Oral Consumption of Bread from an RNAi Wheat Line with Strongly Silenced Gliadins Elicits No Immunogenic Response in a Pilot Study with Celiac Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
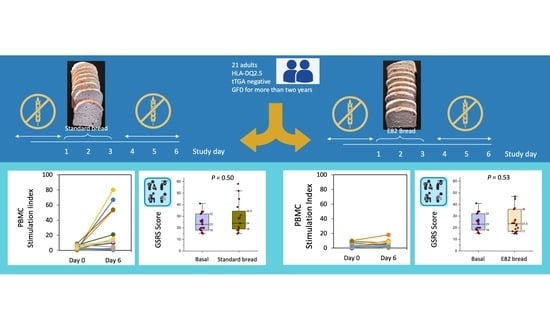
2.1. Patients and In Vivo Challenge
2.2. Preparation of Bread Types
2.3. Protein, Starch, Fructans, and Gluten Content Determination
2.4. Detection of IFN-γ Secreting Cells in Peripheral Blood Mononuclear Cells (PBMCs) by ELISPOT
2.5. Quantification of the Gluten Immunogenic Peptides (GIP) in Stool Samples
2.6. Statistical Analysis
3. Results
3.1. IFN-γ Producing T Cells (ELISPOT)
3.2. Gluten Immunogenic Peptides (GIP) in Human Stool
3.3. Gastrointestinal Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sollid, L.M. Coeliac disease: Dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2002, 2, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bearzi, I.; Holmes, G.K.T. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology 2005, 128, S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Montserrat, V.; Mujico, J.R.; Loh, K.L.; Beringer, D.X.; Van Lummel, M.; Thompson, A.; Mearin, M.L.; Schweizer, J.; Kooy-Winkelaar, Y.; et al. T-cell receptor recognition of HLA-DQ2-gliadin complexes associated with celiac disease. Nat. Struct. Mol. Biol. 2014, 21, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Sollid, L.M.; Tye-Din, J.A.; Qiao, S.W.; Anderson, R.P.; Gianfrani, C.; Koning, F. Update 2020: Nomenclature and listing of celiac disease-relevant gluten epitopes recognized by CD4+ T cells. Immunogenetics 2020, 72, 85–88. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Stewart, J.A.; Dromey, J.A.; Beissbarth, T.; Van Heel, D.A.; Tatham, A.; Henderson, K.; Mannering, S.I.; Gianfrani, C.; Jewell, D.P.; et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2010, 2, 41ra51. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.P.; Degano, P.; Godkin, A.J.; Jewell, D.P.; Hill, A.V.S. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 2000, 6, 337–342. [Google Scholar] [CrossRef]
- Anderson, R.P.; Van Heel, D.A.; Tye-Din, J.A.; Barnardo, M.; Salio, M.; Jewell, D.P.; Hill, A.V.S. T cells in peripheral blood after gluten challenge in coeliac disease. Gut 2005, 54, 1217–1223. [Google Scholar] [CrossRef]
- Kurki, A.; Kemppainen, E.; Laurikka, P.; Kaukinen, K.; Lindfors, K. The use of peripheral blood mononuclear cells in celiac disease diagnosis and treatment. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Picascia, S.; Camarca, A.; Malamisura, M.; Mandile, R.; Galatola, M.; Cielo, D.; Gazza, L.; Mamone, G.; Auricchio, S.; Troncone, R.; et al. In celiac disease patients the in vivo challenge with the diploid Triticum monococcum elicits a reduced immune response compared to hexaploid wheat. Mol. Nutr. Food Res. 2020, 64, 1901032. [Google Scholar] [CrossRef] [PubMed]
- Picascia, S.; Mandile, R.; Auricchio, R.; Troncone, R.; Gianfrani, C. Gliadin-specific T-cells mobilized in the peripheral blood of coeliac patients by short oral gluten challenge: Clinical applications. Nutrients 2015, 7, 10020–10031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvester, J.A.; Comino, I.; Kelly, C.P.; Sousa, C.; Duerksen, D.R.; Bernstein, C.N.; Cebolla, A.; Dominguez, M.R.; Graff, L.A.; Green, K.H.; et al. Most patients with celiac disease on gluten-free diets consume measurable amounts of gluten. Gastroenterology 2020, 158, 1497–1499.e1. [Google Scholar] [CrossRef] [PubMed]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–1308. [Google Scholar] [CrossRef]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A review on the gluten-free diet: Technological and nutritional challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef] [Green Version]
- Caio, G.; Ciccocioppo, R.; Zoli, G.; De Giorgio, R.; Volta, U. Therapeutic options for coeliac disease: What else beyond gluten-free diet? Dig. Liver Dis. 2020, 52, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosell, C.M.; Barro, F.; Sousa, C.; Mena, M.C. Cereals for developing gluten-free products and analytical tools for gluten detection. J. Cereal Sci. 2014, 59, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Gil-Humanes, J.; Pistón, F.; Tollefsen, S.; Sollid, L.M.; Barro, F. Effective shutdown in the expression of celiac disease-related wheat gliadin T-cell epitopes by RNA interference. Proc. Natl. Acad. Sci. USA 2010, 107, 17023–17028. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-León, S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 6213. [Google Scholar] [CrossRef]
