Developmental Vitamin D Deficiency in Pregnant Rats Does Not Induce Preeclampsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
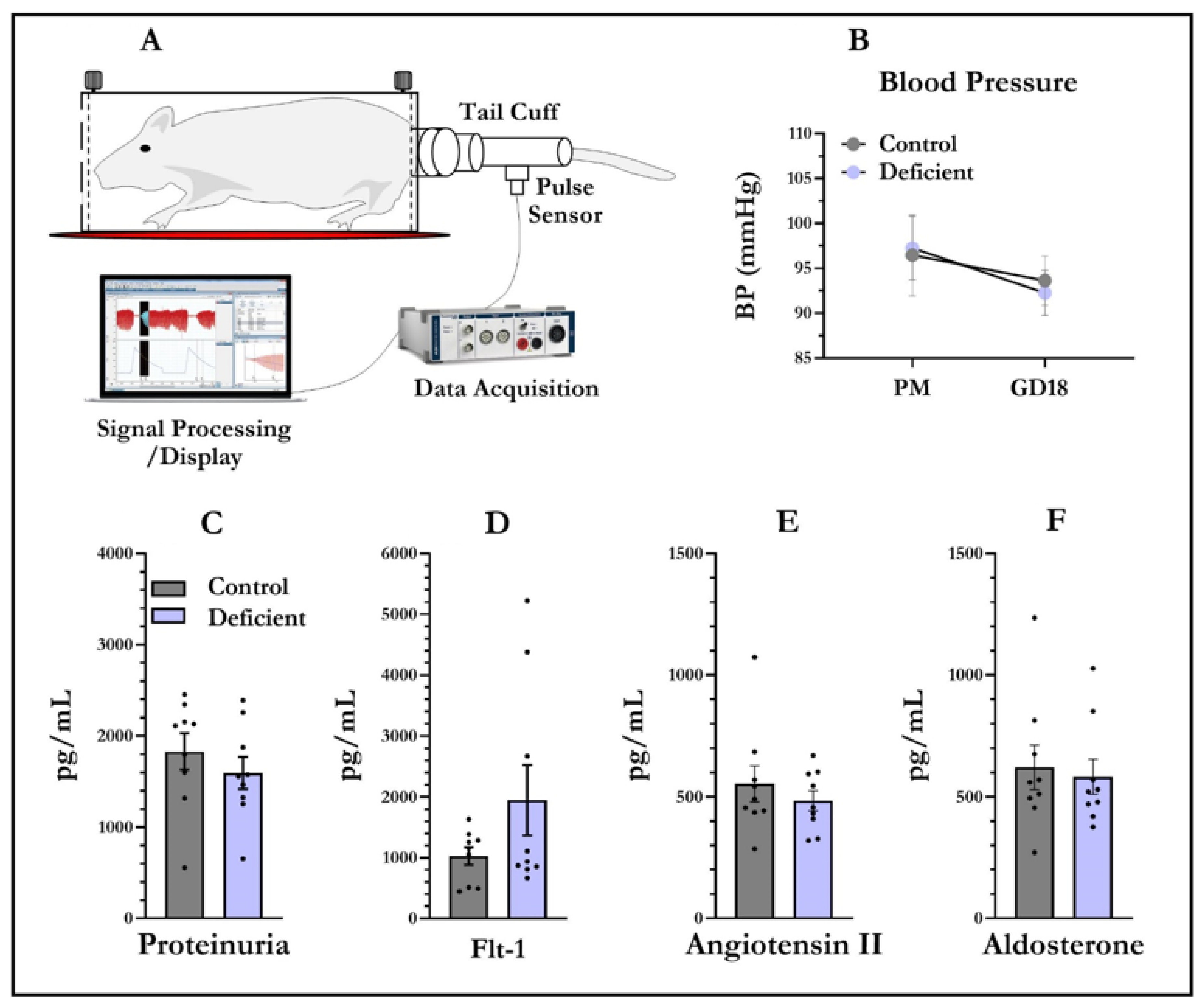
2.2. Blood Pressure Measurements and Tissue Collection
2.3. Angiotensin II, Flt-1, and Aldosterone Assays
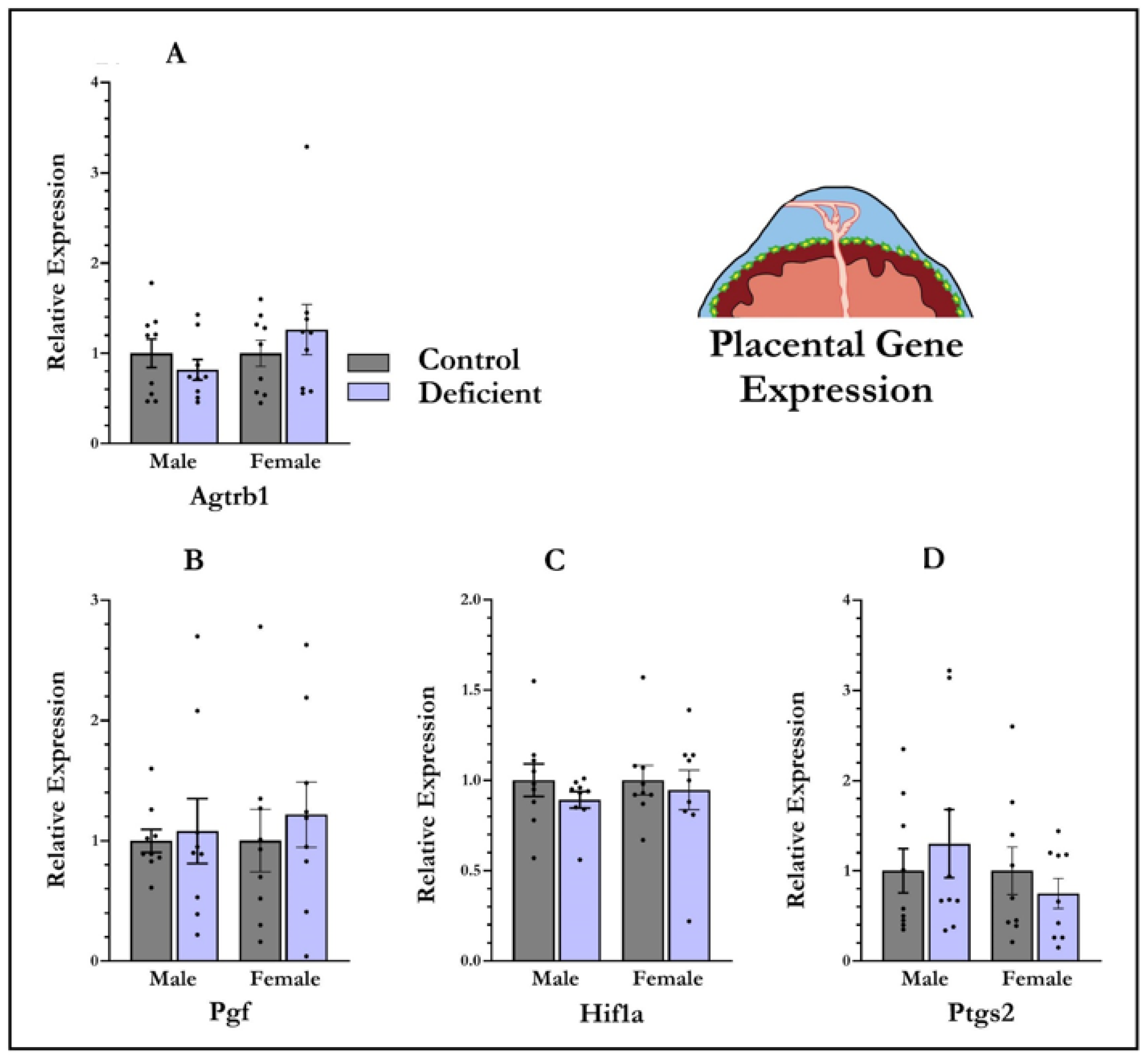
2.4. Quantitative Polymerase Chain Reaction (qPCR)
2.5. Statistical Analysis
3. Results
3.1. Blood Pressure and Proteinuria
3.2. Angiotensin II, Flt-1, and Aldosterone Levels in Dam Sera
3.3. Placental Weight and Angiotensin II, Flt-1, and Aldosterone Levels in Placenta
3.4. mRNA Expression in Placenta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gumusoglu, S.B.; Chilukuri, A.S.; Santillan, D.A.; Santillan, M.K.; Stevens, H.E. Neurodevelopmental Outcomes of Prenatal Preeclampsia Exposure. Trends Neurosci. 2020, 43, 253–268. [Google Scholar] [CrossRef]
- Dachew, B.A.; Mamun, A.; Maravilla, J.C.; Alati, R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: Meta-analysis. Br. J. Psychiatry 2018, 212, 142–147. [Google Scholar] [CrossRef]
- Mol, B.W.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; De Groot, C.J.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Gou, W.; Li, C.; Wu, M.; Han, Z.; Li, X.; Chen, Q. Proteinuria in preeclampsia: Not essential to diagnosis but related to disease severity and fetal outcomes. Pregnancy Hypertens. 2017, 8, 60–64. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K. Incidence of preeclampsia: Risk factors and outcomes associated with early-versus late-onset disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. Placental Origins of Preeclampsia. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef]
- Perez-Sepulveda, A.; España-Perrot, P.P.; Norwitz, E.R.; Illanes, S.E. Metabolic Pathways Involved in 2-Methoxyestradiol Synthesis and Their Role in Preeclampsia. Reprod. Sci. 2013, 20, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Gammill, H.S. Preeclampsia: Recent insights. Hypertension 2005, 46, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Kendall, R.L.; Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10705–10709. [Google Scholar] [CrossRef]
- Athanassiades, A.; Lala, P.K. Role of placenta growth factor (PIGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta 1998, 19, 465–473. [Google Scholar] [CrossRef]
