Yeast Beta-Glucans Ingestion Does Not Influence Body Weight: A Systematic Review and Meta-Analysis of Pre-Clinical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Registration Protocol and Study Design
2.2. Focused Question
2.3. Eligibility Criteria
2.3.1. Search Strategy
2.3.2. Inclusion Criteria and Study Selection
2.3.3. Articles Selection and Data Extraction
2.3.4. Risk of Bias (RoB) Assessment
2.3.5. Methodological Quality Assessment
2.4. Data Analysis
3. Results
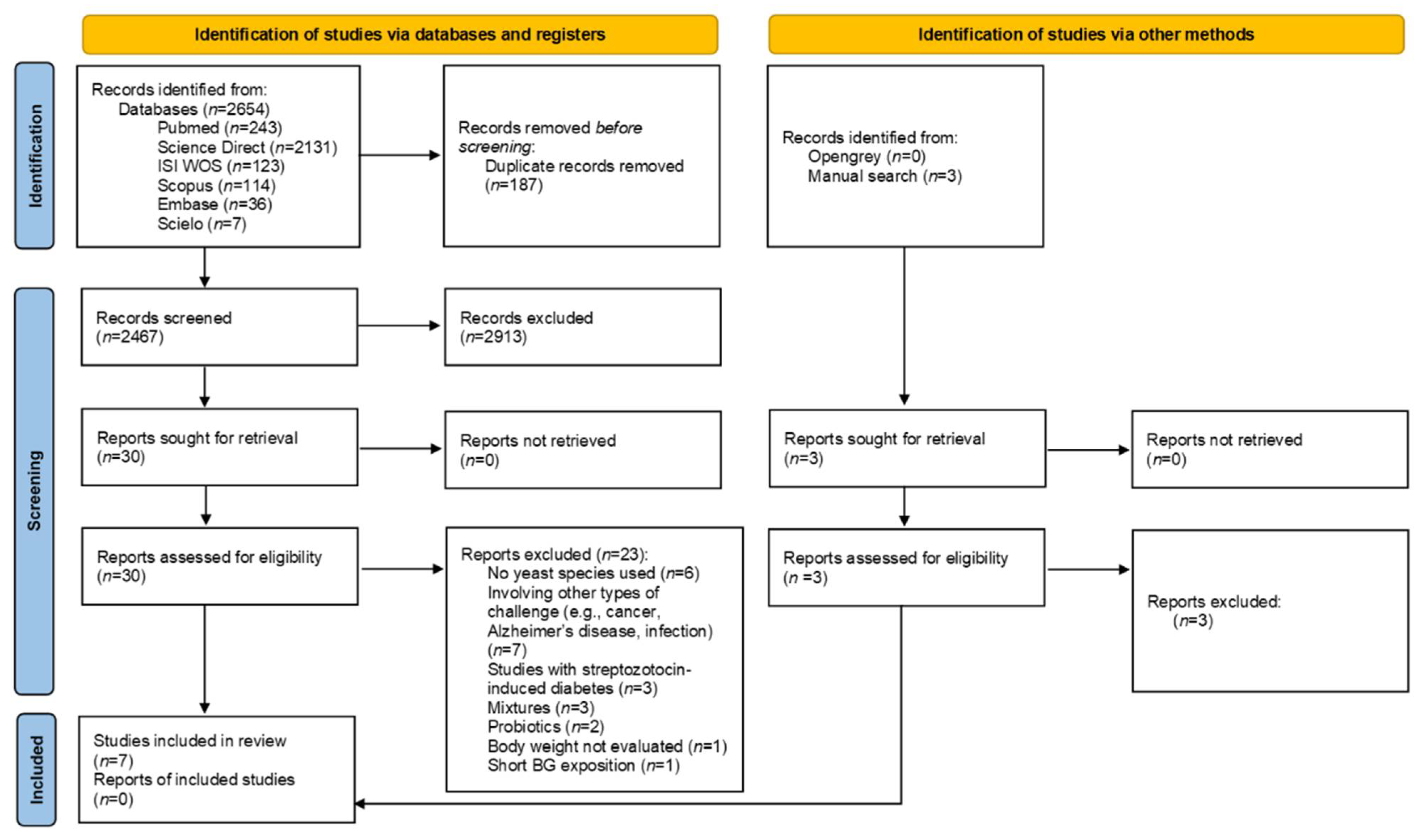
3.1. Study Selection and Characteristics
3.2. Results for Individual Studies
3.3. Bias of Risk and Methodological Quality Assessments
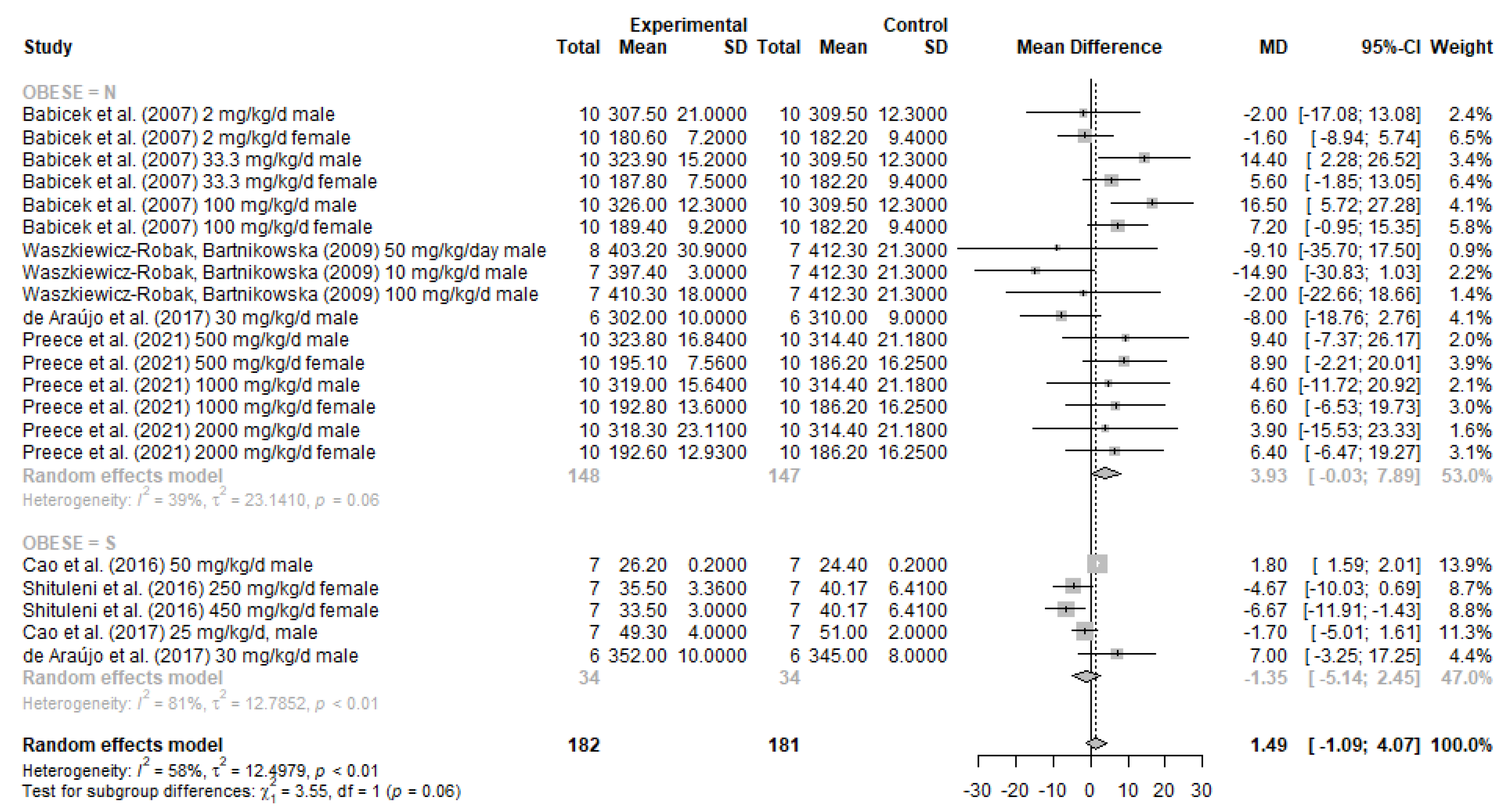
3.4. Meta-Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2019, 1461, 37–52. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K. Prevalence of Overweight and Obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 2016, 22, 1–203. [Google Scholar] [CrossRef] [Green Version]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleymani, T.; Daniel, S.; Garvey, W.T. Weight maintenance: Challenges, tools and strategies for primary care physicians. Obes. Rev. 2016, 17, 81–93. [Google Scholar] [CrossRef]
- Thompson, S.V.; Hannon, B.A.; An, R.; Holscher, H.D. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 1514–1528. [Google Scholar] [CrossRef] [Green Version]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.A.; Singh, G.S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94–103. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.L.; Zhao, T.; Zhou, Y.; Shi, X.; Zou, Y.; Zhao, G. Effect of Oat β-Glucan Intake on Glycaemic Control and Insulin Sensitivity of Diabetic Patients: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Mejia, S.B.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.J. Efecto de los beta-glucanos en el control de los niveles de glucosa en. Nutr. Hosp. 2015, 31, 170–177. [Google Scholar] [CrossRef]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of beta-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, V.D.O.; Lobato, R.V.; Andrade, E.F.; De Macedo, C.G.; Napimoga, J.T.C.; Napimoga, M.H.; Messora, M.R.; Murata, R.M.; Pereira, L.J. β-Glucans (Saccharomyces cereviseae) Reduce Glucose Levels and Attenuate Alveolar Bone Loss in Diabetic Rats with Periodontal Disease. PLoS ONE 2015, 10, e0134742. [Google Scholar] [CrossRef]
- Pereira, L.J. Effects of β-glucans (Saccharomyces cerevisae) per os administration in rats with streptozotocin-induced diabetes. Nutr. Hosp. 2015, 32, 256–264. [Google Scholar] [CrossRef]
