N-3 Polyunsaturated Fatty Acids Ameliorate Neurobehavioral Outcomes Post-Mild Traumatic Brain Injury in the Fat-1 Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Ethics
2.3. Administration of Traumatic Brain Injury and Evaluating Time to First Movement
2.4. Assessment of Motor and Neurobehavioral Function
2.5. Brain Fatty Acid Analysis
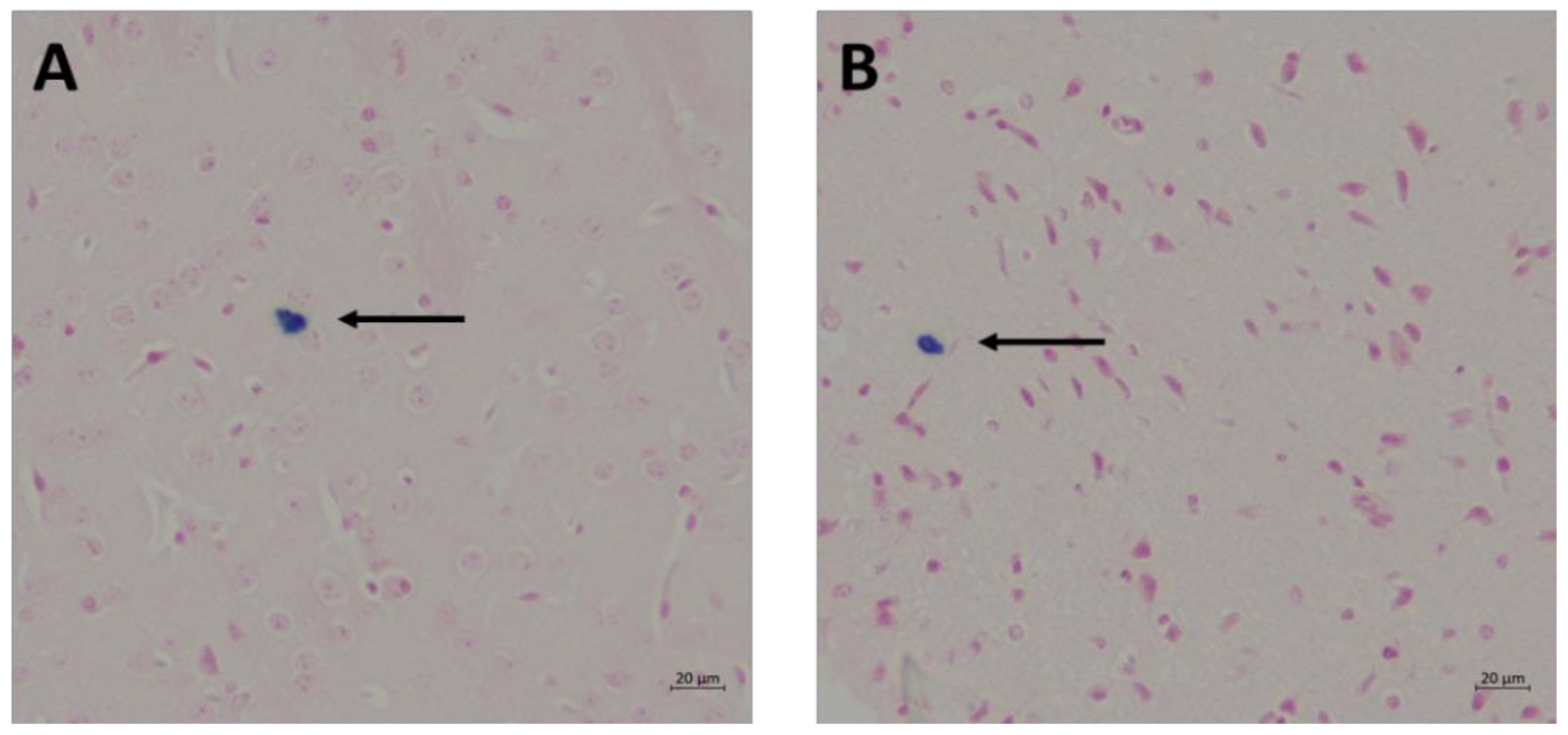
2.6. Prussian Blue and Nuclear Fast Red Staining for Detection of Cerebral Microhemorrhages
2.7. Statistical Analysis
3. Results
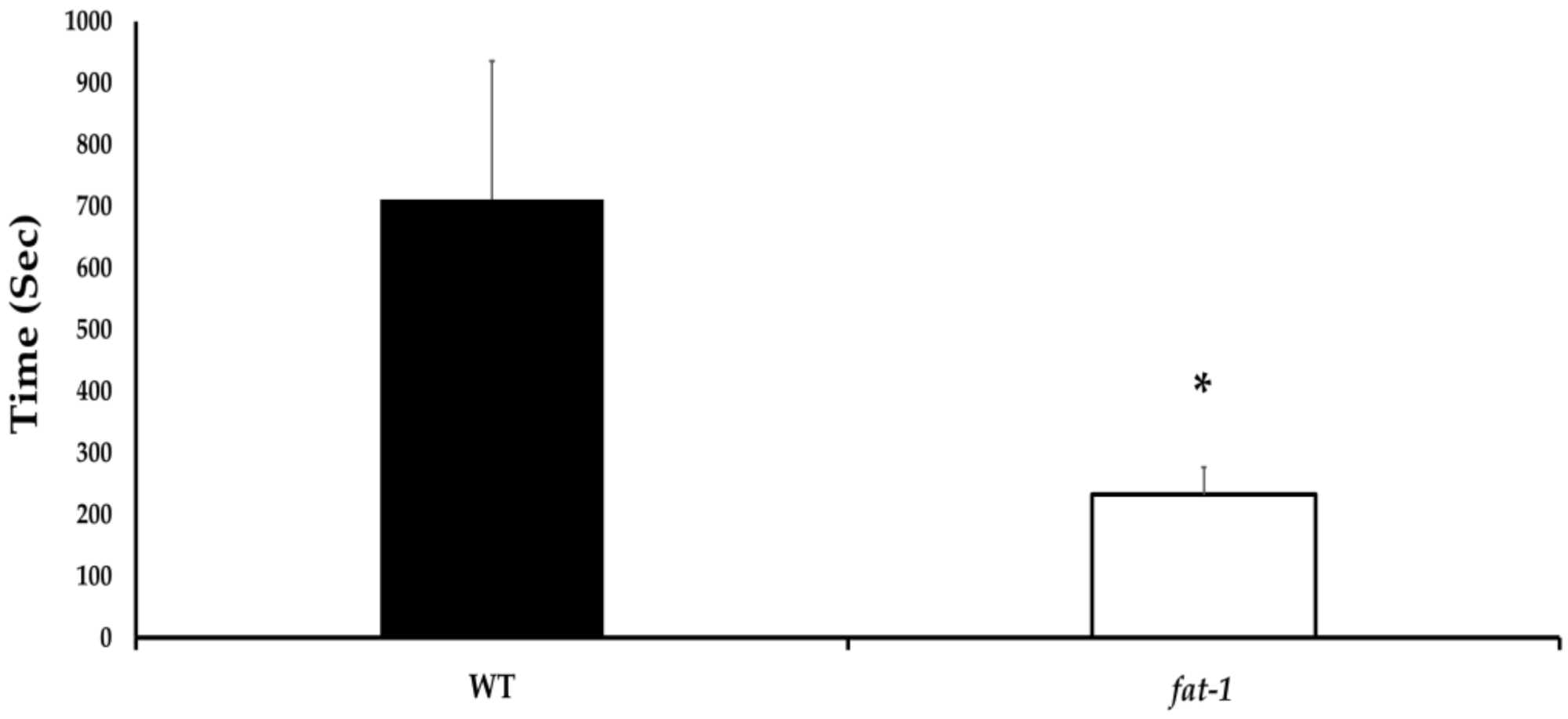
3.1. Time to First Movement
