Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives
Abstract
1. Introduction
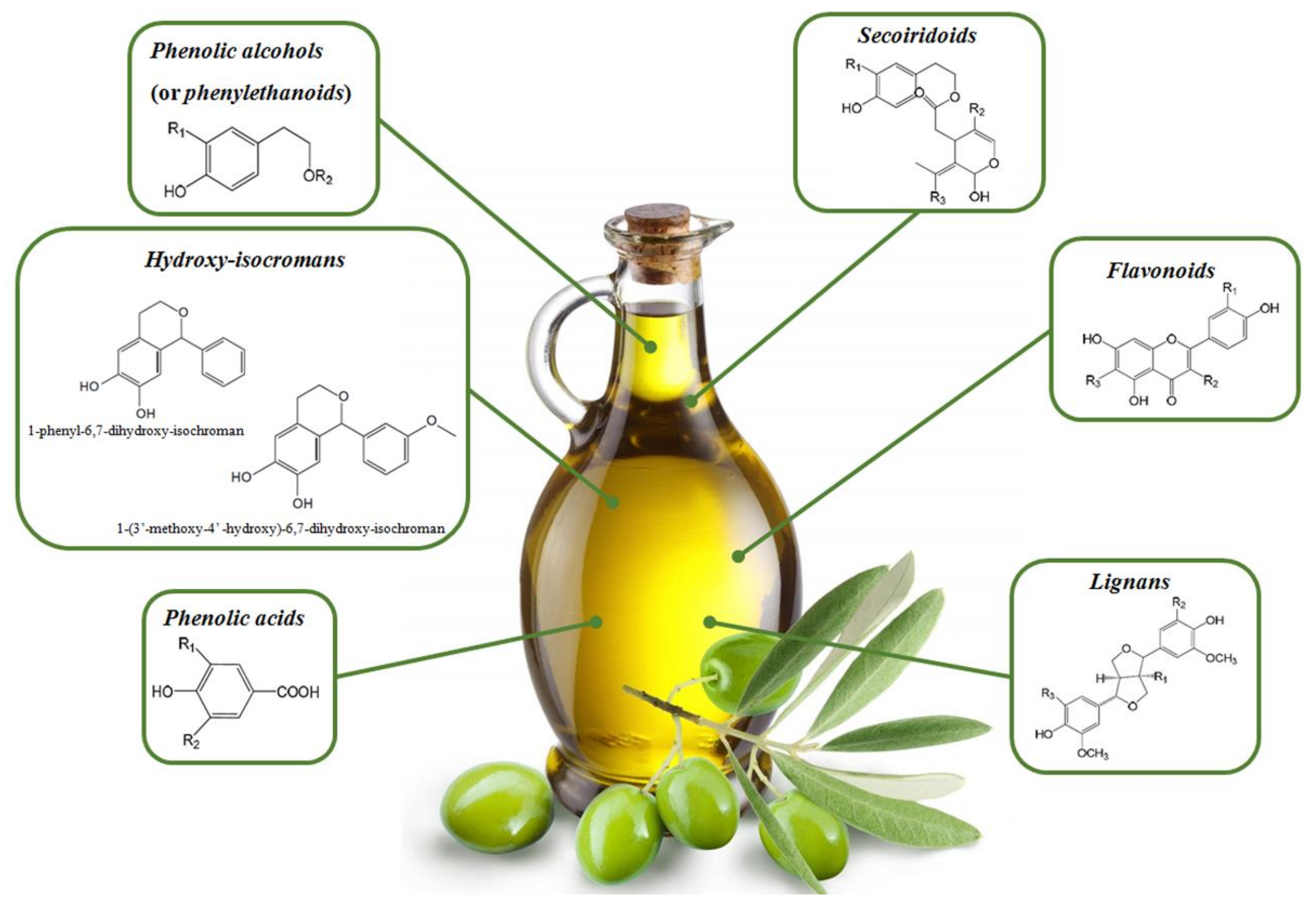
2. Olive Oil Polyphenols
- Secoiridoids are phenolic compounds found in abundance in O. europea with respect to other plant species. They are chemically characterized by a phenyl ethyl alcohol (3,4-DHPEA or p-HPEA) linked to elenolic acid or its derivates; in most cases, they are glycosylated [7]. Secoiridoids are one of the most important micronutrients in EVOO [24]. Demethyloleuropein, oleuropein (Ole), and ligstroside (Lig) are the main glycosides present in olive fruit and their aglycones, accounting for 90% of the phenolic compounds in EVOO [28]. Interestingly, the bitter taste of OO is the result of the secoiridoid content, especially the dialdehydic form of Ole aglycone [29].