- De Araújo, T.V.; Andrade, E.F.; Lobato, R.V.; Orlando, D.R.; Gomes, N.F.; De Sousa, R.V.; Zangeronimo, M.; Pereira, L.J. Effects of beta-glucans ingestion (Saccharomyces cerevisiae) on metabolism of rats receiving high-fat diet. J. Anim. Physiol. Anim. Nutr. 2016, 101, 349–358. [Google Scholar] [CrossRef]
- Bacha, U.; Nasir, M.; Iqbal, S.; Anjum, A.A. Nutraceutical, Anti-Inflammatory, and Immune Modulatory Effects ofβ-Glucan Isolated fromYeast. BioMed Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mosikanon, K.; Arthan, D.; Kettawan, A.; Tungtrongchitr, R.; Prangthip, P. Yeast β–Glucan Modulates Inflammation and Waist Circumference in Overweight and Obese Subjects. J. Diet. Suppl. 2016, 14, 173–185. [Google Scholar] [CrossRef]
- Rahmani, J.; Miri, A.; Černevičiūtė, R.; Thompson, J.; De Souza, N.N.A.; Sultana, R.; Varkaneh, H.K.; Mousavi, S.M.; Hekmatdoost, A. Effects of cereal beta-glucan consumption on body weight, body mass index, waist circumference and total energy intake: A meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 43, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Strączkowski, M.; Nikołajuk, A.; Majewski, R.; Filarski, R.; Stefanowicz, M.; Matulewicz, N.; Karczewska-Kupczewska, M. The effect of moderate weight loss, with or without (1, 3)(1, 6)-β-glucan addition, on subcutaneous adipose tissue inflammatory gene expression in young subjects with uncomplicated obesity. Endocrine 2018, 61, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.R.; De Lima, N.V.; Rezende, K.S.; Santos, I.C.M.; Silva, I.S.; Guimarães, R.D.C.A. Animal models of obesity in rodents. An integrative review. Acta Cir. Bras. 2016, 31, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; Group NCRRGW. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Kellesarian, S.; Abduljabbar, T.; Abduljabbar, A.; Akram, Z.; Vohra, F.; Rahman, I.; Romanos, G. Influence of involuntary cigarette smoke inhalation on osseointegration: A systematic review and meta-analysis of preclinical studies. Int. J. Oral Maxillofac. Surg. 2017, 47, 764–772. [Google Scholar] [CrossRef]
- Schwarzer, G.; Mair, P.; Hatzinger, R. meta: An R Package for Meta-Analysis. R News 2007, 7, 40–45. [Google Scholar]
- Development Core Team. R Core Team: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Sterne, J.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.; Schmid, C.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [Green Version]
- Babíček, K.; Čechová, I.; Simon, R.; Harwood, M.; Cox, D. Toxicological assessment of a particulate yeast (1,3/1,6)-β-d-glucan in rats. Food Chem. Toxicol. 2007, 45, 1719–1730. [Google Scholar] [CrossRef]