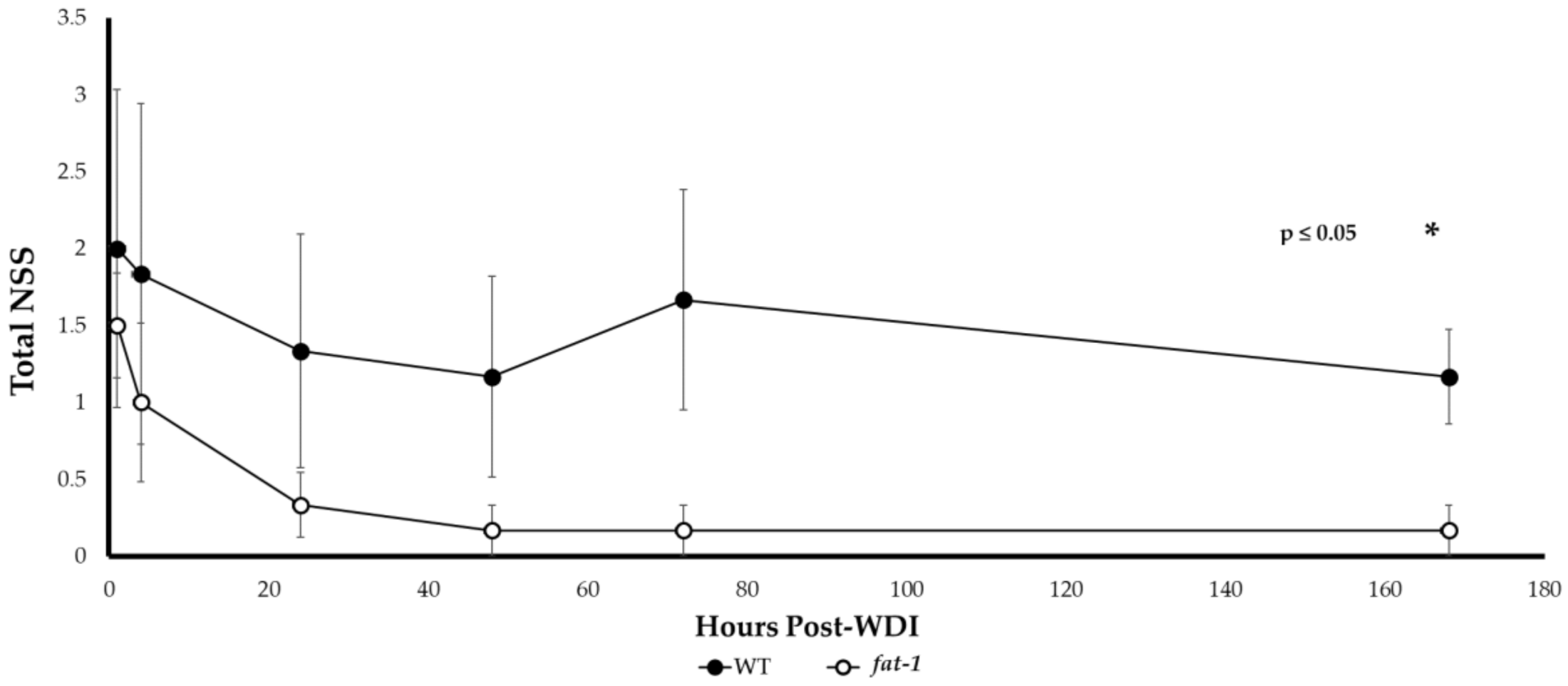
3.2. Neurological Severity Score (NSS)
3.3. Brain Fatty Acid Composition
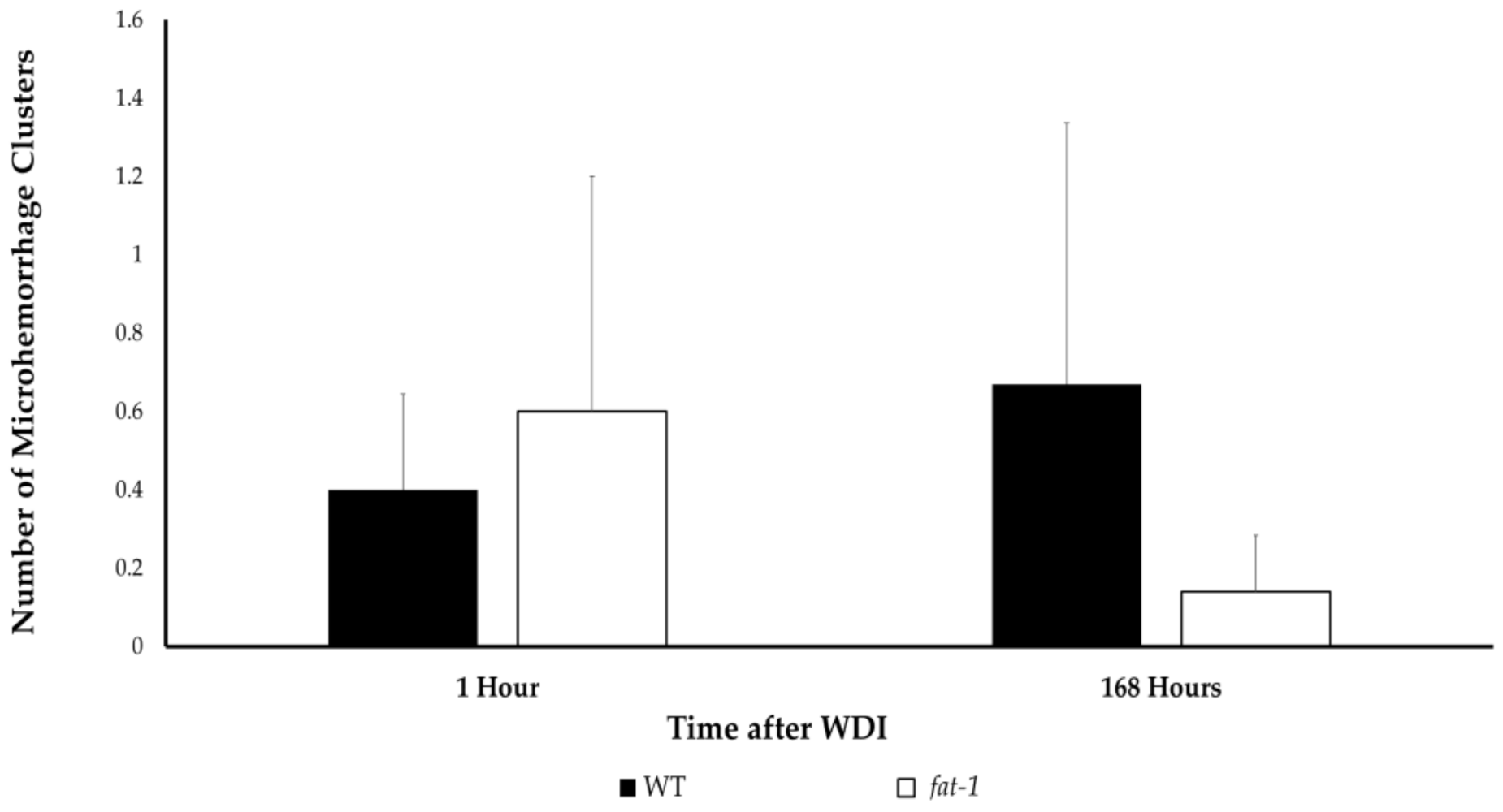
3.4. Cerebral Microhemorrhage
4. Discussion
4.1. Motor and Behavioral Function
4.2. Time to First Movement
4.3. Cerebral Microhemorrhage
4.4. Methodology to Study the Prophylactic Effect of n-3 PUFA in Concussion Prevention
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Rodríguez-Rodríguez, A.; Egea-Guerrero, J.J.; Murillo-Cabezas, F.; Carrillo-Vico, A. Oxidative Stress in Traumatic Brain Injury. Curr. Med. Chem. 