High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Triacylglycerol Content
2.3. Cytokine Analyses
2.4. Histological Parameters
2.5. Western Blot Analyses
2.6. Gene Expression
2.7. Metabolomics Analysis
2.8. Statistical Analyses
3. Results
3.1. Liver Histology, Serum Biochemistry and Body Composition
3.2. Triacylglycerol Content
3.3. Cytokine Analyses
3.4. Histological Parameters
3.5. Western Blot Analyses
3.6. Gene Expression
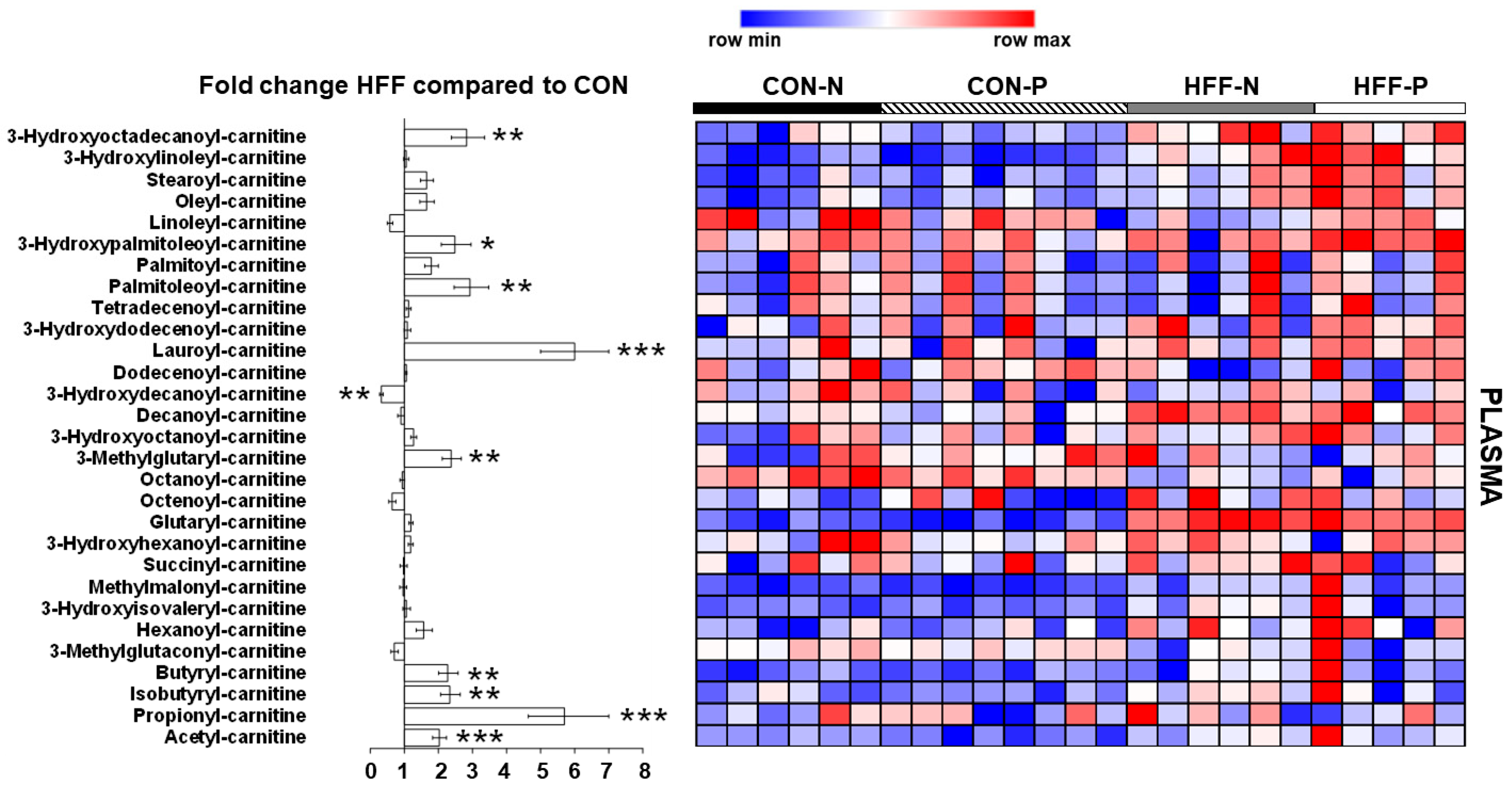
3.7. Metabolomics Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| CON | Control |
| HFF | High-fructose high-fat |
| NASH | Non-alcoholic steatohepatitis |
| M | Male |
| F | Female |
| BW | Body weight |
| CON-N | Control |
| ME | Metabolizable energy |
| CON-P | Control with probiotics |
| HFF-N | High-fructose high-fat |
| HFF-P | High-fructose high-fat with probiotics |
| BCA | Bicinchoninic acid |
| TNFα | Tumor necrosis factor alpha |
| IL-1α | Interleukin 1 alpha |
| PKB | Protein kinase B |
| LC3 | Microtubule-associated protein light chain 3 |
| p62 | Ubiquitin-binding protein |
| RT | Room temperature |
| TBST | Tris-buffered saline with tween |
| UPLC | Ultra-performance liquid chromatography |
| PBS | Phosphate-buffered saline |
| ORO | Oil red O |
| AF | Alexa Fluor |
| TAG | Triacylglycerol |
| SE | Standard error |
| PCA | Principal component analysis |
| IMCL | Intramyocellular lipid |
| EMCL | Extramyocellular lipid |
| CPT1 | Carnitine palmitoyltransferase I |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| GLUT4 | Glucose transporter type 4 |
| TOP2B | DNA topoisomerase 2β |
References
- Kohli, R.; Sunduram, S.; Mouzaki, M.; Ali, S.; Sathya, P.; Abrams, S.; Xanthakos, S.A.; Vos, M.; Schwimmer, J.B. Pediatric Nonalcoholic Fatty Liver Disease: A Report from the Expert Committee on Nonalcoholic Fatty Liver Disease (ECON). J. Pediatr. 2016, 172, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Spengler, E.K.; Loomba, R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin. Proc. 2015, 90, 1233–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Alferink, L.J.M.; Trajanoska, K.; Erler, N.; Schoufour, J.D.; De Knegt, R.J.; Ikram, M.A.; Janssen, H.L.A.; Franco, O.H.; Metselaar, H.J.; Rivadeneira, F.; et al. Nonalcoholic Fatty Liver Disease in The Rotterdam Study: About Muscle Mass, Sarcopenia, Fat Mass, and Fat Distribution. J. Bone Miner. Res. 2019, 34, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Bhanji, R.A.; Narayanan, P.; Allen, A.M.; Malhi, H.; Watt, K.D. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017, 66, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Song, X.; Chen, Y.; Chen, X.; Yu, C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol. Int. 2019, 14, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, L.; Perla, F.M.; Andreoli, G.; Grieco, R.; Pierimarchi, P.; Chiesa, C. Nonalcoholic Fatty Liver Disease Is Associated with Low Skeletal Muscle Mass in Overweight/Obese Youths. Front. Pediatr. 2020, 8, 158. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Lee, S.; Lee, Y.; Jun, J.E.; Ahn, J.; Bae, J.C.; Jin, S.; Hur, K.Y.; Jee, J.H.; Lee, M.; et al. Relationship between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology 2018, 68, 1755–1768. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Jung, K.S.; Kim, S.U.; Yoon, H.-J.; Yun, Y.J.; Lee, B.-W.; Kang, E.S.; Han, K.-H.; Lee, H.C.; Cha, B.-S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef]