- Waszkiewicz-Robak, B.; Bartnikowska, E. Effects of spent brewer’s yeast and biological β-glucans on selected parameters of lipid metabolism in blood and liver in rats. J. Anim. Feed. Sci. 2009, 18, 699–708. [Google Scholar] [CrossRef]
- Preece, K.E.; Glávits, R.; Murbach, T.S.; Endres, J.R.; Hirka, G.; Vértesi, A.; Szakonyiné, I.P. Assessment of toxicological potential of sodium carboxymethyl beta-glucan, a novel beta-glucan. Food Chem. Toxicol. 2021, 152, 112226. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Y.; Zou, S.; Li, M.; Xu, X. Orally Administered Baker’s Yeast β-Glucan Promotes Glucose and Lipid Homeostasis in the Livers of Obesity and Diabetes Model Mice. J. Agric. Food Chem. 2017, 65, 9665–9674. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zou, S.; Xu, H.; Li, M.; Tong, Z.; Xu, M.; Xu, X. Hypoglycemic activity of the Baker’s yeast β-glucan in obese/type 2 diabetic mice and the underlying mechanism. Mol. Nutr. Food Res. 2016, 60, 2678–2690. [Google Scholar] [CrossRef] [PubMed]
- Shituleni, S.A.; Gan, F.; Nido, S.A.; Mengistu, B.M.; Khan, A.Z.; Liu, Y.; Huang, K. Effects of yeast polysaccharide on biochemical indices, antioxidant status, histopathological lesions and genetic expressions related with lipid metabolism in mice fed with high fat diet. Bioact. Carbohydrates Diet. Fibre 2016, 8, 51–57. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Suzuki, T.; Suzuki, T.; Sakanaka, M. Inhibitory effects of water-soluble low-molecular-weight β-(1,3–1,6) d-glucan purified from Aureobasidium pullulans GM-NH-1A1 strain on food allergic reactions in mice. Int. Immunopharmacol. 2007, 7, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Markovina, N.; Banjari, I.; Popovic, V.B.; Kadic, A.J.; Puljak, L. Efficacy and safety of oral and inhalation commercial beta-glucan products: Systematic review of randomized controlled trials. Clin. Nutr. 2019, 39, 40–48. [Google Scholar] [CrossRef]
- Guilarducci, J.D.S.; Marcelino, B.A.R.; Konig, I.; Orlando, T.M.; Varaschin, M.S.; Pereira, L.J. Therapeutic effects of different doses of prebiotic (isolated from Saccharomyces cerevisiae) in comparison to n-3 supplement on glycemic control, lipid profiles and immunological response in diabetic rats. Diabetol. Metab. Syndr. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nat. Cell Biol. 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Kim, Y.; Cho, M. β-glucan, “the knight of health sector”: Critical insights on physiochemical heterogeneities, action mechanisms and health implications. Crit. Rev. Food Sci. Nutr. 2021, 1–37. [Google Scholar] [CrossRef]