- Shojaati, K.; Causevic, M.; Kadereit, B.; Dick, B.; Imobersteg, J.; Schneider, H.; Beinder, E.; Kashiwagi, M.; Frey, B.M.; Frey, F.J.; et al. Evidence for compromised aldosterone synthase enzyme activity in preeclampsia. Kidney Int. 2004, 66, 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Escher, G.; Mohaupt, M. Role of aldosterone availability in preeclampsia. Mol. Asp. Med. 2007, 28, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.; Powers, R.W.; Roberts, J.M. Maternal Vitamin D Deficiency Increases the Risk of Preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.M.; Haeri, S.; Camargo, C.A.; Espinola, J.A.; Stuebe, A.M. A Nested Case-Control Study of Midgestation Vitamin D Deficiency and Risk of Severe Preeclampsia. J. Clin. Endocrinol. Metab. 2010, 95, 5105–5109. [Google Scholar] [CrossRef]
- Robinson, C.J.; Alanis, M.C.; Wagner, C.L.; Hollis, B.W.; Johnson, D.D. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am. J. Obstet. Gynecol. 2010, 203, 366.e1–366.e6. [Google Scholar] [CrossRef] [PubMed]
- Bakacak, M.; Serin, S.; Ercan, O.; Köstü, B.; Avci, F.; Kılınç, M.; Kıran, H.; Kiran, G. Comparison of Vitamin D levels in cases with preeclampsia, eclampsia and healthy pregnant women. Int. J. Clin. Exp. Med. 2015, 8, 16280–16286. [Google Scholar]
- Wei, S.Q.; Audibert, F.; Hidiroglou, N.; Sarafin, K.; Julien, P.; Wu, Y.; Luo, Z.C.; Fraser, W.D. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 832–839. [Google Scholar] [CrossRef]
- Seto, T.L.; Tabangin, M.E.; Langdon, G.; Mangeot, C.; Dawodu, A.; Steinhoff, M.; Narendran, V. Racial disparities in cord blood vitamin D levels and its association with small-for-gestational-age infants. J. Perinatol. 2016, 36, 623–628. [Google Scholar] [CrossRef]
- Bryant, A.S.; Seely, E.W.; Cohen, A.; Lieberman, E. Patterns of Pregnancy-Related Hypertension in Black and White Women. Hypertens Pregnancy 2005, 24, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Shand, A.; Nassar, N.; Von Dadelszen, P.; Innis, S.; Green, T. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG: Int. J. Obstet. Gynaecol. 2010, 117, 1593–1598. [Google Scholar] [CrossRef]
- Powe, C.E.; Seely, E.W.; Rana, S.; Bhan, I.; Ecker, J.; Karumanchi, S.A.; Thadhani, R. First Trimester Vitamin D, Vitamin D Binding Protein, and Subsequent Preeclampsia. Hypertension 2010, 56, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Wetta, L.A.; Biggio, J.R.; Cliver, S.; Abramovici, A.; Barnes, S.; Tita, A.T. Is midtrimester vitamin D status associated with spontaneous preterm birth and preeclampsia? Am. J. Perinatol. 2014, 31, 541–546. [Google Scholar]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and Diabetes. Curr. Diabetes Rep. 2015, 15, 9. [Google Scholar] [CrossRef]