2014, 21, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, C.; Cassidy, J.D.; Côté, P.; Hincapié, C.A.; Hartvigsen, J.; Carroll, L.J.; Marras, C.; Boyle, E.; Kristman, V.; Hung, R.; et al. Protocol for a Systematic Review of Prognosis After Mild Traumatic Brain Injury: An Update of the WHO Collaborating Centre Task Force Findings. Syst. Rev. 2012, 1, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronado, V.G.; McGuire, L.C.; Sarmiento, K.; Bell, J.; Lionbarger, M.R.; Jones, C.D.; Geller, A.I.; Khoury, N.; Xu, L. Trends in Traumatic Brain Injury in the U.S. And the Public Health Response: 1995–2009. J. Saf. Res. 2012, 43, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.D.; Carroll, L.J.; Peloso, P.M.; Borg, J.; Holst, H.V.; Holm, L.; Kraus, J.; Coronado, V.G. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, 43, 28–60. [Google Scholar] [CrossRef] [Green Version]
- Mayer, A.R.; Quinn, D.K.; Master, C.L. The spectrum of mild traumatic brain injury. Neurology 2017, 89, 623–632. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Available online: http://www.cdc.gov/ncipc/tbi/Physicians_Tool_Kit.htm (accessed on 21 June 2021).