- Ozuna, C.V.; Barro, F. Safety evaluation of transgenic low-gliadin wheat in Sprague Dawley rats: An alternative to the gluten free diet with no subchronic adverse effects. Food Chem. Toxicol. 2017, 107, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-León, S.; Giménez, M.J.; Comino, I.; Sousa, C.; López Casado, M.Á.; Torres, M.I.; Barro, F. Stimulatory response of celiac disease peripheral blood mononuclear cells induced by RNAi wheat lines differing in grain protein composition. Nutrients 2019, 11, 2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulich, K.R.; Piqué, J.M.; Vegazo García, O.; Jiménez, J.; Zapardiel, J.; Carlsson, J.; Wiklund, I. Psychometric validation of translation to spanish of the gastrointestinal symptoms rating scale (GSRS) and quality of life in reflux and dyspepsia (QOLRAD) in patients with gastroesophageal reflux disease. Revista Clinica Espanola 2005, 205, 588–594. [Google Scholar] [CrossRef]
- Dumas, J.B.A. Procedes de l’analyse organique. Ann. Chim. Phys. 1831, 247, 198–213. [Google Scholar]
- ICC. Determination of Starch Content by Hydrochloric Acid Dissolution; Method No. 123/121; International Association for Cereal Science and Technology: Vienna, Austria, 1994. [Google Scholar]
- Ferre, S.; García, E.; Méndez, E. Measurement of hydrolysed gliadins by a competitive ELISA based on monoclonal antibody R5: Analysis of syrups and beers. In Proceedings of the 18th Meeting Working Group on Prolamin Analysis and Toxicity; Stern, M., Ed.; Verlag Wissenschafliche Scripten: Zwickau, Germany, 2004; pp. 65–69. [Google Scholar]
- Vaquero, L.; Comino, I.; Vivas, S.; Rodríguez-Martín, L.; Giménez, M.J.; Pastor, J.; Sousa, C.; Barro, F. Tritordeum: A novel cereal for food processing with good acceptability and significant reduction in gluten immunogenic peptides in comparison with wheat. J. Sci. Food Agric. 2018, 98, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Comino, I.; Fernández-Bañares, F.; Esteve, M.; Ortigosa, L.; Castillejo, G.; Fambuena, B.; Ribes-Koninckx, C.; Sierra, C.; Rodríguez-Herrera, A.; Salazar, J.C.; et al. Fecal gluten peptides reveal limitations of serological tests and food questionnaires for monitoring gluten-free diet in celiac disease patients. Am. J. Gastroenterol. 2016, 111, 1456–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2017; Volume 2, ISBN 3900051070. [Google Scholar]
- Jnawali, P.; Kumar, V.; Tanwar, B. Celiac disease: Overview and considerations for development of gluten-free foods. Food Sci. Hum. Wellness 2016, 5, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Haro, C.; Villatoro, M.; Vaquero, L.; Pastor, J.; Giménez, M.J.; Ozuna, C.V.; Sánchez-León, S.; García-Molina, M.D.; Segura, V.; Comino, I.; et al. The dietary intervention of transgenic low-gliadin wheat bread in patients with non-celiac gluten sensitivity (NCGS) showed no differences with gluten free diet (GFD) but provides better gut microbiota profile. Nutrients 2018, 10, 1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Humanes, J.; Pistón, F.; Altamirano-Fortoul, R.; Real, A.; Comino, I.; Sousa, C.; Rosell, C.M.; Barro, F. Reduced-gliadin wheat bread: An alternative to the gluten-free diet for consumers suffering gluten-related pathologies. PLoS ONE 2014, 9, e90898. [Google Scholar] [CrossRef] [Green Version]
- Gil-Humanes, J.; Pistón, F.; Barro, F.; Rosell, C.M. The shutdown of celiac disease-related gliadin epitopes in bread wheat by RNAi provides flours with increased stability and better tolerance to over-mixing. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Barro, F.; Iehisa, J.C.M.; Giménez, M.J.; García-Molina, M.D.; Ozuna, C.V.; Comino, I.; Sousa, C.; Gil-Humanes, J. Targeting of prolamins by RNAi in bread wheat: Effectiveness of seven silencing-fragment combinations for obtaining lines devoid of coeliac disease epitopes from highly immunogenic gliadins. Plant Biotechnol. J. 2016, 14, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Cebolla, Á.; Moreno, M.d.L.; Coto, L.; Sousa, C. Gluten immunogenic peptides as standard for the evaluation of potential harmful prolamin content in food and human specimen. Nutrients 2018, 10, 1927. [Google Scholar] [CrossRef] [Green Version]