- Shao, Y.; Qiu, J.; Huang, H.; Mao, B.; Dai, W.; He, X.; Cui, H.; Lin, X.; Lv, L.; Wang, D.; et al. Pre-pregnancy BMI, gestational weight gain and risk of preeclampsia: A birth cohort study in Lanzhou, China. BMC Pregnancy Childbirth 2017, 17, 400. [Google Scholar] [CrossRef] [PubMed]
- Amraei, M.; Mohamadpour, S.; Sayehmiri, K.; Mousavi, S.F.; Shirzadpour, E.; Moayeri, A. Effects of Vitamin D Deficiency on Incidence Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-analysis. Front. Endocrinol. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2011, 36, 387–396. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Roberts, J.M.; Simhan, H.N. Prepregnancy Obesity Predicts Poor Vitamin D Status in Mothers and Their Neonates. J. Nutr. 2007, 137, 2437–2442. [Google Scholar] [CrossRef]
- McGrath, J.J.; Eyles, D.W.; Pedersen, C.B.; Anderson, C.; Ko, P.; Burne, T.H.; Norgaard-Pedersen, B.; Hougaard, D.M.; Mortensen, P.B. Neonatal vitamin d status and risk of schizophrenia: A population-based case-control study. Schizophr. Res. 2010, 117, 312. [Google Scholar] [CrossRef][Green Version]
- Eyles, D.; Trzaskowski, M.; Vinkhuyzen, A.A.E.; Mattheisen, M.; Meier, S.; Gooch, H.; Anggono, V.; Cui, X.; Tan, M.C.; Burne, T.H.J.; et al. The association between neonatal vitamin D status and risk of schizophrenia. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Eyles, D.W.; Magnusson, C.; Newschaffer, C.J.; McGrath, J.J.; Kvaskoff, D.; Ko, P.; Dalman, C.; Karlsson, H.; Gardner, R.M. Developmental vitamin D and autism spectrum disorders: Findings from the Stockholm Youth Cohort. Mol. Psychiatry 2019, 26, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- Vinkhuyzen, A.; Eyles, D.; Burne, T.; Blanken, L.M.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism-related traits: The Generation R Study. Mol. Psychiatry 2016, 23, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D. Vitamin D and brain development. Endocr. Abstr. 2016. [Google Scholar] [CrossRef]
- Eyles, D.W.; Burne, T.H.; McGrath, J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocr. 2013, 34, 47–64. [Google Scholar] [CrossRef]
- Eyles, D.W.; Dean, A.J. Chapter 15—Maternal Nutritional Deficiencies and Schizophrenia: Lessons from Animal Models with a Focus on Developmental Vitamin D Deficiency. In Handbook of Behavioral Neuroscience; Pletnikov, M.V., Waddington, J.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 243–264. [Google Scholar]