- Wilson, J.M.; Loenneke, J.P.; Jo, E.; Wilson, G.J.; Zourdos, M.C.; Kim, J.-S. The Effects of Endurance, Strength, and Power Training on Muscle Fiber Type Shifting. J. Strength Cond. Res. 2012, 26, 1724–1729. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Mammalian skeletal muscle fiber type transitions. Int. Rev. Cytology 1997, 170, 143–223. [Google Scholar]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Fitts, R.H.; Brimmer, C.J.; Heywood-Cooksey, A.; Timmerman, R.J. Single muscle fiber enzyme shifts with hindlimb suspension and immobilization. Am. J. Physiol. Physiol. 1989, 256, C1082–C1091. [Google Scholar] [CrossRef]
- Adams, G.R.; Hather, B.M.; Baldwin, K.M.; Dudley, G.A. Skeletal muscle myosin heavy chain composition and resistance training. J. Appl. Physiol. 1993, 74, 911–915. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Burrington, C.M.; Graff, E.C.; Zhang, J.; Judd, R.L.; Suksaranjit, P.; Kaewpoowat, Q.; Davenport, S.K.; O’Neill, A.M.; Greene, M.W. Metabolic phenotype and adipose and liver features in a high-fat Western diet-induced mouse model of obesity-linked NAFLD. Am. J. Physiol. Metab. 2016, 310, E418–E439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shieh, K.; Gilchrist, J.M.; Promrat, K. Frequency and predictors of nonalcoholic fatty liver disease in myotonic dystrophy. Muscle Nerve 2010, 41, 197–201. [Google Scholar] [CrossRef]
- Bhardwaj, R.R.; Duchini, A. Non-Alcoholic Steatohepatitis in Myotonic Dystrophy: DMPK Gene Mutation, Insulin Resistance and Development of Steatohepatitis. Case Rep. Gastroenterol. 2010, 4, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Donini, L.M.; Lenzi, A.; Chiesa, C.; Pacifico, L. Non-alcoholic fatty liver disease connections with fat-free tissues: A focus on bone and skeletal muscle. World J. Gastroenterol. 2017, 23, 1747–1757. [Google Scholar] [CrossRef]
- De Bandt, J.-P.; Jegatheesan, P.; Tennoune-El-Hafaia, N. Muscle Loss in Chronic Liver Diseases: The Example of Nonalcoholic Liver Disease. Nutrients 2018, 10, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, G.V.; Smith, V.A.; Melnyk, M.; Burd, M.A.; Sprayberry, K.A.; Edwards, M.S.; Peterson, D.G.; Bennet, D.C.; Fanter, R.K.; Columbus, D.A.; et al. Dysregulated FXR-FGF19 signaling and choline metabolism are associated with gut dysbiosis and hyperplasia in a novel pig model of pediatric NASH. Am. J. Physiol. Liver Physiol. 2020, 318, G582–G609. [Google Scholar] [CrossRef]
