Abstract
Royal jelly (RJ) demand is growing every year and so is the market for functional foods in general. RJ is formed by different substances, mainly carbohydrates, proteins, and lipids, but also vitamins, minerals, and phenolic or volatile compounds in lower proportion. Major royal jelly proteins (MRJP) are, together with 10-hydroxy-2-decenoic acid (10-HDA), key substances of RJ due to their different biological properties. In particular, 10-HDA is a unique substance in this product. RJ has been historically employed as health enhancer and is still very relevant in China due to the traditional medicine and the apitherapy. Nowadays, it is mainly consumed as a functional food or is found in supplements and other formulations for its health-beneficial properties. Within these properites, anti-lipidemic, antioxidant, antiproliferative, antimicrobial, neuroprotective, anti-inflammatory, immunomodulatory, antiaging, and estrogenic activities have been reported for RJ or its specific components. This manuscript is aimed at reviewing the current knowledge on RJ components, their assessment in terms of authenticity, their biological activities, and related health applications.
1. Introduction
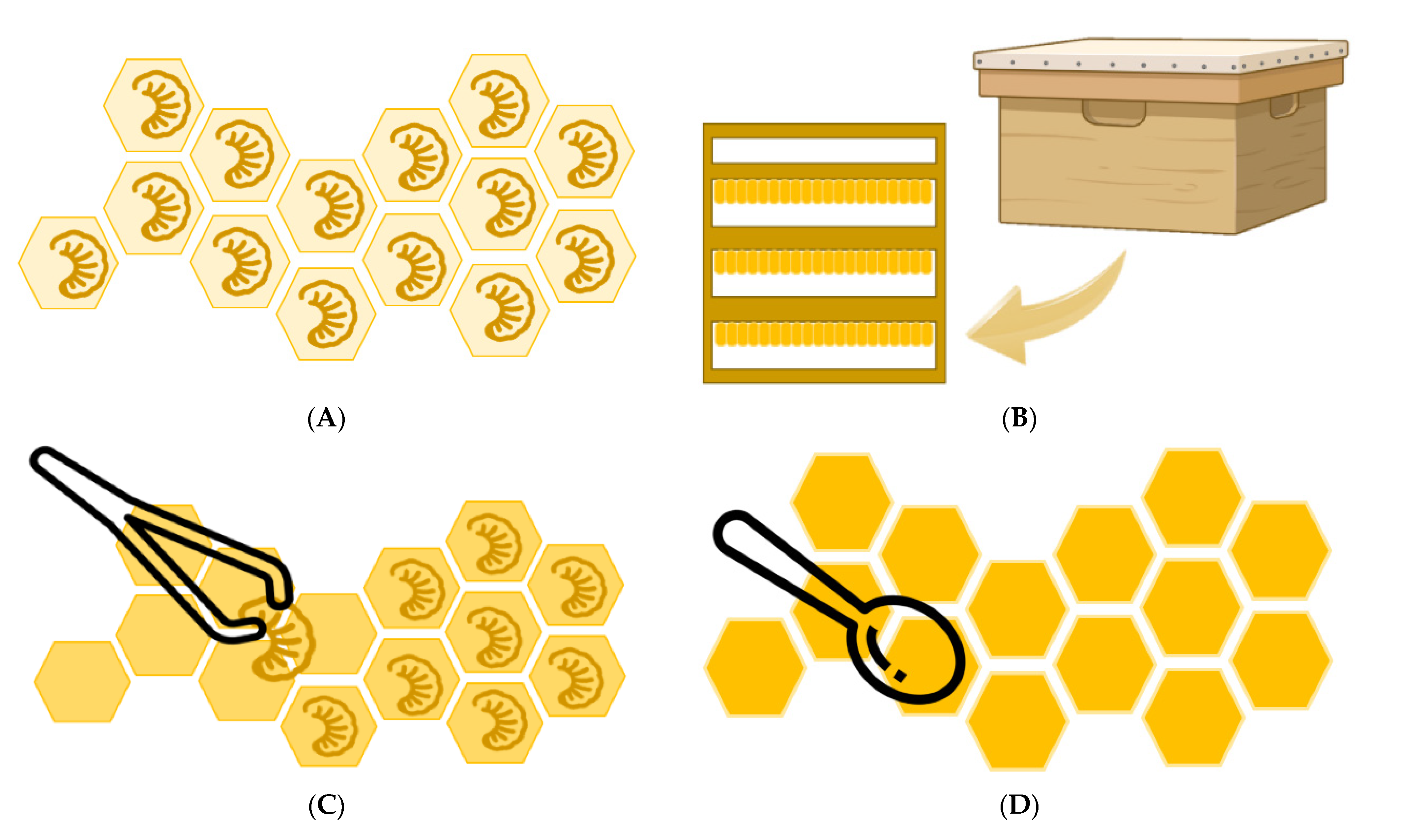
Royal jelly (RJ) is a yellowish-white, creamy, acidic secretion from the mandibular and hypopharyngeal glands of young worker bees of the Apis mellifera species [1,2,3]. Although the literature usually states that all bee larvae, workers, and queens are fed with jelly for the first three days after hatching, and only the queen larvae continue to be fed with RJ throughout their development, a clarification must be stated. During the first 3 days, nurse bees provide two different larval foods for queen and workers—the former is called the RJ and the latter is the worker jelly. Nurse bees provide a larger quantity and higher quality of food to the larvae in the queen cells, which causes the larvae to synthesize large amounts of juvenile hormone at 3 days old, thus leading to the development of the queen bee. The unique composition of RJ causes changes in gene expression, allowing, for example, full ovarian development to continue in the queen. Thanks to the RJ, the queen can live for up to five years, while the workers usually live about 45 days, and can lay about 2500 eggs a day [2,4,5,6,7,8]. The main method of production of RJ consists in the grafting of artificial larvae. Worker bee larvae, 12 to 18 h after hatching, are transferred using a grafting pen, to artificial queen