- Phenolic alcohols (or phenylethanoids) possess a hydroxyl group attached to an aromatic hydrocarbon group. The main molecules encompassed in this class are hydroxytyrosol (3,4-dihydroxyphenyl ethanol or 3,4 DHPEA; HTyr), tyrosol (p-Hydroxyphenyl ethanol or p-HPEA; Tyr), and oleocanthal [8]. Htyr and Tyr are present in low concentrations in fresh OO, but their amount increases substantially during the storage process because of the hydrolysis of secoiridoids [30].
- Flavonoids have a chemical structure composed of two benzene rings joined by three linear carbon chains. These molecules undergo further modifications, such as glycosylation, giving rise to other compounds divided in other groups, i.e., flavones, flavonols, flavanones, and flavanols [8]. The first flavonoids identified in virgin OO were flavones; their freeform, luteolin and apigenin, are the most concentrated compounds [31].
- Lignans are chemically characterized by the condensation of aromatic aldehydes. The pulp of olives as well as the woody portion of the seed contain lignans; these molecules are released into the oil during the extraction process without biochemical modifications [31]. (+)-pinoresinol and (+)-1-Acetoxypinoresinol are the lignans most concentrated in EVOO [24].
- Phenolic acids are further subdivided into two groups of hydroxybenzoic acid derivatives (e.g., p-hydroxybenzoic, protocatechuic, vanillic, syringic, and gallic acid) and hydroxycinnamic acid derivatives (e.g., p-coumaric, ferulic, cinnamic caffeic, and synaptic acid [7].
- Hydroxy-isocromans consists of the only two molecules characterized in commercial virgin OO, i.e., 1-phenyl-6,7-dihydroxy-isochroman and 1-(3′-methoxy-4′ -hydroxy)-6,7-dihydroxy-isochroman. These compounds are formed from the HTyr reaction with benzaldehyde and vanillin, respectively [25].
3. The Endogenous and Exogenous Factors Influencing the Biosynthesis of OOPs
4. Absorption and Metabolism of OOPs
4.1. Absorption
4.2. Metabolism
4.2.1. HTyr
4.2.2. Tyr
4.2.3. Ole
4.2.4. Others
5. OOPs and Clinical Trials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruskovska:, T.; Maksimova, V.; Milenkovic, D. Polyphenols in human nutrition: From the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability—An overview and perspective. Br. J. Nutr. 2020, 123, 241–254. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Mozaffarian, D. Nutrition′s dark matter of polyphenols and health. Nat. Food 2021, 2, 139–140. [Google Scholar] [CrossRef]
- do Valle, I.F.; Roweth, H.G.; Malloy, M.W.; Moco, S.; Barron, D.; Battinelli, E.; Loscalzo, J.; Barabasi, A.L. Network medicine framework shows that proximity of polyphenol targets and disease proteins predicts therapeutic effects of polyphenols. Nat. Food 2021, 2, 143. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordonez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lopez, P.; Lozano-Sanchez, J.; Borras-Linares, I.; Emanuelli, T.; Menendez, J.A.; Segura-Carretero, A. Structure-Biological Activity Relationships of Extra-Virgin Olive Oil Phenolic Compounds: Health Properties and Bioavailability. Antioxidants 2020, 9, 685. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef]
- Guerrero Maldonado, N.; López, M.J.; Caudullo, G.; de Rigo, D. Olea europaea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; European Commision: Luxembourg, 2016; p. e01534b+. [Google Scholar]
- Lambardi, M.; Ozudogru, E.A.; Roncasaglia, R. In vitro propagation of olive (Olea europaea L.) by nodal segmentation of elongated shoots. Methods Mol. Biol. 2013, 11013, 33–44. [Google Scholar] [CrossRef]