- Johansen, H.N.; Knudsen, K.E.; Sandström, B.; Skjøth, F. Effects of varying content of soluble dietary fibre from wheat flour and oat milling fractions on gastric emptying in pigs. Br. J. Nutr. 1996, 75, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Aravind, N.; Sissons, M.; Egan, N.; Fellows, C.M.; Blazek, J.; Gilbert, E.P. Effect of β-Glucan on Technological, Sensory, and Structural Properties of Durum Wheat Pasta. Cereal Chem. J. 2012, 89, 84–93. [Google Scholar] [CrossRef]
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces boulardii Administration Changes Gut Microbiota and Reduces Hepatic Steatosis, Low-Grade Inflammation, and Fat Mass in Obese and Type 2 Diabetic db/db Mice. mBio 2014, 5, e01011-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudi, R.; Suber, J.; Brown, R.; Johnson, B.M.; Vasu, C. Pretreatment with Yeast-Derived Complex Dietary Polysaccharides Suppresses Gut Inflammation, Alters the Microbiota Composition, and Increases Immune Regulatory Short-Chain Fatty Acid Production in C57BL/6 Mice. J. Nutr. 2019, 150, 1291–1302. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Effects of yeast-derived β-glucans on blood cholesterol and macrophage functionality. J. Immunotoxicol. 2009, 6, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, M.; Vetvicka, V. β-Glucans, History, and the Present: Immunomodulatory Aspects and Mechanisms of Action. J. Immunotoxicol. 2008, 5, 47–57. [Google Scholar] [CrossRef]
- Manners, D.J.; Masson, A.J.; Patterson, J.C.; Björndal, H.; Lindberg, B. The structure of a β-(1→6)-d-glucan from yeast cell walls. Biochem. J. 1973, 135, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Han, M.D.; Han, Y.S.; Hyun, S.H.; Shin, H.W. Solubilization of water-insoluble beta-glucan isolated from Ganoderma lucidum. J. Environ. Biol. 2008, 29, 237–242. [Google Scholar] [PubMed]
- Battilana, P.; Ornstein, K.; Minehira, K.; Schwarz, J.M.; Acheson, K.; Schneiter, P.; Burri, J.; Jéquier, E.; Tappy, L. Mechanisms of action of beta-glucan in postprandial glucose metabolism in healthy men. Eur. J. Clin. Nutr. 2001, 55, 327–333. [Google Scholar] [CrossRef] [Green Version]
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Wani, S.M.; Gani, A.; Mir, S.A.; Masoodi, F.A.; Khanday, F.A. β-Glucan: A dual regulator of apoptosis and cell proliferation. Int. J. Biol. Macromol. 2021, 182, 1229–1237. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Y.; Zou, S.; Duan, B.; Sun, M.; Xu, X. Yeast β-Glucan Suppresses the Chronic Inflammation and Improves the Microenvironment in Adipose Tissues of ob/ob Mice. J. Agric. Food Chem. 2018, 66, 621–629. [Google Scholar] [CrossRef]