cell bases to induce colony worker bees to produce RJ to feed the larvae. After 68–72 h (3 days), the larvae are removed with tweezers from the bases and the RJ is collected and transferred to a RJ bottle for storage. Larval grafting is a time-consuming and labor-intensive step, so a new method for the production of RJ that does not require larval grafting was described in recent years [5]. Figure 1 shows the production process of RJ in queen cell bases. RJ has a complex composition of water, proteins, carbohydrates, fatty acids and lipids, minerals and small amounts of vitamins, free amino acids, and volatile compounds [7,9,10,11,12]. However, it is difficult to gather data collected by several authors because of the different locations of the RJ samples and their inhomogeneous nature [10]. The most characteristic compound of the RJ is trans-10-hydroxy-2-decenoic acid (10-HDA), which is a unique active substance present in RJ [8,13,14]. In addition, proteins are the most abundant compounds in RJ and are usually called major royal jelly proteins (MRJPs) [11].
Figure 1.
Royal jelly (RJ) production in royal cells inside a hive. (A) RJ production in queen cell base with worker bee larvae inside. (B) Hive for breeding honeybees and queen bees for RJ production. (C) Removal of worker larvae from royal cells. (D) Collection of RJ from the cells.
There are no official data on the RJ market, but China is undoubtedly recognized as the largest producer of RJ [10]. It produces around 3500 tons/year, which accounts for more than 60% of the world’s production, and is exported almost entirely to the United States, Europe, and Japan [7,15,16]. Other countries such as Korea, Japan, and Taiwan are also major producers and exporters of RJ. In Europe, more RJ is produced in Eastern European countries than in Western Europe [7,10]. The lack of quality criteria and control of authenticity and geographical origin deters beekeepers from expanding their businesses, causing the industry to grow very slowly, and these growths are Chinese imports that cover local demand in many countries with highly competitive prices [12,17]. There are currently no European or international standards for bee products other than honey, but many countries have established their own national standards. The first country that set the criteria for RJ was Argentina in 1979, followed by Bulgaria in 1984, Poland in 1996, Turkey in 2000, Brazil in 2001, Serbia in 2003, Switzerland in 2005, Japan and China in 2008, India in 2012, and Korea in 2014 [18].
