U.S. Montmorency Tart Cherry Juice Decreases Bone Resorption in Women Aged 65–80 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Medical History, Physical Activity, Calcium Intake and Sun Exposure Questionnaires
2.4. Anthropometric Measurements
2.5. Serum Biomarkers
2.6. Statistical Analysis
3. Results
3.1. Participant Demographic and Lifestyle Factors
3.2. Anthropometric, Diet Analysis and Physical Activity
3.3. Vitamin D Status
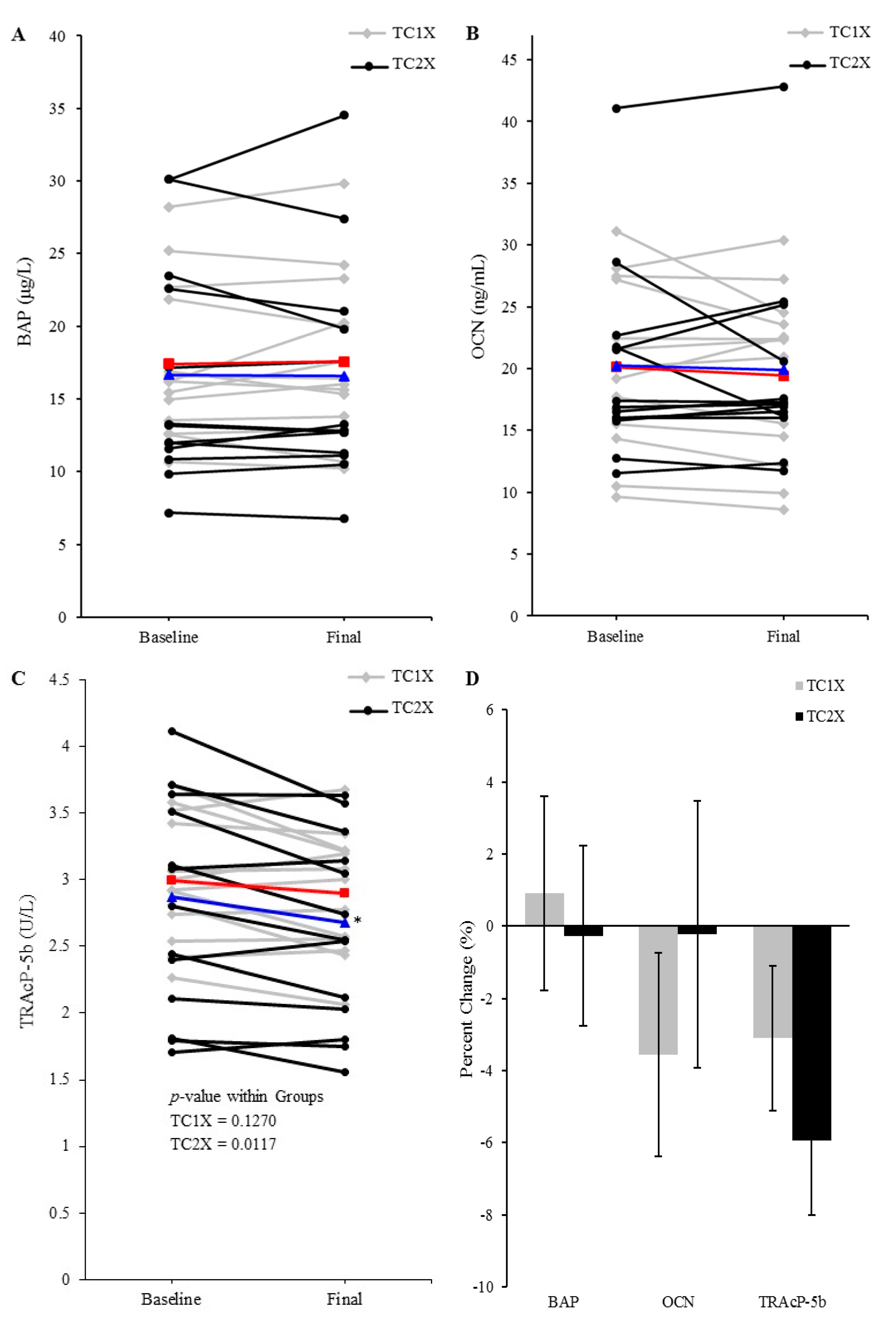
3.4. Serum Bone Formation and Resorption Indicators
3.5. Serum Indicators of Inflammation and Oxidative Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Statement of Human Rights
References
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of Osteoporosis at the Primary Health-Care Level. In World Health Organization Collaborating Centre for Metabolic Bone Diseases; University of Sheffield: Sheffield, UK, 2007; p. 66. [Google Scholar]
- Khosla, S. Pathogenesis of Osteoporosis. Transl. Endocrinol. Metab. Metab. Surg. Update 2010, 1, 55–86. [Google Scholar] [CrossRef]
- Khosla, S. Pathogenesis of Age-Related Bone Loss in Humans. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013, 68, 1226–1235. [Google Scholar] [CrossRef]
- Qaseem, A.; Forciea, M.A.; McLean, R.M.; Denberg, T.D. For the Clinical Guidelines Committee of the American College of Physicians Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann. Intern. Med. 2017, 166, 818–839. [Google Scholar] [CrossRef]
- Dolzhenko, A.T.; Sagalovsky, S. Cellular and molecular mechanisms of osteoporosis: Current concepts and future direction treatment. Mod. Rheumatol. J. 2016, 10, 56–63. [Google Scholar] [CrossRef][Green Version]
- Qiu, R.; Cao, W.-T.; Tian, H.-Y.; He, J.; Chen, G.-D.; Chen, Y.-M. Greater Intake of Fruit and Vegetables Is Associated with Greater Bone Mineral Density and Lower Osteoporosis Risk in Middle-Aged and Elderly Adults. PLoS ONE 2017, 12, e0168906. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Kern, M.; Metti, D.; Shamloufard, P.; Chai, S.C.; Johnson, S.A.; Payton, M.E.; Arjmandi, B.H. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: A randomized, controlled trial. Osteoporos. Int. 2016, 27, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Rendina, E.; Hembree, K.D.; Davis, M.R.; Marlow, D.; Clarke, S.L.; Halloran, B.P.; Lucas, E.A.; Smith, B.J. Dried Plum’s Unique Capacity to Reverse Bone Loss and Alter Bone Metabolism in Postmenopausal Osteoporosis Model. PLoS ONE 2013, 8, e60569. [Google Scholar] [CrossRef]
- Smith, B.J.; Crockett, E.K.; Chongwatpol, P.; Graef, J.L.; Clarke, S.L.; Rendina-Ruedy, E.; Lucas, E.A. Mont-morency tart cherry protects against age-related bone loss in female C57BL/6 mice and demonstrates some an-abolic effects. Eur. J. Nutr. 2018, 58, 3035–3046. [Google Scholar] [CrossRef]