- Zeltser, N.; Meyer, I.; Hernandez, G.V.; Trahan, M.J.; Fanter, R.K.; Abo-Ismail, M.; Glanz, H.; Strand, C.R.; Burrin, D.G.; La Frano, M.R.; et al. Neurodegeneration in juvenile Iberian pigs with diet-induced nonalcoholic fatty liver disease. Am. J. Physiol. Metab. 2020, 319, E592–E606. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Alloosh, M.; Saxena, R.; Van Alstine, W.; Watkins, B.A.; Klaunig, J.E.; Sturek, M.; Chalasani, N. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology 2009, 50, 56–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, R.S.; Huff, E.W.; Kapur, A. Compositions and Methods for Improving Human Health and Nutrition. BiOWiSH Technologies, Inc. U.S. Patent No. 10022409, 11 July 2017. Available online: https://patents.justia.com/patent/10022409 (accessed on 6 September 2020).
- Jouihan, H. Measurement of Liver Triglyceride Content. Bio-Protocol 2012, 2, 13. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Stipanuk, M.H. Growing Rats Respond to a Sulfur Amino Acid–Deficient Diet by Phosphorylation of the α Subunit of Eukaryotic Initiation Factor 2 Heterotrimeric Complex and Induction of Adaptive Components of the Integrated Stress Response. J. Nutr. 2010, 140, 1080–1085. [Google Scholar] [CrossRef] [Green Version]
- Presnell, J.K.; Schreibman, M.P.; Humason, G.L. Humason’s Animal Tissue Techniques, 5th ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1997; pp. 154–196. [Google Scholar]
- Piepho, H. Data Transformation in Statistical Analysis of Field Trials with Changing Treatment Variance. Agron. J. 2009, 101, 865–869. [Google Scholar] [CrossRef]
- Crescenzo, R.; Bianco, F.; Coppola, P.; Mazzoli, A.; Cigliano, L.; Liverini, G.; Iossa, S. The effect of high-fat–high-fructose diet on skeletal muscle mitochondrial energetics in adult rats. Eur. J. Nutr. 2014, 54, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Olver, T.D.; Grunewald, Z.I.; Jurrissen, T.J.; MacPherson, R.; Leblanc, P.J.; Schnurbusch, T.R.; Czajkowski, A.M.; Laughlin, M.H.; Rector, R.S.; Bender, S.B.; et al. Microvascular insulin resistance in skeletal muscle and brain occurs early in the development of juvenile obesity in pigs. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R252–R264. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Wang, M.-E.; Jiang, Y.-F.; Liu, H.-C.; Chen, Y.-C.; Chiu, C.-H. Long-term feeding of high-fat plus high-fructose diet induces isolated impaired glucose tolerance and skeletal muscle insulin resistance in miniature pigs. Diabetol. Metab. Syndr. 2017, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Günther, P.; Lauterbach, M.A.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.-J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175.e14. [Google Scholar] [CrossRef] [Green Version]
- Barton, M. Childhood obesity: A life-long health risk. Acta Pharmacol. Sin. 2012, 33, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Fryar, C.D. Prevalence of Overweight, Obesity, and Severe Obesity among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 through 2015–2016. NCHS Health E-stats, CDC Stacks Puclic Health Publications 2018, Hyattsville, MD, USA. Available online: https://stacks.cdc.gov/view/cdc/58670 (accessed on 18 October 2021).