Since ancient times, RJ has been used in traditional medicine, especially in Asian apitherapy and in ancient Egypt [11]. However, over the last few years, the interest of consumers and food industry in healthy natural products has gradually increased in order to promote health and reduce disease [8]. Due to its excellent biological properties, RJ is one of the most attractive functional foods, being used as a dietary supplement and in various industries, such as pharmaceutical, food, and cosmetics [7,19]. Several studies reported RJ biological activities such as antimicrobial [1,20], antitumor [21], hepatoprotective [22], immunomodulatory and anti-hypercholesterolemic [23], antioxidant [24], and antidiabetic [24].
The purpose of this study is to carry out a review of the main constituents of RJ and their biological and health promoting properties. In addition, a wide variety of applications in the nutraceutical and cosmetic industries are addressed in the manuscript together with a brief revision of the main issues associated with RJ authentication.
2. Composition of Royal Jelly
RJ is an acidic colloid, whose pH usually ranges between 3.6 and 4.2, although other authors have expanded this range between 3.4 and 4.5 [25,26]. Water constitutes the major component (50–70%), followed by proteins (9–18%), carbohydrates (7–18%), lipids (3–8%), trace minerals (0.8–3%), vitamins, phenols, and amino acids [2,7,10,15]. Hence, the different values obtained by different authors fluctuate within a certain range. This is due to the heterogeneous nature of RJ, and the different samples taken from different locations at different times of production. However, the most variable parameters are sugars and lipids [10]. Depending on multiple factors (season, location, botanical origin, among others), RJ sugar content significantly varies from sample to sample. French RJ has lower contents of sucrose and erlose [12,25]. Lipid content is affected by RJ type as well. Italian samples have a higher lipid content than commercial samples. The total amount of fatty acids is not influenced by the RJ type of sample, as is the total amount of sterols. However, there is a high degree of variability according to the predominant sterol type between Italian and commercial samples [27]. Environmental conditions significantly affect the chemical composition. During the rainy season, water, and carbohydrates reach maximum levels, while the highest level of lipids reaches the maximum in the dry season. Protein content was lightly altered throughout the year, while mineral content and pH value were constant [28]. The main composition of RJ and its different biological activities are described below and collected in Table 1.

Table 1.
Components of RJ related to their abundance and biological activities.
2.1. Carbohydrates
The sugar portion accounts for between 7 and 18% of the total in fresh RJ and about 30% of the dry matter [10,49]. Overall, the total fructose and glucose content accounts for 90% of total sugars. The average concentrations are: fructose (2.3–7.6%, mean 4.9%) and glucose (2.9–8.1%, mean 5.5%) [12]. Whatever the RJ’s origin, fructose and glucose contents are in the range of 2.3–7.8% and 3.4–8.2%, respectively [75,76,77].
On the contrary, large differences are observed in the content of minor sugars, playing a providential role in the control of the origin and authenticity of the product [10,49]. Sucrose is always present but often in variable concentrations: <0.1–2.1%, mean 1.0% [77], 0.8–3.6%, mean 1.87% [75], 0.0–1.6%, mean 0.6% [17]. Sucrose and erlose contents in French RJ are between 0.0 and 1.8% (mean 0.5%) and 0.0–0.4% (mean 0.1%), respectively, as they reach 3.9 and 2.0% in some commercial samples and 7.7 and 1.7% in RJ produced by sugarcane feeding. Maltose and maltotriose contents are 0.0–1.0% (mean 0.3%) and 0.0–0.2% (mean 0.02%) in French RJ, and they attain levels of 2.6 and 0.4% in commercial samples, and by feeding bees with starch hydrolysate syrups can reach 5.5% and 1.7%. These differences in percentages are due to the effects of different types of feeding on bees [12]. In addition, more minor sugars, such as galactose, mannitol, maltulose, turanose, trehalose, palatinose, isomaltose, gentiobiose, and melezitose, and gluconic acid, a product derived from glucose oxidation, have also been detected [12,76].