- Hardy, M.Y.; Girardin, A.; Pizzey, C.; Cameron, D.J.; Watson, K.A.; Picascia, S.; Auricchio, R.; Greco, L.; Gianfrani, C.; La Gruta, N.L.; et al. Consistency in polyclonal T-cell responses to gluten between children and adults with celiac disease. Gastroenterology 2015, 149, 1541–1552.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamone, G.; Camarca, A.; Fierro, O.; Sidney, J.; Mazzarella, G.; Addeo, F.; Auricchio, S.; Troncone, R.; Sette, A.; Gianfrani, C. Immunogenic peptides can be detected in whole gluten by transamidating highly susceptible glutamine residues: Implication in the search for gluten-free cereals. J. Agric. Food Chem. 2013, 61, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Roncoroni, L.; Hils, M.; Pasternack, R.; Barisani, D.; Terrani, C.; Vaira, V.; Ferrero, S.; Bardella, M.T. Immunological effects of transglutaminase-treated gluten in coeliac disease. Hum. Immunol. 2012, 73, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Comino, I.; Real, A.; Vivas, S.; Síglez, M.Á.; Caminero, A.; Nistal, E.; Casqueiro, J.; Rodríguez-Herrera, A.; Cebolla, Á.; Sousa, C. Monitoring of gluten-free diet compliance in celiac patients by assessment of gliadin 33-mer equivalent epitopes in feces. Am. J. Clin. Nutr. 2012, 95, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Dieterich, W.; Zopf, Y. Gluten and FODMAPS-sense of a restriction/when is restriction necessary? Nutrients 2019, 11, 1957. [Google Scholar] [CrossRef] [Green Version]
- Menezes, L.A.A.; Molognoni, L.; de Sá Ploêncio, L.A.; Costa, F.B.M.; Daguer, H.; Lindner, J.D.D. Use of sourdough fermentation to reducing FODMAPs in breads. Eur. Food Res. Technol. 2019, 245, 1183–1195. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Anderson, R.P.; Ffrench, R.A.; Brown, G.J.; Hodsman, P.; Siegel, M.; Botwick, W.; Shreeniwas, R. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin. Immunol. 2010, 134, 289–295. [Google Scholar] [CrossRef]
- Mandile, R.; Picascia, S.; Parrella, C.; Camarca, A.; Gobbetti, M.; Greco, L.; Troncone, R.; Gianfrani, C.; Auricchio, R. Lack of immunogenicity of hydrolysed wheat flour in patients with coeliac disease after a short-term oral challenge. Aliment. Pharmacol. Ther. 2017, 46, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Catassi, C.; Fabiani, E.; Iacono, G.; D’Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; De Vitis, I.; et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr. 2007, 85, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Silvester, J.A.; Comino, I.; Rigaux, L.N.; Segura, V.; Green, K.H.; Cebolla, A.; Weiten, D.; Dominguez, R.; Leffler, D.A.; Leon, F.; et al. Exposure sources, amounts and time course of gluten ingestion and excretion in patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 2020, 52, 1469–1479. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-López, M.H.; Sánchez-León, S.; Marín-Sanz, M.; Comino, I.; Segura, V.; Vaquero, L.; Rivero-Lezcano, O.M.; Pastor, J.; Sousa, C.; Vivas, S.; et al. Oral Consumption of Bread from an RNAi Wheat Line with Strongly Silenced Gliadins Elicits No Immunogenic Response in a Pilot Study with Celiac Disease Patients. Nutrients 2021, 13, 4548. https://doi.org/10.3390/nu13124548
Guzmán-López MH, Sánchez-León S, Marín-Sanz M, Comino I, Segura V, Vaquero L, Rivero-Lezcano OM, Pastor J, Sousa C, Vivas S, et al. Oral Consumption of Bread from an RNAi Wheat Line with Strongly Silenced Gliadins Elicits No Immunogenic Response in a Pilot Study with Celiac Disease Patients. Nutrients. 2021; 13(12):4548. https://doi.org/10.3390/nu13124548
Chicago/Turabian StyleGuzmán-López, María H., Susana Sánchez-León, Miriam Marín-Sanz, Isabel Comino, Verónica Segura, Luis Vaquero, Octavio M. Rivero-Lezcano, Jorge Pastor, Carolina Sousa, Santiago Vivas, and et al. 2021. "Oral Consumption of Bread from an RNAi Wheat Line with Strongly Silenced Gliadins Elicits No Immunogenic Response in a Pilot Study with Celiac Disease Patients" Nutrients 13, no. 12: 4548. https://doi.org/10.3390/nu13124548
APA StyleGuzmán-López, M. H., Sánchez-León, S., Marín-Sanz, M., Comino, I., Segura, V., Vaquero, L., Rivero-Lezcano, O. M., Pastor, J., Sousa, C., Vivas, S., & Barro, F. (2021). Oral Consumption of Bread from an RNAi Wheat Line with Strongly Silenced Gliadins Elicits No Immunogenic Response in a Pilot Study with Celiac Disease Patients. Nutrients, 13(12), 4548. https://doi.org/10.3390/nu13124548