. Global Action Plan for the Prevention and Control of NCDs 2013–2020; World Health Organization: Geneva, Switzerland, 2013; Available online: http://www.who.int/nmh/events/ncd_action_plan/en/ (accessed on 12 September 2021).
- Stuckler, D.; McKee, M.; Ebrahim, S.; Basu, S. Manufacturing epidemics: The role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med. 2012, 9, e1001235. [Google Scholar] [CrossRef] [Green Version]
- Capewell, S.; Lloyd-Williams, F. The role of food industry in health: Lessons from tobbacco? Br. Med. Bull. 2018, 125, 131–143. [Google Scholar] [CrossRef]
- Gilmore, L.A.; Duhé, A.F.; Frost, E.A.; Redman, L.M. The technology boom: A new era in obesity management. J. Diabetes Sci. Technol. 2014, 8, 596–608. [Google Scholar] [CrossRef] [Green Version]
- Wongvibulsin, S.; Martin, S.S.; Saria, S.; Zeger, S.I.; Murphy, S.A. An individualized, data- driven digital approach for precision behavior change. Am. J. Lifestyle Med. 2019, 14, 289–293. [Google Scholar] [CrossRef]
- Harrison, K.; Taylor, L.D.; Marske, A.L. Women’s and Men’s Eating Behavior Following Exposure to Ideal-Body Images and Text. Commun. Res. 2006, 33, 507–529. [Google Scholar] [CrossRef]
- Brunner, T.A.; Siegrist, M. Reduced food intake after exposure to subtle weight-related cues. Appetite 2012, 58, 1109–1112. [Google Scholar] [CrossRef]
- Pacanowski, C.R.; Bertz, F.; Levitsky, D.A. Daily self-weighing to control body weight in adults: A critical review of the literature. SAGE Open 2014, 4, 1–16. [Google Scholar] [CrossRef]
- McGuire, M.T.; Wing, R.R.; Klem, M.L.; Seagle, H.M.; Hill, J.O. Long-term maintenance of weight loss: Do people who lose weight through various weight-loss methods use different behaviors to maintain their wight? Int. J. Obes. 1998, 22, 572–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacanowski, C.R.; Levitsky, D.A. Frequent Self-Weighing and Visual Feedback for Weight Loss in Overweight Adults. J. Obes. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitsky, D.A.; Pacanowski, C. Free will and the obesity epidemic. Public Health Nutr. 2011, 15, 126–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, V.; Marchetti, C.M.; Krishnan, R.K.; Stetzer, B.P.; Gonzalez, F.; Kirwan, J.P. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J. Appl. Physiol. 2006, 100, 1584–1589. [Google Scholar] [CrossRef]











| Tasks | Old Order Amish Way | Contemporary Way |
|---|---|---|
| Transportation | Horse and buggy, Walking | Car, public transit, some active transportation |
| Food provision | Farming, gardening, milking cattle by hand | Convenience and grocery stores, some gardening |
| Food preparation | Home cooking with wood-burning stoves | Restaurants, ready-to eat food, some home cooking |
| Food storage | Ice blocks cut from frozen lake ice | Refrigerators and freezers |
| House cleaning | Brooms and mops | Vacuum cleaners |
| Laundry | Hand washing, wringing, and air drying | Washing machines and dryers |
| Heating | Chopping firewood | HVAC |
| Mowing grass | Scythe, hand mowers | Motorized lawn mowers |
| Removing fallen leaves | Hand raking | Motorized air blowers |
| Spiritual life and Socializing | Church, mutual communal assistance | Television, internet, social media, some church |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borer, K.T. Why We Eat Too Much, Have an Easier Time Gaining Than Losing Weight, and Expend Too Little Energy: Suggestions for Counteracting or Mitigating These Problems. Nutrients 2021, 13, 3812. https://doi.org/10.3390/nu13113812
Borer KT. Why We Eat Too Much, Have an Easier Time Gaining Than Losing Weight, and Expend Too Little Energy: Suggestions for Counteracting or Mitigating These Problems. Nutrients. 2021; 13(11):3812. https://doi.org/10.3390/nu13113812
Chicago/Turabian StyleBorer, Katarina T. 2021. "Why We Eat Too Much, Have an Easier Time Gaining Than Losing Weight, and Expend Too Little Energy: Suggestions for Counteracting or Mitigating These Problems" Nutrients 13, no. 11: 3812. https://doi.org/10.3390/nu13113812
APA StyleBorer, K. T. (2021). Why We Eat Too Much, Have an Easier Time Gaining Than Losing Weight, and Expend Too Little Energy: Suggestions for Counteracting or Mitigating These Problems. Nutrients, 13(11), 3812. https://doi.org/10.3390/nu13113812