- Parra, M.M.O.; Elangovan, S.; Lee, C.-T.; Satheesh, E. Specialized pro-resolving lipid mediators in experimental periodontitis: A systematic review. Oral Dis. 2018, 25, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Parsons, N.; Kadyszewski, E.; Festing, M.F.W.; Cuthill, I.C.; Fry, D.; Hutton, J.; Altman, D.G. Survey of the Quality of Experimental Design, Statistical Analysis and Reporting of Research Using Animals. PLoS ONE 2009, 4, e7824. [Google Scholar] [CrossRef]
- Ma, B.; Xu, J.-K.; Wu, W.-J.; Liu, H.-Y.; Kou, C.-K.; Liu, N.; Zhao, L. Survey of basic medical researchers on the awareness of animal experimental designs and reporting standards in China. PLoS ONE 2017, 12, e0174530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, K.H.; Hill, C.L.; Whittle, S. Quality of reporting of interventional animal studies in rheumatology: A systematic review using the ARRIVE guidelines. Int. J. Rheum. Dis. 2015, 18, 488–494. [Google Scholar] [CrossRef]
- Nicolosi, R.; Bell, S.J.; Bistrian, B.R.; Greenberg, I.; Forse, R.A.; Blackburn, G.L. Plasma lipid changes after supplementation with beta-glucan fiber from yeast. Am. J. Clin. Nutr. 1999, 70, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Santas, J.; Lázaro, E.; Cuñé, J. Effect of a polysaccharide-rich hydrolysate from Saccharomyces cerevisiae (LipiGo®) in body weight loss: Randomised, double-blind, placebo-controlled clinical trial in overweight and obese adults. J. Sci. Food Agric. 2017, 97, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Perel, P.; Roberts, I.; Sena, E.S.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.R.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMJ 2006, 334, 197. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; IntHout, J.; Ritskes-Hoitinga, M.; Rovers, M. Meta-Analyses of Animal Studies: An Introduction of a Valuable Instrument to Further Improve Healthcare. ILAR J. 2014, 55, 418–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesterinen, H.; Sena, E.S.; Egan, K.; Hirst, T.; Churolov, L.; Currie, G.; Antonic-Baker, A.; Howells, D.; Macleod, M.R. Meta-analysis of data from animal studies: A practical guide. J. Neurosci. Methods 2014, 221, 92–102. [Google Scholar] [CrossRef] [Green Version]
| References | Animal Model (Specie, Sex, Age) and Randomization | Specie and Purity | Groups and Dose of BG | Experimental Period | Body Weight Evaluation | Statistical Analysis # | Effects of BG on the Body Weight | Obesity Status |
|---|---|---|---|---|---|---|---|---|