- Lewis, M.D. Concussions, Traumatic Brain Injury, and the Innovative Use of Omega-3s. J. Am. Coll. Nutr. 2016, 35, 469–475. [Google Scholar] [CrossRef]
- Lauritzen, L.; Hansen, H.S.; Jørgensen, M.H.; Michaelsen, K.F. The Essentiality of Long Chain n-3 Fatty Acids in Relation to Development and Function of the Brain and Retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.L.; Haagsma, J.A.; Diaz-Arrastia, R.; von Steinbuechel, N. A Multidimensional Approach to Post-concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 1113. [Google Scholar] [CrossRef] [Green Version]
- Barrett, E.C.; McBurney, M.I.; Ciappio, E.D. ω-3 Fatty Acid Supplementation as a Potential Therapeutic Aid for the Recovery from Mild Traumatic Brain Injury/Concussion. Adv. Nutr. 2014, 5, 268–277. [Google Scholar] [CrossRef]
- Kane, M.J.; Angoa- Pérez, M.; Briggs, D.I.; Viano, D.C.; Kreipke, C.W.; Kuhn, D.M. A mouse model of human repetitive mild traumatic brain injury. J. Neurosci. Methods 2012, 203, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Flierl, M.A.; Stahel, P.F.; Beauchamp, K.M.; Morgan, S.J.; Smith, W.R.; Shohami, E. Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 2009, 4, 1328–1337. [Google Scholar] [CrossRef]
- Kriger, B. Complete Works, 1st ed.; Altaspera Publishing & Literary Agency Inc.: Huntsville, ON, Canada, 2012; p. 653. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Fisher, M.; Vasilevko, V.; Passos, G.F.; Ventura, C.; Quiring, D.; Cribbs, D.H. Therapeutic Modulation of Cerebral Microhemorrhage in a Mouse Model of Cerebral Amyloid Angiopathy. Stroke 2011, 42, 3300–3303. [Google Scholar] [CrossRef] [Green Version]
- Yarnell, A.M.; Barry, E.S.; Mountney, A.; Shear, D.; Tortella, F.; Grunberg, N.E. The Revised Neurobehavioral Severity Scale (NSS-R) for Rodents. Neuroscience 2016, 75, 9.52.1–9.52.16. [Google Scholar]
- Tsenter, J.; Beni-Adani, L.; Assaf, Y.; Alexandrovich, A.G.; Trembovler, V.; Shohami, E. Dynamic Changes in the Recovery After Traumatic Brain Injury in Mice: Effect of Injury Severity on T2-Weighted MRI Abnormalities, And Motor And Cognitive Functions. J. Neurotrauma 2008, 25, 324–333. [Google Scholar] [CrossRef]
- Orr, S.K.; Tong, J.Y.M.; Kang, J.X.; Ma, D.W.L.; Bazinet, R.P. The fat-1 mouse has brain docosahexaenoic acid levels achievable through fish oil feeding. Neurochem. Res. 2010, 35, 811–819. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The Salutary Effects of DHA Dietary Supplementation on Cognition, Neuroplasticity, and Membrane Homeostasis after Brain Trauma. J. Neurotrauma 2011, 25, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Guskiewicz, K.M. Balance Assessment in the Management of Sport-Related Concussion. Clin. Sports Med. 2011, 30, 89–102. [Google Scholar] [CrossRef]
- McCrory, P.; Meeuwisse, W.H.; Aubry, M.; Cantu, R.C.; Dvořák, J.; Echemendia, R.J.; Engebretsen, L.; Johnston, K.; Kutcher, J.S.; Raftery, M.; et al. Consensus Statement on Concussion in Sport: The 4th International Conference on Concussion in Sport, Zurich, November 2012. J. Athl. Train. 2013, 48, 554–575. [Google Scholar] [CrossRef] [Green Version]