- Moon, N.; Effiong, L.; Song, L.; Gardner, T.R.; Soung, D.Y. Tart Cherry Prevents Bone Loss through Inhibition of RANKL in TNF-Overexpressing Mice. Nutrients 2018, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; South, S.; Vijayagopal, P.; Juma, S. Effect of Tart Cherry Polyphenols on Osteoclast Differentiation and Activity. J. Med. Food 2020, 23, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; McHugh, M.P.; Stevenson, E.; Howatson, G. The role of cherries in exercise and health. Scand. J. Med. Sci. Sports 2013, 24, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.C.; Davis, K.; Zhang, Z.; Zha, L.; Kirschner, K.F. Effects of Tart Cherry Juice on Biomarkers of Inflam-mation and Oxidative Stress in Older Adults. Nutrients 2019, 11, 228. [Google Scholar] [CrossRef]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; George, T.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus Cerasus L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef]
- Schumacher, H.; Pullman-Mooar, S.; Gupta, S.; Dinnella, J.; Kim, R.; McHugh, M. Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthr. Cartil. 2013, 21, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Bioactive and functional properties of sour cherry juice (Prunus cerasus). Food Funct. 2016, 7, 4675–4682. [Google Scholar] [CrossRef]
- Dimitriou, L.; Hill, J.A.; Jehnali, A.; Dunbar, J.; Brouner, J.; McHugh, M.P.; Howatson, G. Influence of a montmorency cherry juice blend on indices of exercise-induced stress and upper respiratory tract symptoms following marathon running—A pilot investigation. J. Int. Soc. Sports Nutr. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2009, 20, 843–852. [Google Scholar] [CrossRef]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef]
- Levers, K.; Dalton, R.; Galvan, E.; O’Connor, A.; Goodenough, C.; Simbo, S.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; Riechman, S.; et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J. Int. Soc. Sports Nutr. 2016, 13, 1–23. [Google Scholar] [CrossRef]
- DiPietro, L.; Caspersen, C.J.; Ostfeld, A.M.; Nadel, E.R. A survey for assessing physical activity among older adults. Med. Sci. Sports Exerc. 1993, 25, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Sebring, N.G.; Denkinger, B.I.; Menzie, C.M.; Yanoff, L.B.; Parikh, S.J.; Yanovski, J.A. Validation of Three Food Frequency Questionnaires to Assess Dietary Calcium Intake in Adults. J. Am. Diet. Assoc. 2007, 107, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, H.; Vieth, R.; Cole, D.; Scillitani, A.; Modoni, S.; Frusciante, V.; Ritrovato, G.; Chiodini, I.; Minisola, S.; Carnevale, V. Sun exposure questionnaire predicts circulating 25-hydroxyvitamin D concentrations in Caucasian hospital workers in southern Italy. J. Steroid Biochem. Mol. Biol. 2010, 121, 334–337. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey III: Body Measurements (Anthropometry). In 3.3 Examination Procedures; Westat, Inc.: Rockville, MD, USA, 1988; pp. 3–16. [Google Scholar]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2015.