- Hua, N.; Takahashi, H.; Yee, G.M.; Kitajima, Y.; Katagiri, S.; Kojima, M.; Anzai, K.; Eguchi, Y.; Hamilton, J.A. Influence of muscle fiber type composition on early fat accumulation under high-fat diet challenge. PLoS ONE 2017, 12, e0182430. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.S.; Molnar, M.Z.; Tayek, J.A.; Ix, J.H.; Noori, N.; Benner, D.; Heymsfield, S.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a cross-sectional study and review of literature. J. Cachex-Sarcopenia Muscle 2013, 4, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Machann, J.; Haring, H.; Schick, F.; Stumvoll, M. Intramyocellular lipids and insulin resistance. Diabetes Obes. Metab. 2004, 6, 239–248. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Zierath, J.R.; Hawley, J. Skeletal Muscle Fiber Type: Influence on Contractile and Metabolic Properties. PLoS Biol. 2004, 2, e348. [Google Scholar] [CrossRef] [PubMed]
- Howald, H.; Hoppeler, H.; Claassen, H.; Mathieu, O.; Straub, R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflüg. Arch. 1985, 403, 369–376. [Google Scholar] [CrossRef]
- Jansson, E.; Sjödin, B.; Tesch, P. Changes in muscle fibre type distribution in man after physical training: A sign of fibre type transformation? Acta Physiol. Scand. 1978, 104, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism—Emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef] [Green Version]
- Eaton, S. Control of mitochondrial β-oxidation flux. Prog. Lipid Res. 2002, 41, 197–239. [Google Scholar] [CrossRef]
- Storlien, L.; Oakes, N.D.; Kelley, D.E. Metabolic flexibility. Proc. Nutr. Soc. 2004, 63, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. 2015, 29, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Xiao, Q. The Common Mechanisms of Sarcopenia and NAFLD. BioMed Res. Int. 2017, 2017, 6297651. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M. Autophagy in skeletal muscle. FEBS Lett. 2010, 584, 1411–1416. [Google Scholar] [CrossRef]
- Sha, W.; Da Costa, K.; Fischer, L.M.; Milburn, M.V.; Lawton, K.A.; Berger, A.; Jia, W.; Zeisel, S.H. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. FASEB J. 2010, 24, 2962–2975. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H.; da Costa, K.-A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Rinella, M.E.; Elias, M.S.; Smolak, R.R.; Fu, T.; Borensztajn, J.; Green, R.M. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J. Lipid Res. 2008, 49, 1068–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizki, G.; Arnaboldi, L.; Gabrielli, B.; Yan, J.; Lee, G.S.; Ng, R.K.; Turner, S.M.; Badger, T.M.; Pitas, R.E.; Maher, J.J. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J. Lipid Res. 2006, 47, 2280–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hove, E.L.; Copeland, D.H. Progressive Muscular Dystrophy in Rabbits as a Result of Chronic Choline Deficiency. J. Nutr. 1954, 53, 391–405. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.F.; Hood, D.A. Denervation-induced oxidative stress and autophagy signaling in muscle. Autophagy 2009, 5, 230–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, S.K.; Brown, L. Rodent Models for Metabolic Syndrome Research. J. Biomed. Biotechnol. 2010, 2011, 351982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klurfeld, D.M.; Hekler, E.B.; Nebeker, C.; Patrick, K.; Khoo, C.S.H. Technology Innovations in Dietary Intake and Physical Activity Assessment: Challenges and Recommendations for Future Directions. Am. J. Prev. Med. 2018, 55, e117–e122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padulo, J.; Oliva, F.; Frizziero, A.; Maffulli, N. Muscles, ligaments and tendons journal—Basic principles and recommendations in clinical and field science research: 2016 Update. Muscles Ligaments Tendons J. 2016, 6, 1–5. [Google Scholar] [CrossRef]
- Harrison Laboratory; The Jackson Laboratory. Life Span as a Biomarker. Available online: https://www.jax.org/research-and-faculty/research-labs/the-harrison-lab/gerontology/life-span-as-a-biomarker (accessed on 7 September 2020).