| Babíček et al. (2007) [34] | Acute model: Brl- Han:WIST@Jcl rats male and female 5 weeks old Sub-chronic model: SPF Fisher CDF (F-344)/CrlBR rats (sub-chronic model) male and female 5–6 weeks old | Saccharomyces cerevisiae Purity: >75% | Acute toxicity study: Control group Intervention group: dose: 2000 mg/kg body weight (BW)/day n = 10 (5/group) Sub-chronic toxicity study: Control group Intervention groups: dose: 2 mg/kg BW/day dose: 33.3 mg/kg BW/day dose: 100 mg/kg BW/day n = 120 60 male and 60 female were randomly selected according to weight criteria and allocated in 4 groups (ou 10/sex/group ?) | 14 days 91 days | once a week | t-test ANOVA | no statistically significant difference | non-obese |
| Waszkiewicz-Robak et al. (2009) [35] | Wistar rats male age not mentioned | Saccharomyces cerevisiae Purity: 92% | Control: standard diet Intervention groups: BG 10 mg/kg BW/day BG 100 mg/kg BW/day Dried spent brewer’s yeast 50 mg/kg BW/day n = 29 (dried spent brewer’s yeast group = 8; the other = 7/group). After eating, all rats were fed ad libitum diet containing cholesterol | 42 days | daily | ANOVA | no statistically significant difference | non-obese |
| Araújo et al. (2017) [19] | Wistar rats male 3 weeks old | Saccharomyces cerevisiae Purity: >60% | Group C: control diet Group CB: control diet treated with BG 30 mg/kg/day Group O: obese, high-fat diet Group OB: obese, high-fat diet treated with BG 30 mg/kg/day n = 24 (6/group) | 28 days (after 60 days of obesity induction) | after 60 days of obesity induction and after 28 days of intervention | paired t-test | no statistically significant difference Obs: comparison of Groups CB × C | non-obese |
| Preece et al. (2021) [36] | Han:WIST rats male and female age not mentioned | Saccharomyces cerevisiae 90% | 40 male and 40 female divided separately into 4 groups: 0 (control group) BG 500 mg/kg BW/day BG 1000 mg/kg BW/day BG 2000 mg/kg BW/day n = 80 (10/sex/group) | 28 days | twice a week | one-way ANOVA followed by Duncan’s multiple range test | no statistically significant difference Obs: transitorily between 21 and 24 days it was a difference in weight gain in female using middle-dose (1000 mg) | non-obese |
| Cao et al. (2016) [38] | C57BL/6 mice male and female 7 weeks old | Baker’s yeast β-(1 → 3)-glucan (BYG) (Saccharomyces cerevisiae) Purity: 99% | ND group (normal diet), n = 10 HF group (high-fat), n = 30 PRE group (high-fat + BG 50 mg/kg/day), n = 10 After 30 days, streptozotocin-induced diabetes in mice of the HF and PRE groups. Then, HF group was subdivided into three new groups. MODEL group (high-fat diet + saline), n = 8 POST group (high-fat diet + BG 50 mg/kg/day), n = 8 MET (high-fat diet + metformin 50 mg/kg/day), n = 8 | first phase: 30 days (period of evaluation) streptozotocin diabetes induction: from day 31 to day 40 second phase: day 41 to day 120 | at the beginning and end of the first phase (30 days) | Paired-samples t-test (among two groups) and one-way ANOVA with Bonferroni’s post hoc test (among multiple groups) | statistically significant decrease Obs: body weight of the PRE (high-fat/BG) group was significantly lowered compared with HF group (high-fat) in the day 30 (first phase), before streptozotocin-induced diabetes. | obese |