- Lozano-Castellon, J.; Lopez-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero Del Castillo-Alba, J.; Vallverdu-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventos, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Scholl, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Estruch, R.; Corella, D.; Fito, M.; Ros, E.; Predimed, I. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remon, A.; Martinez-Gonzalez, M.A.; de la Torre, R.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; Aros, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remon, A.; Martinez-Gonzalez, M.A.; Lopez-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Ozcelik, B.; Frank, J.; Rimbach, G. Comprehensive analysis of polyphenols in 55 extra virgin olive oils by HPLC-ECD and their correlation with antioxidant activities. Plant Foods Hum. Nutr. 2012, 67, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Karkovic Markovic, A.; Toric, J.; Barbaric, M.; Jakobusic Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef]
- Fabiani, R. Anti-cancer properties of olive oil secoiridoid phenols: A systematic review of in vivo studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenco-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Souza, P.A.L.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef]
- Perona, J.S.; Cabello-Moruno, R.; Ruiz-Gutierrez, V. The role of virgin olive oil components in the modulation of endothelial function. J. Nutr. Biochem. 2006, 17, 429–445. [Google Scholar] [CrossRef]
- de la Torre-Carbot, K.; Jauregui, O.; Gimeno, E.; Castellote, A.I.; Lamuela-Raventos, R.M.; Lopez-Sabater, M.C. Characterization and quantification of phenolic compounds in olive oils by solid-phase extraction, HPLC-DAD, and HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Cert, A.; Pérez-Camino, C.M.; García, J.M. Evaluation of Virgin Olive Oil Bitterness by Quantification of Secoiridoid Derivatives. J. Am. Oil Chem. Soc. 2004, 81, 71–75. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and biotransformations of phenol-conjugated oleosidic secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167–1179. [Google Scholar] [CrossRef]
- de Torres, A.; Espínola, F.; Moya, M.X.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT—Food Sci. Technol. 2018, 80, 22–30. [Google Scholar] [CrossRef]
- EFSA. EFSA Panel on Dietetic Products, Nutrition and allergies (NDA). Scientific opinion on the substantiation of health claims related to polyphenols in olive oil and protection of LDL particles from oxidative damage. EFSA J. 2011, 10, 2848. [Google Scholar]
- UE. European Commission Regulation EC No. 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children′s Development and Health; Official Journal of the European Union: Brussels, Belgium, 2012. [Google Scholar]
- Ortega-García, F.; Blanco, S.; Peinado, M.A.; Peragón, J. Chapter 25—Polyphenol Oxidase and Oleuropein in Olives and their Changes During Olive Ripening. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier: London, UK, 2010; pp. 233–238. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Hbaieb, R.H.; Kotti, F.; Mugnozza, G.S.; Gargouri, M. Mechanical Strategies to Increase Nutritional and Sensory Quality of Virgin Olive Oil by Modulating the Endogenous Enzyme Activities. Compr. Rev. Infood Sci. Food Saf. 2014, 13, 135–154. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Influence of enzymes and technology on virgin olive oil composition. Crit. Rev. Food Sci. Nutr. 2017, 57, 3104–3126. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Yerena, A.; Lozano-Castellon, J.; Olmo-Cunillera, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Jimenez, B.; Perez, M.; Vallverdu-Queralt, A. Effects of Organic and Conventional Growing Systems on the Phenolic Profile of Extra-Virgin Olive Oil. Molecules 2019, 24, 1986. [Google Scholar] [CrossRef] [PubMed]