- Ali, A.; Vasileva, S.; Langguth, M.; Alexander, S.; Cui, X.; Whitehouse, A.; McGrath, J.J.; Eyles, D. Developmental Vitamin D Deficiency Produces Behavioral Phenotypes of Relevance to Autism in an Animal Model. Nutrients 2019, 11, 1187. [Google Scholar] [CrossRef]
- Kesby, J.; Turner, K.; Alexander, S.; Eyles, D.; McGrath, J.; Burne, T.H. Developmental vitamin D deficiency alters multiple neurotransmitter systems in the neonatal rat brain. Int. J. Dev. Neurosci. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Golzarand, M.; Shab-Bidar, S.; Koochakpoor, G.; J, R.S.; Djafarian, K. Effect of vitamin D3 supplementation on blood pressure in adults: An updated meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 663–673. [Google Scholar] [CrossRef]
- Forman, J.P.; Williams, J.; Fisher, N.D. Plasma 25-Hydroxyvitamin D and Regulation of the Renin-Angiotensin System in Humans. Hypertension 2010, 55, 1283–1288. [Google Scholar] [CrossRef]
- Ma, S.-L.; Tian, X.-Y.; Wang, Y.-Q.; Zhang, H.-F.; Zhang, L. Vitamin D Supplementation Prevents Placental Ischemia Induced Endothelial Dysfunction by Downregulating Placental Soluble FMS-Like Tyrosine Kinase-1. DNA Cell Biol. 2017, 36, 1134–1141. [Google Scholar] [CrossRef]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Grammer, T.; Drechsler, C.; Boehm, B.O.; März, W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin. Chim. Acta 2010, 411, 1354–1360. [Google Scholar] [CrossRef]
- Boxer, R.S.; Hoit, B.D.; Schmotzer, B.J.; Stefano, G.; Gomes, A.; Negrea, L. The Effect of Vitamin D on Aldosterone and Health Status in Patients with Heart Failure. J. Card. Fail. 2014, 20, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Burne, T.H.; Alexander, S.; Cui, X.; McGrath, J.J. The Developmental Vitamin D (DVD) Model of Schizophrenia. In Animal Models of Schizophrenia and Related Disorders; Humana Press: Totowa, NJ, USA, 2011. [Google Scholar]
- Sathishkumar, K.; Elkins, R.; Chinnathambi, V.; Gao, H.; Hankins, G.D.; Yallampalli, C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod. Biol. Endocrinol. 2011, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, J.; Deb, D.K.; Chang, A.; Li, Y.C. Vitamin D Receptor Attenuates Renal Fibrosis by Suppressing the Renin-Angiotensin System. J. Am. Soc. Nephrol. 2010, 21, 966–973. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, F.; Cao, K.; Xu, D.I.; Goltzman, D.; Miao, D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008, 74, 170–179. [Google Scholar] [CrossRef]