| Shituleni et al. (2016) [39] | ICR mice female 4 weeks old | Saccharomyces cerevisiae Purity: >25% | Group A: control diet Group B: high-fat diet (HFD) Group C: HFD + 250 mg/kg yeast polysaccharide (YPS) 3 times a week HFD + 450 mg/kg YPS 3 times a week n = 60 (15/group) (n = 7/group for the body weight evaluation) | 49 days | once a week | one-way ANOVA followed by the Student–Newman–Keuls post hoc test | statistically significant decrease | obese |
| Cao et al. (2017) [37] | ob/ob mice C57BLKS.B6.V-Lepob/Nju male 11–12 weeks old | Baker’s yeast β-(1 → 3)-glucan (BYG) (Saccharomyces cerevisiae) Purity: 99% | Control group: water Treated group: BYG 25 mg/kg/day n = 14 (7/group) | 28 to 35 days with BYG diet; sacrificed at the age of 4−5 months | at the beginning and after 25 days of use of the BYG | Student’s t-test | statistically significant decrease | obese |
| Araújo et al. (2017) [19] | Wistar rats male 3 weeks old | Saccharomyces cerevisiae Purity: >60% | Group C: control diet Group CB: control diet treated with BG 30 mg/kg/day Group O: obese, high-fat diet Group OB: obese, high-fat diet treated with BG 30 mg/kg/day n = 24 (6/group) | 28 days (after 60 days of obesity induction) | after 60 days to obesity induction and after 4 weeks of intervention | paired t-test | no statistically significant decrease Obs: comparison of Groups OB × O | obese |
| Studies | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-obese animals | ||||||||||
| Babíček et al. (2007) [34] | + | + | - | - | - | + | - | + | + | ? |
| Waszkiewicz-Robak et al. (2009) [35] | + | + | - | - | - | + | - | + | + | ? |
| Araújo et al. (2017) [19] | + | + | + | - | - | + | - | + | + | + |
| Preece et al. (2021) [36] | + | + | - | - | - | + | - | + | + | + |
| Obese animals | ||||||||||
| Cao et al. (2016) [38] | + | - | - | - | - | + | - | ? | + | + |
| Shituleni et al. (2016) [39] | + | + | + | - | - | + | - | - | ? | ? |
| Cao et al. (2017) [37] | + | + | - | - | - | + | - | + | + | ? |
| Araújo et al. (2017) [19] | + | + | + | - | - | + | - | + | + | + |
| Studies | ARRIVE Items | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | Total | |
| Non-Obese Animals | |||||||||||||||||||||
| Babíček et al. (2007) [34] | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 30 |
| Waszkiewicz-Robak et al. (2009) [35] | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 28 |