- De Torres, A.; Espínola, F.; Moya, M.; Castro, E. Composition of secoiridoid derivatives from Picual virgin olive oil using response surface methodology with regard to malaxation conditions, fruit ripening, and irrigation management. Eur. Food Res. Technol. 2016, 242, 1709–1718. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Quantitative measurement of major secoiridoid derivatives in olive oil using qNMR. Proof of the artificial formation of aldehydic oleuropein and ligstroside aglycon isomers. J. Agric. Food Chem. 2014, 62, 600–607. [Google Scholar] [CrossRef]
- Lukić, I.; Krapac, M.; Horvat, I.; Godena, S.; Kosić, U.; Bubola, K.B. Three-factor approach for balancing the concentrations of phenols and volatiles in virgin olive oil from a late-ripening olive cultivar. LWT—Food Sci. Technol. 2018, 87, 194–202. [Google Scholar] [CrossRef]
- Bakhouche, A.; Lozano-Sánchez, J.; Beltrán-Debón, R.; Joven, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic characterization and geographical classification of commercial Arbequina extra-virgin olive oils produced in southern Catalonia. Food Res. Int. 2013, 50, 401–408. [Google Scholar] [CrossRef]
- Lia, F.; Zammit-Mangion, M.; Farrugia, C.A. First Description of the Phenolic Profile of EVOOs from the Maltese Islands Using SPE and HPLC: Pedo-Climatic Conditions Modulate Genetic Factors. Agriculture 2019, 9, 107. [Google Scholar] [CrossRef]
- Kotsiou, K.; Tasioula-Margari, M. Monitoring the phenolic compounds of Greek extra-virgin olive oils during storage. Food Chem. 2016, 200, 255–262. [Google Scholar] [CrossRef]
- De la Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jia, X.; Zheng, Z.; Lu, X.; Zheng, Y.; Zheng, B.; Xiao, J. Chemical composition and nutritional function of olive (Olea europaea L.): A review. Phytochem. Rev. 2018, 17, 1091–1110. [Google Scholar] [CrossRef]
- López de las Hazas, M.C.; Piñol, C.; Macià, A.; Romero, M.P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Corona, G.; Tzounis, X.; Assunta Dessi, M.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Deiana, M.; Serra, G.; Corona, G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef]
- Manna, C.; Galletti, P.; Maisto, G.; Cucciolla, V.; D′Angelo, S.; Zappia, V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. 2000, 470, 341–344. [Google Scholar] [CrossRef]
- Gonzalez-Santiago, M.; Fonolla, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharm. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Grande, S.; Colonnelli, K.; Patelli, C.; Galli, G.; Caruso, D. Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J. Nutr. 2003, 133, 2612–2615. [Google Scholar] [CrossRef]
- Edgecombe, S.C.; Stretch, G.L.; Hayball, P.J. Oleuropein, an antioxidant polyphenol from olive oil, is poorly absorbed from isolated perfused rat intestine. J. Nutr. 2000, 130, 2996–3002. [Google Scholar] [CrossRef]