- Liu, N.Q.; Ouyang, Y.; Bulut, Y.; Lagishetty, V.; Chan, S.; Hollis, B.W.; Wagner, C.; Equils, O.; Hewison, M. Dietary Vitamin D Restriction in Pregnant Female Mice Is Associated with Maternal Hypertension and Altered Placental and Fetal Development. Endocrinology 2013, 154, 2270–2280. [Google Scholar] [CrossRef]
- Andersen, L.B.; Golic, M.; Przybyl, L.; Sorensen, G.L.; Jørgensen, J.S.; Fruekilde, P.; von Versen-Höynck, F.; Herse, F.; Højskov, C.S.; Dechend, R.; et al. Vitamin D depletion does not affect key aspects of the preeclamptic phenotype in a transgenic rodent model for preeclampsia. J. Am. Soc. Hypertens. 2016, 10, 597–607.e1. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Dixon, G.; Robertson, W.; Brosens, I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1, 3–19. [Google Scholar] [CrossRef]
- Marshall, S.A.; Hannan, N.J.; Jelinic, M.; Nguyen, T.P.; Girling, J.; Parry, L.J. Animal models of preeclampsia: Translational failings and why. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R499–R508. [Google Scholar] [CrossRef]
- Silva, J.F.; Serakides, R. Intrauterine trophoblast migration: A comparative view of humans and rodents. Cell Adhes. Migr. 2016, 10, 88–110. [Google Scholar] [CrossRef]
- Ain, R.; Canham, L.N.; Soares, M.J. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: Novel endocrine phenotype and regulation. Dev. Biol. 2003, 260, 176–190. [Google Scholar] [CrossRef]
- Vianna, P.; Bauer, M.; Dornfeld, D.; Chies, J. Distress conditions during pregnancy may lead to pre-eclampsia by increasing cortisol levels and altering lymphocyte sensitivity to glucocorticoids. Med. Hypotheses 2011, 77, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, Z.; Liu, H.; Chen, Z.; Wu, J.; Zhang, Y.; Yu, Y. Association between mental stress and gestational hypertension/preeclampsia: A meta-analysis. Obstet. Gynecol. Surv. 2013, 68, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Tesic, D.; Hawes, J.E.; Zosky, G.R.; Wyrwoll, C.S. Vitamin D Deficiency in BALB/c Mouse Pregnancy Increases Placental Transfer of Glucocorticoids. Endocrinology 2015, 156, 3673–3679. [Google Scholar] [CrossRef]