| Araújo et al. (2017) [19] | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 30 |
| Preece et al. (2021) [36] | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 30 |
| Obese Animals | |||||||||||||||||||||
| Cao et al. (2016) [38] | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 0 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 29 |
| Shituleni et al. (2016) [39] | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 30 |
| Cao et al. (2017) [37] | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 0 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 28 |
| Araújo et al. (2017) [19] | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 30 |
| Category Score (Quality Obtained) | 8 | 9 | 16 | 8 | 15 | 8 | 14 | 14 | 11 | 8 | 8 | 14 | 15 | 8 | 13 | 16 | 10 | 12 | 14 | 14 | 235 |
| Maximum Score Expected (Quality Expected) | 8 | 16 | 16 | 8 | 16 | 16 | 16 | 16 | 16 | 16 | 8 | 16 | 16 | 8 | 16 | 16 | 16 | 16 | 16 | 16 | 288 |
| Ratio Quality Score/Maximum Score | 1 | 0.56 | 1 | 1 | 0.94 | 0.50 | 0.88 | 0.88 | 0.69 | 0.50 | 1 | 0.88 | 0.94 | 1 | 0.94 | 1 | 0.63 | 0.75 | 0.88 | 0.88 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canaan, M.M.; Reis-Canaan, J.C.; Zangerônimo, M.G.; Andrade, E.F.; Gonçalves, T.M.S.V.; Pereira, M.C.A.; Lima, R.R.; Pardi, V.; Murata, R.M.; Pereira, L.J. Yeast Beta-Glucans Ingestion Does Not Influence Body Weight: A Systematic Review and Meta-Analysis of Pre-Clinical Studies. Nutrients 2021, 13, 4250. https://doi.org/10.3390/nu13124250
Canaan MM, Reis-Canaan JC, Zangerônimo MG, Andrade EF, Gonçalves TMSV, Pereira MCA, Lima RR, Pardi V, Murata RM, Pereira LJ. Yeast Beta-Glucans Ingestion Does Not Influence Body Weight: A Systematic Review and Meta-Analysis of Pre-Clinical Studies. Nutrients. 2021; 13(12):4250. https://doi.org/10.3390/nu13124250
Chicago/Turabian StyleCanaan, Marcelo M., Juliana C. Reis-Canaan, Márcio G. Zangerônimo, Eric F. Andrade, Thais M. S. V. Gonçalves, Michel C. A. Pereira, Renato R. Lima, Vanessa Pardi, Ramiro M. Murata, and Luciano J. Pereira. 2021. "Yeast Beta-Glucans Ingestion Does Not Influence Body Weight: A Systematic Review and Meta-Analysis of Pre-Clinical Studies" Nutrients 13, no. 12: 4250. https://doi.org/10.3390/nu13124250
APA StyleCanaan, M. M., Reis-Canaan, J. C., Zangerônimo, M. G., Andrade, E. F., Gonçalves, T. M. S. V., Pereira, M. C. A., Lima, R. R., Pardi, V., Murata, R. M., & Pereira, L. J. (2021). Yeast Beta-Glucans Ingestion Does Not Influence Body Weight: A Systematic Review and Meta-Analysis of Pre-Clinical Studies. Nutrients, 13(12), 4250. https://doi.org/10.3390/nu13124250