- DuBose, D.F.; Herman, D.C.; Jones, D.L.; Tillman, S.M.; Clugston, J.R.; Pass, A.; Hernandez, J.A.; Vasilopoulos, T.; Horodyski, M.; Chmielewski, T.L. Lower Extremity Stiffness Changes following Concussion in Collegiate Football Players. Med. Sci. Sports Exerc. 2017, 49, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Fino, P.C. A Preliminary Study of Longitudinal Differences in Local Dynamic Stability Between Recently Concussed and Healthy Athletes During Single and Dual-Task Gait. J. Biomech. 2016, 49, 1983–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorman, J.C.; Valentine, V.D.; Munce, T.A.; Tjarks, B.J.; Thompson, P.A.; Bergeron, M.F. Tracking postural stability of young concussion patients using dual- task interference. J. Sci. Med. Sport 2015, 18, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.D.; Turtzo, L.C.; Parikh, G.Y.; Tolpygo, A.; Lodato, Z.; Moses, A.D.; Nair, G.; Perl, D.P.; Edwards, N.A.; Dardzinski, B.J.; et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019, 142, 3550–3564. [Google Scholar] [CrossRef] [PubMed]
- Guskiewicz, K.M.; Mhalik, J.P.; Shankar, V.; Marshall, S.W.; Crowell, D.H.; Oliaro, S.M.; Ciocca, M.F.; Hooker, D.N. Measurement of Head Impacts in Collegiate Football Players: Relationship Between Head Impact Biomechanics and Acute Clinical Outcome After Concussion. Neurosurgery 2007, 61, 1244–1252. [Google Scholar] [CrossRef]
- Meaney, D.F.; Smith, D.H. Biomechanics of Concussion. Clin. Sports Med. 2014, 30, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Pellman, E.J.; Viano, D.C.; Tucker, A.M.; Casson, I.R. Concussion in Professional Football: Location and Direction of Helmet Impacts—Part 2. Neurosurgery 2003, 53, 1328–1341. [Google Scholar] [CrossRef]
- Pellman, E.J.; Viano, D.C.; Tucker, A.M.; Casson, I.R.; Waeckerle, J.F. Concussion in Professional Football: Reconstruction of Game Impacts and Injuries. Neurosurgery 2003, 53, 799–814. [Google Scholar] [CrossRef]
- Viano, D.C.; Casson, I.R.; Pellman, E.J. Concussion in Professional Football: Biomechanics of The Struck Player—Part 14. Neurosurgery 2007, 61, 313–328. [Google Scholar] [CrossRef]
- Viano, D.C.; Casson, I.R.; Pellman, E.J.; Bir, C.A.; Zhang, L.; Sherman, D.C.; Boitano, M.A. Concussion in Professional Football: Comparison with Boxing Head Impacts—Part 10. Neurosurgery 2005, 57, 1154–1172. [Google Scholar] [CrossRef]
- Viano, D.C.; Hamberger, A.; Bolouri, H.; Säljö, A. Concussion in Professional Football: Animal Model of Brain Injury—Part 15. Neurosurgery 2009, 64, 1162–1173. [Google Scholar] [CrossRef]
- Viano, D.C.; Pellman, E.J. Concussion in Professional Football: Biomechanics of the Striking Player—Part 8. Neurosurgery 2005, 56, 266–280. [Google Scholar] [CrossRef]
- Thompson, H.J.; Lifshitz, J.; Marklund, N.; Grady, M.S.; Graham, D.I.; Hovda, D.A.; McIntosh, T.K. Lateral Fluid Percussion Brain Injury: A 15-year Review and Evaluation. J. Neurotrauma 2005, 22, 42–75. [Google Scholar] [CrossRef]