- Ali, A.A.; Cui, X.; Pertile, R.A.N.; Li, X.; Medley, G.; Alexander, S.A.; Whitehouse, A.J.O.; McGrath, J.J.; Eyles, D.W. Developmental vitamin D deficiency increases foetal exposure to testosterone. Mol. Autism 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Rogers, F.; Buller, K.; McGrath, J.J.; Ko, P.; French, K.; Burne, T.H. Developmental vitamin D (DVD) deficiency in the rat alters adult behaviour independently of HPA function. Psychoneuroendocrinology 2006, 31, 958–964. [Google Scholar] [CrossRef]
- Li, J.; Lamarca, B.; Reckelhoff, J.F. A model of preeclampsia in rats: The reduced uterine perfusion pressure (RUPP) model. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1–H8. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; Kassab, S.E.; Miller, M.T.; Abram, S.R.; Reckelhoff, J.F.; Bennett, W.A.; Granger, J.P. Reduced Uterine Perfusion Pressure During Pregnancy in the Rat Is Associated with Increases in Arterial Pressure and Changes in Renal Nitric Oxide. Hypertension 2001, 37, 1191–1195. [Google Scholar] [CrossRef]
- LaMarca, B.B.D.; Bennett, W.A.; Alexander, B.T.; Cockrell, K.; Granger, J.P. Hypertension produced by reductions in uterine perfusion in the pregnant rat: Role of tumor necrosis factor-α. Hypertension 2005, 46, 1022–1025. [Google Scholar] [CrossRef]
- Intapad, S.; Warrington, J.P.; Spradley, F.T.; Palei, A.C.; Drummond, H.A.; Ryan, M.J.; Granger, J.P.; Alexander, B.T. Reduced uterine perfusion pressure induces hypertension in the pregnant mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1353–R1357. [Google Scholar] [CrossRef] [PubMed]
- Goksu Erol, A.Y.; Nazli, M.; Elis Yildiz, S. Expression levels of cyclooxygenase-2, tumor necrosis factor-α and inducible NO synthase in placental tissue of normal and preeclamptic pregnancies. J. Matern.-Fetal Neonatal Med. 2012, 25, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Rath, G.; Aggarwal, R.; Jawanjal, P.; Tripathi, R.; Batra, A. HIF-1 Alpha and Placental Growth Factor in Pregnancies Complicated with Preeclampsia: A Qualitative and Quantitative Analysis. J. Clin. Lab. Anal. 2014, 30, 75–83. [Google Scholar] [CrossRef]
- Chen, X.; Xi, X.; Cui, F.; Wen, M.; Hong, A.; Hu, Z.; Ni, J. Abnormal expression and clinical significance of 25-hydroxyvitamin D and sFlt-1 in patients with preeclampsia. J. Int. Med Res. 2019, 47, 4673–4682. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shoshan, M.; Amir, S.; Dang, D.T.; Dang, L.H.; Weisman, Y.; Mabjeesh, N.J. 1α, 25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 2007, 6, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zheng, W.; Teegarden, D. 1α, 25-Dihydroxyvitamin D regulates hypoxia-inducible factor-1α in untransformed and Harvey-ras transfected breast epithelial cells. Cancer Lett. 2010, 298, 159–166. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Chen, D.; Zuo, C.; Qin, J.; Wang, H.; Wang, J.; Yu, Y. Vitamin D Inhibits COX-2 Expression and Inflammatory Response by Targeting Thioesterase Superfamily Member 4. J. Biol. Chem. 2014, 289, 11681–11694. [Google Scholar] [CrossRef]
- Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin D and regulation of vascular cell function. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H753–H765. [Google Scholar] [CrossRef]
- Fushima, T.; Sekimoto, A.; Minato, T.; Ito, T.; Oe, Y.; Kisu, K.; Sato, E.; Funamoto, K.; Hayase, T.; Kimura, Y.; et al. Reduced Uterine Perfusion Pressure (RUPP) Model of Preeclampsia in Mice. PLoS ONE 2016, 11, e0155426. [Google Scholar] [CrossRef]
- Buñag, R.D. Facts and fallacies about measuring blood pressure in rats. Clin. Exp. Hypertens. Part A Theory Pr. 1983, 5, 1659–1681. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.T.; Pearson, D.V.; Powell, C.E.; Kirschner, G.L. Thermal stress elevates the systolic blood pressure of spontaneously hypertensive rats. Life Sci. 1978, 22, 359–362. [Google Scholar] [CrossRef]
- Cui, X.; McGrath, J.J.; Burne, T.H.J.; Eyles, D.W. Vitamin D and schizophrenia: 20 years on. Mol. Psychiatry 2021, 26, 2708–2720. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Alexander, S.; Ko, P.; Cuffe, J.S.M.; Whitehouse, A.J.O.; McGrath, J.J.; Eyles, D. Developmental Vitamin D Deficiency in Pregnant Rats Does Not Induce Preeclampsia. Nutrients 2021, 13, 4254. https://doi.org/10.3390/nu13124254
Ali A, Alexander S, Ko P, Cuffe JSM, Whitehouse AJO, McGrath JJ, Eyles D. Developmental Vitamin D Deficiency in Pregnant Rats Does Not Induce Preeclampsia. Nutrients. 2021; 13(12):4254. https://doi.org/10.3390/nu13124254
Chicago/Turabian StyleAli, Asad, Suzanne Alexander, Pauline Ko, James S. M. Cuffe, Andrew J. O. Whitehouse, John J. McGrath, and Darryl Eyles. 2021. "Developmental Vitamin D Deficiency in Pregnant Rats Does Not Induce Preeclampsia" Nutrients 13, no. 12: 4254. https://doi.org/10.3390/nu13124254
APA StyleAli, A., Alexander, S., Ko, P., Cuffe, J. S. M., Whitehouse, A. J. O., McGrath, J. J., & Eyles, D. (2021). Developmental Vitamin D Deficiency in Pregnant Rats Does Not Induce Preeclampsia. Nutrients, 13(12), 4254. https://doi.org/10.3390/nu13124254