- Mosele, J.I.; Martin-Pelaez, S.; Macia, A.; Farras, M.; Valls, R.M.; Catalan, U.; Motilva, M.J. Faecal microbial metabolism of olive oil phenolic compounds: In vitro and in vivo approaches. Mol. Nutr. Food Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Incani, A.; Serra, G.; Atzeri, A.; Melis, M.P.; Serreli, G.; Bandino, G.; Sedda, P.; Campus, M.; Tuberoso, C.I.; Deiana, M. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem. Toxicol. 2016, 90, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M.; Piccirillo, C.; Castro, P.M.; Kalogerakis, N.; Pintado, M.E. Bioconversion of oleuropein to hydroxytyrosol by lactic acid bacteria. World J. Microbiol. Biotechnol. 2012, 28, 2435–2440. [Google Scholar] [CrossRef]
- Medina, E.; Brenes, M.; Romero, C.; Garcia, A.; de Castro, A. Main antimicrobial compounds in table olives. J. Agric. Food Chem. 2007, 55, 9817–9823. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, J.; Kohnen, S.; Hauser, C. Antimicrobial activity of Olea europaea Linne extracts and their applicability as natural food preservative agents. Int. J. Food Microbiol. 2017, 251, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Furneri, P.M.; Marino, A.; Saija, A.; Uccella, N.; Bisignano, G. In vitro antimycoplasmal activity of oleuropein. Int. J. Antimicrob. Agents 2002, 20, 293–296. [Google Scholar] [CrossRef]
- Tranter, H.S.; Tassou, S.C.; Nychas, G.J. The effect of the olive phenolic compound, oleuropein, on growth and enterotoxin B production by Staphylococcus aureus. J. Appl. Bacteriol. 1993, 74, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Farras, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the Gut Microbiota by Olive Oil Phenolic Compounds: Implications for Lipid Metabolism, Immune System, and Obesity. Nutrients 2020, 12, 2200. [Google Scholar] [CrossRef]
- Serra, A.; Rubio, L.; Borras, X.; Macia, A.; Romero, M.P.; Motilva, M.J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef] [PubMed]
- D′Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar]
- Schaffer, S.; Asseburg, H.; Kuntz, S.; Muller, W.E.; Eckert, G.P. Effects of polyphenols on brain ageing and Alzheimer′s disease: Focus on mitochondria. Mol. Neurobiol. 2012, 46, 161–178. [Google Scholar] [CrossRef]
- Schaffer, S.; Muller, W.E.; Eckert, G.P. Cytoprotective effects of olive mill wastewater extract and its main constituent hydroxytyrosol in PC12 cells. Pharmacol. Res. 2010, 62, 322–327. [Google Scholar] [CrossRef]
- Rodriguez-Morato, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Perez-Mana, C.; Khymenets, O.; Fito, M.; Farre, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef]
- Rubio, L.; Macia, A.; Valls, R.M.; Pedret, A.; Romero, M.P.; Sola, R.; Motilva, M.J. A new hydroxytyrosol metabolite identified in human plasma: Hydroxytyrosol acetate sulphate. Food Chem. 2012, 134, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Khymenets, O.; Farré, M.; Pujadas, M.; Ortiz, E.; Joglar, J.; Covas, M.I.; de la Torre, R. Direct analysis of glucuronidated metabolites of main olive oil phenols in human urine after dietary consumption of virgin olive oil. Food Chem. 2011, 126, 306–314. [Google Scholar] [CrossRef]
- Caruso, D.; Visioli, F.; Patelli, R.; Galli, C.; Galli, G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 2001, 50, 1426–1428. [Google Scholar] [CrossRef]
- De la Torre, R.; Corella, D.; Castaner, O.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Vila, J.; Estruch, R.; Sorli, J.V.; Aros, F.; Fiol, M.; et al. Protective effect of homovanillyl alcohol on cardiovascular disease and total mortality: Virgin olive oil, wine, and catechol-methylation. Am. J. Clin. Nutr. 2017, 105, 1297–1304. [Google Scholar] [CrossRef]