- Lighthall, J.W.; Dixon, C.E.; Anderson, T.E. Experimental Models of Brain Injury. J. Neurotrauma 1989, 6, 83–97. [Google Scholar] [CrossRef]
- Masson, F.; Thicoipe, M.; Aye, P.; Mokni, T.; Senjean, P.; Schmitt, V.; Dessalles, P.H.; Casaugade, M.; Labadens, P. Epidemiology of Severe Brain Injuries: A Prospective Population-Based Study. J. Trauma. 2001, 51, 481–489. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Cooper, D.J.; Finfer, S.R.; Venkatesh, B.; Jones, D.; Higgins, A.; Bishop, N.; Higlett, T. Epidemiology And 12-Month Outcomes from Traumatic Brain Injury in Australia And New Zealand. J. Trauma. 2008, 64, 854–862. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Zhuo, L.; Fu, C.; Hui, G.; Wang, Y.; Yang, W.; Teng, L.; Lu, S.; Xu, G. Epidemiology of Traumatic Brain Injury In Eastern China, 2004: A Prospective Large Case Study. J. Trauma. 2008, 64, 1313–1319. [Google Scholar] [CrossRef]
- Statler, K.D.; Alexander, H.; Vagni, V.; Holubkov, R.; Dixon, C.E.; Clark, R.S.B.; Jenkins, L.; Kochanek, P.M. Isoflurane Exerts Neuroprotective Actions at or Near the Time of Severe Traumatic Brain Injury. Brain Res. 2006, 1076, 216–224. [Google Scholar] [CrossRef]
- Kang, J.X. Fat-1 Transgenic Mice: A New Model for Omega-3 Research. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Thau-Zuchman, O.; Ingram, R.; Harvey, G.G.; Cooke, T.; Palmas, F.; Pallier, P.N.; Brook, J.; Priestley, J.V.; Dalli, J.; Tremoleda, J.L.; et al. A Single Injection of Docosahexaenoic Acid Induces a Pro-Resolving Lipid Mediator Profile in the Injured Tissue and a Long-Lasting Reduction in Neurological Deficit after Traumatic Brain Injury in Mice. J. Neurotrauma 2020, 37, 66–79. [Google Scholar] [CrossRef]
| Fatty Acid | WT | fat-1 |
|---|---|---|
| 16:0 | 28.25 ± 1.07 | 24.34 ± 0.31 * |
| 18:0 | 28.29 ± 1.02 | 25.79 ± 0.25 |
| 18:1c9 | 17.56 ± 0.71 | 19.13 ± 0.61 |
| 18:2n6 | 1.28 ± 0.01 | 1.41 ± 0.15 |
| 20:3n6 | 0.33 ± 0.01 | 0.46 ± 0.03 * |
| 20:4n6 | 17.90 ± 3.16 | 11.03 ± 0.03 * |
| 20:5n3 | 0.15 ± 0.02 | 0.26 ± 0.02 * |
| 22:4n6 | 3.52 ± 0.07 | 2.50 ±0.16 * |
| 22:5n6 | 10.27 ± 0.91 | 0.43 ± 0.11 * |
| 22:5n3 | 0.22 ± 0.02 | 0.27 ± 0.05 |
| 22:6n3 | 6.56 ± 0.98 | 18.04 ± 0.34 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecques, J.-D.; Kerr, B.J.K.; Hillyer, L.M.; Kang, J.X.; Robinson, L.E.; Ma, D.W.L. N-3 Polyunsaturated Fatty Acids Ameliorate Neurobehavioral Outcomes Post-Mild Traumatic Brain Injury in the Fat-1 Mouse Model. Nutrients 2021, 13, 4092. https://doi.org/10.3390/nu13114092
Lecques J-D, Kerr BJK, Hillyer LM, Kang JX, Robinson LE, Ma DWL. N-3 Polyunsaturated Fatty Acids Ameliorate Neurobehavioral Outcomes Post-Mild Traumatic Brain Injury in the Fat-1 Mouse Model. Nutrients. 2021; 13(11):4092. https://doi.org/10.3390/nu13114092
Chicago/Turabian StyleLecques, Jessica-Dominique, Brynna J. K. Kerr, Lyn M. Hillyer, Jing X. Kang, Lindsay E. Robinson, and David W. L. Ma. 2021. "N-3 Polyunsaturated Fatty Acids Ameliorate Neurobehavioral Outcomes Post-Mild Traumatic Brain Injury in the Fat-1 Mouse Model" Nutrients 13, no. 11: 4092. https://doi.org/10.3390/nu13114092