- King Orchards. 32 oz. Montmorency Tart Cherry Juice Concentrate. Available online: https://www.kingorchards.com/product/1-quart-32-oz-montmorency-tart-cherry-juice-concentrate/ (accessed on 1 February 2020).
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Dietary Reference Intakes for Calcium and Vitamin D; Institute of Medicine: Washington, DC, USA; National Academies Press (US): Washington, DC, USA, 2011.
- Richard, M.J.; Portal, B.; Meo, J.; Coudray, C.; Hadjian, A.; Favier, A. Malondialdehyde kit evaluated for de-termining plasma and lipoprotein fractions that react with thiobarbituric acid. Clin. Chem. 1992, 38, 704–709. [Google Scholar] [CrossRef]
- Martin, K.R.; Burrell, L.; Bopp, J. Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: A randomized, crossover pilot study. Food Funct. 2018, 9, 5290–5300. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.C.; Davis, K.; Wright, R.S.; Zhang, Z.; Luo, J.; Lee, K. Tart cherry juice improves visual sustained atten-tion and subjective memory ability in older adults. FASEB J. 2017, 31, 298. [Google Scholar]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Hooshmand, S.; Brisco, J.R.Y.; Arjmandi, B.H. The effect of dried plum on serum levels of receptor activator of NF-κB ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: A randomised controlled trial. Br. J. Nutr. 2014, 112, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Simonavice, E.; Liu, P.-Y.; Ilich, J.Z.; Kim, J.-S.; Arjmandi, B.; Panton, L.B. The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl. Physiol. Nutr. Metab. 2014, 39, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, N.S.; Saadat, R.L.; Hooshmand, S.; Chai, S.C.; Johnson, S.A.; Pourafshar, S.; Arjmandi, B.H. Dried plum consumption modulates bone turnover biomarkers in postmenopausal women with osteopenia. FASEB J. 2017, 31, 645. [Google Scholar]
- Chai, S.C.; Davis, K.; Wright, R.S.; Kuczmarski, M.F.; Zhang, Z.; Lee, K.; Luo, J. Tart cherry juice reduces sys-tolic blood pressure in older adults. FASEB J. 2017, 31, 966. [Google Scholar]
- Johnson, S.A.; Navaei, N.; Pourafshar, S.; Jaime, S.J.; Akhavan, N.S.; Alvarez-Alvarado, S.; Litwin, N.S.; Elam, M.L.; Payton, M.E.; Arjmandi, B.H. Effects of tart cherry juice on brachial and aortic hemodynamics, arterial stiffness, and blood biomarkers of cardiovascular health in adults with metabolic syndrome. FASEB J. 2017, 31, lb325. [Google Scholar]
- Szalóki-Dorkó, L.; Végvári, G.; Ladányi, M.; Ficzek, G.; Stéger-Máté, M. Degradation of Anthocyanin Content in Sour Cherry Juice during Heat Treatment. Food Technol. Biotechnol. 2015, 53, 354–360. [Google Scholar] [CrossRef]