- Kotronoulas, A.; Pizarro, N.; Serra, A.; Robledo, P.; Joglar, J.; Rubio, L.; Hernaez, A.; Tormos, C.; Motilva, M.J.; Fito, M.; et al. Dose-dependent metabolic disposition of hydroxytyrosol and formation of mercapturates in rats. Pharmacol. Res. 2013, 77, 47–56. [Google Scholar] [CrossRef]
- Covas, M.I.; Miro-Casas, E.; Fito, M.; Farre-Albadalejo, M.; Gimeno, E.; Marrugat, J.; De La Torre, R. Bioavailability of tyrosol, an antioxidant phenolic compound present in wine and olive oil, in humans. Drugs Exp. Clin. Res. 2003, 29, 203–206. [Google Scholar]
- Muriana, F.J.G.; Montserrat-de la Paz, S.; Lucas, R.; Bermudez, B.; Jaramillo, S.; Morales, J.C.; Abia, R.; Lopez, S. Tyrosol and its metabolites as antioxidative and anti-inflammatory molecules in human endothelial cells. Food Funct. 2017, 8, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Perez-Mana, C.; Farre, M.; Pujadas, M.; Mustata, C.; Menoyo, E.; Pastor, A.; Langohr, K.; de la Torre, R. Ethanol induces hydroxytyrosol formation in humans. Pharmacol. Res. 2015, 95–96, 27–33. [Google Scholar] [CrossRef]
- Perez-Mana, C.; Farre, M.; Rodriguez-Morato, J.; Papaseit, E.; Pujadas, M.; Fito, M.; Robledo, P.; Covas, M.I.; Cheynier, V.; Meudec, E.; et al. Moderate consumption of wine, through both its phenolic compounds and alcohol content, promotes hydroxytyrosol endogenous generation in humans. A randomized controlled trial. Mol. Nutr. Food Res. 2015, 59, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Tacker, M.; Creaven, P.J.; McIsaac, W.M. Alteration in tyramine metabolism by ethanol. Biochem. Pharmacol. 1970, 19, 604–607. [Google Scholar] [CrossRef]
- Rodriguez-Morato, J.; Robledo, P.; Tanner, J.A.; Boronat, A.; Perez-Mana, C.; Oliver Chen, C.Y.; Tyndale, R.F.; de la Torre, R. CYP2D6 and CYP2A6 biotransform dietary tyrosol into hydroxytyrosol. Food Chem. 2017, 217, 716–725. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Lemonakis, N.; Mougios, V.; Halabalaki, M.; Skaltsounis, A.L.; Gikas, E. A novel bioanalytical method based on UHPLC-HRMS/MS for the quantification of oleuropein in human serum. Application to a pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 2016–2023. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef]
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; Mayas, M.D.; Martínez-Martos, J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci. Technol. 2013, 31, 92–99. [Google Scholar] [CrossRef]
- de Bock, M.; Thorstensen, E.B.; Derraik, J.G.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Aponte, M.; Ungaro, F.; d′Angelo, I.; De Caro, C.; Russo, R.; Blaiotta, G.; Dal Piaz, F.; Calignano, A.; Miro, A. Improving in vivo conversion of oleuropein into hydroxytyrosol by oral granules containing probiotic Lactobacillus plantarum 299v and an Olea europaea standardized extract. Int. J. Pharm. 2018, 543, 73–82. [Google Scholar] [CrossRef]
- Silva, S.; Garcia-Aloy, M.; Figueira, M.E.; Combet, E.; Mullen, W.; Bronze, M.R. High Resolution Mass Spectrometric Analysis of Secoiridoids and Metabolites as Biomarkers of Acute Olive Oil Intake-An Approach to Study Interindividual Variability in Humans. Mol. Nutr. Food Res. 2018, 62, 1700065. [Google Scholar] [CrossRef]
- Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Nevedomskaya, E.; Mayboroda, O.A.; Deelder, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Exploratory analysis of human urine by LC-ESI-TOF MS after high intake of olive oil: Understanding the metabolism of polyphenols. Anal. Bioanal. Chem. 2010, 398, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 15 March 2021).