- Jackson, S.J.; Andrews, N.; Ball, D.; Bellantuono, I.; Gray, J.; Hachoumi, L.; Holmes, A.; Latcham, J.; Petrie, A.; Potter, P.; et al. Does age matter? The impact of rodent age on study outcomes. Lab. Anim. 2017, 51, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Katsumata, M.; Yamaguchi, T.; Ishida, A.; Ashihara, A. Changes in muscle fiber type and expression of mRNA of myosin heavy chain isoforms in porcine muscle during pre-and postnatal development. Anim. Sci. J. 2017, 88, 364–371. [Google Scholar] [CrossRef]
- 2012 National Health Interview Survey (NHIS): Public Use Data Release. NHIS Survey. Available online: http://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2012/srvydesc.pdf (accessed on 6 September 2020).
- Hulston, C.J.; Churnside, A.A.; Venables, M. Probiotic supplementation prevents high-fat, overfeeding-induced insulin resistance in human subjects. Br. J. Nutr. 2015, 113, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Osterberg, K.L.; Boutagy, N.E.; McMillan, R.P.; Stevens, J.R.; Frisard, M.I.; Kavanaugh, J.W.; Davy, B.M.; Davy, K.P.; Hulver, M.W. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity 2015, 23, 2364–2370. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; DeSimone, C.; Song, X.; Diehl, A.M. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 2003, 37, 343–350. [Google Scholar] [CrossRef]
- Ma, X.; Hua, J.; Li, Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J. Hepatol. 2008, 49, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Rijkers, G.T.; Bengmark, S.; Enck, P.; Haller, D.; Herz, U.; Kalliomaki, M.; Kudo, S.; Lenoir-Wijnkoop, I.; Mercenier, A.; Myllyluoma, E.; et al. Guidance for Substantiating the Evidence for Beneficial Effects of Probiotics: Current Status and Recommendations for Future Research. J. Nutr. 2010, 140, 671S–676S. [Google Scholar] [CrossRef] [Green Version]
- Clark, B.A.; Alloosh, M.; Wenzel, J.W.; Sturek, M.; Kostrominova, T.Y. Effect of diet-induced obesity and metabolic syndrome on skeletal muscles of Ossabaw miniature swine. Am. J. Physiol. Metab. 2011, 300, E848–E857. [Google Scholar] [CrossRef] [Green Version]
- Guillerm-Regost, C.; Louveau, I.; Sébert, S.P.; Damon, M.; Champ, M.M.; Gondret, F. Cellular and Biochemical Features of Skeletal Muscle in Obese Yucatan Minipigs. Obesity 2006, 14, 1700–1707. [Google Scholar] [CrossRef]
- Ruan, J.; Zhang, Y.; Yuan, J.; Xin, L.; Xia, J.; Liu, N.; Mu, Y.; Chen, Y.; Yang, S.; Li, K. A long-term high-fat, high-sucrose diet in Bama minipigs promotes lipid deposition and amyotrophy by up-regulating the myostatin pathway. Mol. Cell. Endocrinol. 2016, 425, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Hintze, K.J.; Benninghoff, A.D.; Ward, R.E. Formulation of the Total Western Diet (TWD) as a Basal Diet for Rodent Cancer Studies. J. Agric. Food Chem. 2012, 60, 6736–6742. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. Membrane Fatty Acid Transporters as Regulators of Lipid Metabolism: Implications for Metabolic Disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spooner, H.C.; Derrick, S.A.; Maj, M.; Manjarín, R.; Hernandez, G.V.; Tailor, D.S.; Bastani, P.S.; Fanter, R.K.; Fiorotto, M.L.; Burrin, D.G.; et al. High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 4195. https://doi.org/10.3390/nu13124195
Spooner HC, Derrick SA, Maj M, Manjarín R, Hernandez GV, Tailor DS, Bastani PS, Fanter RK, Fiorotto ML, Burrin DG, et al. High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease. Nutrients. 2021; 13(12):4195. https://doi.org/10.3390/nu13124195
Chicago/Turabian StyleSpooner, Heather C., Stefani A. Derrick, Magdalena Maj, Rodrigo Manjarín, Gabriella V. Hernandez, Deepali S. Tailor, Parisa S. Bastani, Rob K. Fanter, Marta L. Fiorotto, Douglas G. Burrin, and et al. 2021. "High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease" Nutrients 13, no. 12: 4195. https://doi.org/10.3390/nu13124195
APA StyleSpooner, H. C., Derrick, S. A., Maj, M., Manjarín, R., Hernandez, G. V., Tailor, D. S., Bastani, P. S., Fanter, R. K., Fiorotto, M. L., Burrin, D. G., La Frano, M. R., Sikalidis, A. K., & Blank, J. M. (2021). High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease. Nutrients, 13(12), 4195. https://doi.org/10.3390/nu13124195