- Repajić, M.; Kovačević, D.B.; Putnik, P.; Dragović-Uzelac, V.; Kušt, J.; Čosić, Z.; Levaj, B. Influence of Cultivar and Industrial Processing on Polyphenols in Concentrated Sour Cherry (Prunus cerasus L.) Juice. Food Technol. Biotechnol. 2015, 53, 215–222. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency Cherries Reduce the Oxidative Stress and Inflammatory Responses to Repeated Days High-Intensity Stochastic Cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef]
- McCormick, R.; Peeling, P.; Binnie, M.; Dawson, B.; Sim, M. Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players. J. Int. Soc. Sports Nutr. 2016, 13, 41. [Google Scholar] [CrossRef]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (Prunus cerasus L.) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2015, 55, 1695–1705. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.E.; Jenner, A.; Roodenrys, S. Acute reduction in blood pressure following consumption of anthocyanin-rich cherry juice may be dose-interval dependant: A pilot cross-over study. Int. J. Food Sci. Nutr. 2015, 67, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R.; Coles, K.M. Consumption of 100% Tart Cherry Juice Reduces Serum Urate in Overweight and Obese Adults. Curr. Dev. Nutr. 2019, 3, nzz011. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.; Browne, R.; Ram, M.; Muti, P.; Freudenheim, J.; Carosella, A.M.; Armstrong, D. Correlates of Markers of Oxidative Status in the General Population. Am. J. Epidemiol. 2001, 154, 348–356. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Kelly, M.E.; Bielinski, D.F.; Fisher, D.R. Tart Cherry Extracts Reduce Inflammatory and Oxidative Stress Signaling in Microglial Cells. Antioxidants 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Stacewicz-Sapuntzakis, M. Dried Plums and Their Products: Composition and Health Effects–An Updated Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1277–1302. [Google Scholar] [CrossRef]

| Characteristic | Total (n = 27) | TC1X 1 (n = 14) | TC2X 1 (n = 13) | p-Value 2 |
|---|---|---|---|---|
| Age (years) | 70.93 ± 4.47 | 70.57 ± 4.15 | 71.31 ± 4.94 | 0.678 |
| Time Post menopause (years) | 22.96 ± 5.58 | 23.86 ± 8.09 | 22.00 ± 4.56 | 0.474 |
| Marital status | 0.432 | |||
| Single (%) | 0.07 (2) | 0 (0) | 15.38 (2) | |
| Married (%) | 70.40 (19) | 71.40 (10) | 69.20 (9) | |
| Widowed (%) | 14.80 (4) | 21.40 (3) | 7.70 (1) | |
| Divorced (%) | 14.80 (4) | 7.10 (1) | 23.10 (3) | |
| Education | 0.409 | |||
| High school diploma (%) | 7.40 (2) | 0 (0) | 13.33 (2) | |
| Some college (%) | 18.52 (5) | 14.29 (2) | 23.08 (3) | |
| College degree (%) | 29.63 (8) | 23.08 (4) | 33.33 (4) | |
| Postgraduate (%) | 44.44 (12) | 57.14 (8) | 30.77 (4) | |
| Ethnicity | 1.000 | |||
| Black or African American (%) | 3.70 (1) | 7.1 (1) | 0 (0) | |
| White (%) | 96.30 (26) | 92.90 (13) | 100.00 (13) | |
| Lifestyle | ||||
| Ever smoked (%) | 29.63 (8) | 30.77 (4) | 28.57 (4) | 1.000 |