- Crespo, M.C.; Tome-Carneiro, J.; Burgos-Ramos, E.; Loria Kohen, V.; Espinosa, M.I.; Herranz, J.; Visioli, F. One-week administration of hydroxytyrosol to humans does not activate Phase II enzymes. Pharmacol. Res. 2015, 95–96, 132–137. [Google Scholar] [CrossRef]
- Filip, R.; Possemiers, S.; Heyerick, A.; Pinheiro, I.; Raszewski, G.; Davicco, M.J.; Coxam, V. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J. Nutr. Health Aging. 2015, 19, 77–86. [Google Scholar] [CrossRef]
- Santiago-Mora, R.; Casado-Diaz, A.; De Castro, M.D.; Quesada-Gomez, J.M. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2011, 22, 675–684. [Google Scholar] [CrossRef]
- Drira, R.; Chen, S.; Sakamoto, K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3 T3-L1 cells. Life Sci. 2011, 89, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig. Liver Dis. 2020, 53, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; de la Torre, R.; Fito, M.; Covas, M.I.; Vilchez, P.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2019, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Lima-Cabello, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Roca, M.; Espejo-Calvo, J.A.; Gil-Extremera, B.; Soria-Florido, M.; de la Torre, R.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Metabolic Syndrome and Endothelial Functional Risk Biomarkers in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2018, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.; Finicelli, M.; Grossi, M.; Vicchio, M.; Alessio, N.; Sante, P.; De Feo, M.; Cotrufo, M.; Berrino, L.; Rossi, F.; et al. DNA damage and repair in a model of rat vascular injury. Clin. Sci. 2010, 118, 473–485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, L.; Wang, Q.; Zhang, S.; Zhang, L. Correlation between serum inflammatory factors TNF-alpha, IL-8, IL-10 and Henoch-Schonlein purpura with renal function impairment. Exp. Ther. Med. 2018, 15, 3924–3928. [Google Scholar] [CrossRef] [PubMed]



| Category | ID | Title | Condition | Study Start |
|---|---|---|---|---|
| Obesity/Mito. disorders | NCT04149288 | Olive Oil Polyphenols and Cardiovascular Health Biomarkers |
| May 2021 |
| NCT03101436 | Extra Virgin Olive Oil, Red Wine Polyphenols and Fecal Microbiota |
| 2 February 2015 | |
| NCT04317079 | Effects of Hydroxytyrosol Administration in Anthropometric Parameters in Overweight and Obese Women |
| 30 October 2017 | |
| NCT04543968 | Clinical Study of Extra-virgin Olive Oil in Mitochondrial Diseases |
| 1 January 2021 | |
| Bone disorders | NCT01828944 | Olive Oil Polyphenols, Vitamin D, Docosahexaenoic Acid (DHA) and Locomotor Function (PolivD3) |
| December 2012 |
| NCT03072108 | Dietary Supplement for Joint: the OLE Study |
| 24 June 2016 | |
| NCT00789425 | Investigating the Effect of Standardized Olive Extract on Bone Turnover Markers in Postmenopausal Women |
| September 2008 | |
| Cancer | NCT04027088 | Effect of Preoperative Immunonutrition in Upper Digestive Tract |
| 10 August 2019 |
| NCT02520739 | New Industrial Procedures for Achieving a Nutritional Added Value of the Olive Oil. The NUTRAOLEUM Study |
| February 2014 | |
| NCT04215367 | Dietary Intervention with High Phenolic EVOO in CLL |
| 15 December 2018 | |
| NCT02068092 | Olive Oil for High Risk Breast Cancer Prevention in Women |
| December 2013 | |
| Diabetes/Hyperglycemia | NCT04764786 | Polyphenol Enriched Extra- Virgin Olive Oil and Postprandial Glycemia in Type 1 Diabetes (DOP) |
| 1 April 2019 |
| NCT02669693 | Effect of Olives on Glycaemic Response in Vivo |