| Consumes any alcohol (%) | 74.07 (20) | 71.40 (10) | 76.90 (10) | 1.000 |
| Frequency (drinks/week) | 1.78 ± 2.44 | 2.34 ± 2.97 | 1.18 ± 1.62 | 0.224 |
| Reproductive history | ||||
| Gravidity | 2.6 ± 0.3 | 2.9 ± 0.4 | 2.3 ± 0.4 | 0.980 |
| Parity | 2.1 ± 0.2 | 2.2 ± 0.3 | 2.0 ± 0.3 | 0.562 |
| Oral contraceptive use (years) | 8.1 ± 1.9 | 10.4 ± 3.2 | 5.7 ± 2.0 | 0.224 |
| Characteristic | Total (n = 27) | TC1X 1 (n = 14) | TC2X 1 (n = 13) | p-Value 2 |
|---|---|---|---|---|
| Any site T-score 3 | 0.490 | |||
| Normal (%) | 7.4 (2) | 14.3 (3) | 0 (0) | |
| Osteopenia (%) | 81.5 (22) | 71.4 (10) | 92.3 (12) | |
| Osteoporosis (%) | 11.1 (3) | 14.3 (2) | 7.7 (1) | |
| Total hip T-score | 0.3180 | |||
| Normal (%) | 33.3 (9) | 42.9 (6) | 23.1 (3) | |
| Osteopenia (%) | 63.0 (17) | 50.0 (7) | 76.9 (10) | |
| Osteoporosis (%) | 3.7 (1) | 7.1 (1) | 0 (0) | |
| Femur neck T-score | 0.088 | |||
| Normal (%) | 14.8 (4) | 28.6 (4) | 0 (0) | |
| Osteopenia (%) | 74.1 (20) | 57.1 (8) | 92.3 (12) | |
| Osteoporosis (%) | 11.1 (3) | 14.3 (2) | 7.7 (1) | |
| Trochanter T-score | 1.000 | |||
| Normal (%) | 37.0 (10) | 35.7 (5) | 38.5 (5) | |
| Osteopenia (%) | 59.3 (16) | 57.2 (8) | 61.5 (8) | |
| Osteoporosis (%) | 3.7 (1) | 7.1 (1) | 0 (0) | |
| Intertrochanter T-score | 0.334 | |||
| Normal (%) | 55.6 (16) | 64.3 (9) | 46.2 (6) | |
| Osteopenia (%) | 40.7 (10) | 28.6 (1) | 53.8 (7) | |
| Osteoporosis (%) | 3.7 (1) | 7.1 (1) | 0 (0) | |
| Lumbar spine T-score | 0.265 | |||
| Normal (%) | 51.9 (14) | 57.1 (8) | 46.2 (6) | |
| Osteopenia (%) | 40.7 (11) | 28.6 (4) | 53.8 (7) | |
| Osteoporosis (%) | 7.4 (2) | 14.3 (2) | 0.0 (0) |
| Parameter | TC1X 1 (n = 14) | TC2X 1 (n = 13) | p-Value 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Final | p-Value 2 | Baseline | Final | p-Value 2 | Baseline | Final | |
| Anthropometrics | ||||||||
| Weight (kg) | 66.7 ± 4.1 | 67.6 ± 4.3 | 0.020 | 74.3 ± 3.5 | 75.4 ± 3.4 | 0.175 | 0.176 | 0.175 |
| Height (cm) | 161.8 ± 1.8 | 161.8 ± 1.7 | 0.855 | 163.9 ± 2.3 | 463.6 ± 2.2 | 0.331 | 0.480 | 0.521 |
| BMI (kg/m2) | 25.4 ± 1.4 | 25.7 ± 1.4 | 0.016 | 27.5 ± 0.9 | 28.0 ± 0.8 | 0.129 | 0.204 | 0.177 |
| Normal (%) | 8 (57.1) | 7 (50.0) | 1.00 | 4 (30.8) | 3 (23.1) | 1.000 | 0.522 | 0.372 |
| Overwt (%) | 4 (28.6) | 5 (35.7) | - | 6 (46.2) | 6 (46.2) | - | - | - |
| Obese (%) | 2 (14.3) | 2 (14.3) | - | 3 (23.1) | 4 (30.8) | - | - | - |
| Waist circ (cm) | 84.9 ± 3.3 | 84.5 ± 3.4 | 0.598 | 88.7 ± 2.2 | 89.8 ± 2.2 | 0.264 | 0.364 | 0.203 |
| Waist/Hip ratio | 0.82 ± 0.01 | 0.81 ± 0.02 | 0.134 | 0.83 ± 0.02 | 0.84 ± 0.02 | 0.790 | 0.670 | 0.296 |
| Body fat (%) | 36.3 ± 1.6 | - | - | 40.1 ± 1.1 | - | - | 0.163 | - |
| Macronutrients | ||||||||
| TEnergy (kcal) | 1729.1 ± 116.8 | 1931.2 ± 119.3 | 0.226 | 1641.8 ± 96.6 | 1787.9 ± 136.2 | 0.344 | 0.573 | 0.435 |