| December 2015 | |
| NCT03093753 | Effect of a Beverage Comprised of Compounds from Olives on Post- prandial Blood Glucose Responses in Healthy Volunteers |
| July 2016 | |
| NCT04419948 | Oleocanthal Rich Olive Oil Acute Effects on Hyperglycemia and Platelet Activation in T2DM |
| 16 May 2019 | |
| Cardiovascular diseases | NCT04760093 | A Multicenter Pilot Study to Evaluate the Effect of EVOO on Lipid Parameters |
| 1 March 2021 |
| NCT01796561 | The Effect of Olive Leaf Extract on Blood Pressure in Overweight Prehypertensives |
| February 2013 | |
| NCT01983943 | Olive Oil and Cardiovascular Health |
| August 2013 | |
| NCT02783989 | Effects on Cardiovascular Risk Factors of the Endogenous Hydroxytyrosol Generation After the Combined Intake of Wine and Tyrosol in Humans |
| 20 January 2016 | |
| NCT04520126 | Effect of Olivomed (Olive Extract) on Endothelial, Cardiac and Vascular Function |
| 1 December 2020 | |
| NCT02421835 | Olive Leaf Extract as Part of a Healthy Lifestyle in the Reduction of Blood Pressure |
| April 2013 | |
| NCT03528603 | Acute Assessment of Platelet Reactivity After the Intake of Oleocanthal |
| 2 April 2018 | |
| NCT02902913 | Impact of Extra Virgin Olive Oil Oleocanthal Content on Platelet Reactivity |
| January 2015 | |
| Healthy | NCT01347515 | Bioactivity of Olive Oils Enriched with Their Own Phenolic Compounds (VOHF1) |
| April 2011 |
| NCT03886597 | Nutritional Intervention with Table Olives in Healthy Volunteers |
| 25 March 2019 | |
| NCT02273622 | Human Study of Hydroxytyrosol on Phase II Enzymes in Healthy Subjects |
| October 2014 | |
| NCT01790672 | Contribution of Wine Components on Hydroxytyrosol Body Concentrations and Biological Effects |
| May 2011 | |
| NCT02042742 | Punicalagin and Hydroxytyrosol Mixture on Different Inflammatory Markers |
| April 2013 | |
| NCT01788670 | Relevance of the Ethanol Dose in the Generation of Endogenous Hydroxytyrosol |
| May 2009 | |
| NCT04328571 | Effects of Enzymatic Digestion and Probiotic on Oleuropein Bioavailability |
| 10 February 2020 | |
| NCT04725955 | Postprandial Responses to Hydroxytyrosol-enriched Bread |
| 31 January 2021 | |
| Colitis | NCT03408847 | Monocultivar Coratina Extra Virgin Olive Oil in UC Patients |
| 20 November 2017 |
| Liver disease | NCT02842567 | Hydroxytyrosol and Vitamin E in Pediatric NASH |
| 1 April 2017 |
| Neurological diseases | NCT04440020 | Management of Dementia with Olive Oil Leaves-GOLDEN |
| 5 January 2019 |
| NCT03362996 | Management of Mild Cognitive Impairment Patients with Extra Virgin Olive Oil-MICOIL |
| 9 November 2016 | |
| NCT03824197 | Auburn University Research on Olive Oil for Alzheimer’s Disease (AU-ROOAD) |
| 7 May 2019 | |
| NCT04787497 | The Effect of Extra Virgin Olive Oil in People with Multiple Sclerosis |
| December 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. https://doi.org/10.3390/nu13113831
Finicelli M, Squillaro T, Galderisi U, Peluso G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients. 2021; 13(11):3831. https://doi.org/10.3390/nu13113831
Chicago/Turabian StyleFinicelli, Mauro, Tiziana Squillaro, Umberto Galderisi, and Gianfranco Peluso. 2021. "Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives" Nutrients 13, no. 11: 3831. https://doi.org/10.3390/nu13113831
APA StyleFinicelli, M., Squillaro, T., Galderisi, U., & Peluso, G. (2021). Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients, 13(11), 3831. https://doi.org/10.3390/nu13113831