| Protein (%kcal) | 18.0 ± 1.4 | 15.7 ± 1.0 | 0.178 | 17.7 ± 1.6 | 17.2 ± 1.2 | 0.757 | 0.880 | 0.313 |
| Carbs (%kcal) | 46.2 ± 3.1 | 43.9 ± 2.2 | 0.390 | 50.1 ± 2.1 | 48.6 ± 2.0 | 0.585 | 0.313 | 0.127 |
| TFat (%kcal) | 36.9 ± 3.0 | 40.6 ± 2.2 | 0.144 | 34.1 ± 1.4 | 35.8 ± 1.6 | 0.392 | 0.425 | 0.103 |
| Daily Calcium Intake | ||||||||
| Diet (mg) | 942.8 ± 71.2 | 888.1 ± 84.7 | 0.425 | 1091.2 ± 121.9 | 1079.7 ± 125.6 | 0.919 | 0.295 | 0.212 |
| Supplement (mg) | 557.3 ± 110.0 | 563.9 ± 107.0 | 0.953 | 616.7 ± 176.0 | 523.8 ± 129.7 | 0.405 | 0.774 | 0.812 |
| Total Ca (mg) | 1500.1 ± 141.6 | 1452.1 ± 130.0 | 0.696 | 1707.9 ± 191.1 | 1603.5 ± 199.6 | 0.502 | 0.386 | 0.525 |
| %Below RDA | 4 (28.6) | 4 (28.6) | 1.000 | 4 (23.1) | 6 (46.2) | 0.411 | 1.000 | 0.440 |
| Daily Vitamin D Intake | ||||||||
| Diet (IU/day) | 141.9 ± 25.2 | 100.7 ± 26.0 | 0.123 | 143.2 ± 22.8 | 115.6 ± 18.1 | 0.288 | 0.970 | 0.646 |
| Supplement (IU/day) | 1585.7 ± 515.1 | 1585.7 ± 515.1 | 1.000 | 1076.9 ± 338.0 | 1076.9 ± 338.0 | 1.000 | 0.424 | 0.424 |
| Total (IU) | 1727.6 ± 509.3 | 1686.4 ± 510.1 | 0.123 | 1220.1 ± 345.3 | 1192.5 ± 341.1 | 0.288 | 0.424 | 0.138 |
| Parameter | TC1X 1 (n = 14) | TC2X 1 (n = 13) | p-Value 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Final | p-Value 2 | Baseline | Final | p-Value 2 | Baseline | Final | |
| Vitamin D Status | ||||||||
| 25(OH)D (ng/mL) | 36.7 ± 3.1 | 35.6 ± 3.2 | 0.595 | 31.2 ± 2.3 | 30.8 ± 2.4 | 0.783 | 0.173 | 0.250 |
| At risk (%) 4 | 4 (28.6) | 5 (35.7) | 1.000 | 4 (30.8) | 6 (46.2) | 0.688 | 1.000 | 0.704 |
| Inflammation | ||||||||
| hsCRP (mg/L) | 2.2 ± 0.5 | 2.4 ± 0.6 | 0.557 | 2.5 ± 0.7 | 4.4 ± 0.2 | 0.593 | 0.739 | 0.642 |
| Oxidative Stress | ||||||||
| TBARS (μM) | 4.6 ± 0.5 | 3.9 ± 0.5 | 0.180 | 5.1 ± 0.6 | 2.8 ± 0.6 | 0.235 | 0.545 | 0.388 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodier, T.; Anderson, K.L.; Bothwell, J.; Hermann, J.; Lucas, E.A.; Smith, B.J. U.S. Montmorency Tart Cherry Juice Decreases Bone Resorption in Women Aged 65–80 Years. Nutrients 2021, 13, 544. https://doi.org/10.3390/nu13020544
Dodier T, Anderson KL, Bothwell J, Hermann J, Lucas EA, Smith BJ. U.S. Montmorency Tart Cherry Juice Decreases Bone Resorption in Women Aged 65–80 Years. Nutrients. 2021; 13(2):544. https://doi.org/10.3390/nu13020544
Chicago/Turabian StyleDodier, Tiffany, Kendall L. Anderson, James Bothwell, Janice Hermann, Edralin A. Lucas, and Brenda J. Smith. 2021. "U.S. Montmorency Tart Cherry Juice Decreases Bone Resorption in Women Aged 65–80 Years" Nutrients 13, no. 2: 544. https://doi.org/10.3390/nu13020544
APA StyleDodier, T., Anderson, K. L., Bothwell, J., Hermann, J., Lucas, E. A., & Smith, B. J. (2021). U.S. Montmorency Tart Cherry Juice Decreases Bone Resorption in Women Aged 65–80 Years. Nutrients, 13(2), 544. https://doi.org/10.3390/nu13020544