It is thought that sugars contribute to RJ’s epigenetic effects, representing a phagostimulant that works through the insulin/insulin-like signaling cascades and the mTOR nutrient detection pathway to derive larval development by increasing amounts of food ingested and increasing intake of nutrients needed for queen development [4,25].
2.2. Proteins
Proteins account for >50% of dry weight and the so-called major proteins of royal jelly (MRJP) constituted about 80–90% of soluble RJ proteins [25,30,78,79]. MRJP are recognized as the main factor involved in specific physiological actions of RJ [42]. The nine members within this family (MRJP 1–9) are heavily homologous and have theoretical molecular masses of 49–87 kDa [5,80]. They have been named according to their molecular weight or order of its discovery [26]. Since the first five MRJPs account for between 82 and 90% of MRJPs, they are thought to have an important nutritional role and they are the most important nitrogen reserve. MRJP 6–8 have no apparent nutritional function [20,25,80].
MRJP1 is a weak acidic glycoprotein which comprises about 48% of all proteins in RJ [15,30,81]. It is found in either monomeric or oligomeric form. The monomeric form, also known as apalbumin I or royalactin, is a 55 kDa protein. The oligomer, known as apisin, has been estimated to be between 280 and 420 kDa [70,82,83,84,85]. Tian et al., (2018) reported that apisin adopts a H-like structure of 254 kDa formed by four MRJP1, four apisimin, and eight 24-methylenecholesterol at neutral pH. Apisimin is a serine-valine-rich 40 kDa peptide only found in honeybees. Apisin could be used to determine the quality of RJ [86,87], although its physiological function is still unclear [82]. Royalactin induces the differentiation of honeybee larvae into queens [88].
MRJPs 2–5 are also glycoproteins of 49, 60–70, 60, and 80 kDa molecular weight, respectively [42,78]. MRJP2 is a lightly basic protein, whilst MRJP 3–5 are almost neutral and MRJP7 is an acidic protein. MRJP8 and 9 are both rare in RJ, but could be detected in the venom gland [5,80,82]. Most of the MRJPs contain high amounts of the 10 essential amino acids in honeybees: Arg, His, Ile, Leu, Lys, Met, Phe, Thr, Trp, Val. The highest essential amino acids content is seen in MRJP5 (51.4%), MRJP1 (48%), and MRJP2 (47%) [78,80].
RJ also contains other proteins than MRJPs, but in a much smaller amount. Royalisin is an antimicrobial protein of the insect defensine family, active against a large spectrum of Gram+ and Gram- bacteria and fungi [48]. Jelleins are four peptides derived from the C-terminus of MRJP1. Jelleins I, II and III have antimicrobial activity against Gram+ and Gram- bacteria and yeasts, whereas jellein-IV has no antimicrobial effect [1,26,49]. Jelleins and royalisin together provide a wide-spectrum antimicrobial protection of the RJ [1,20].
Free amino acids (FAA) are another important component of the RJ. The average content of FAA in fresh RJ is 9.21 mg/g [89]. The major L-series FAAs are proline (2.4–5.4 mg/g), lysine (0.6–2.2), glutamate (0.5–0.9), ß-alanine (0.3–0.5), phenylalanine (0.2–0.6), aspartate (0.2–0.5) and serine (0.1–0.3). The D-Series FAA concentration was below the method detection limit (0.1 mg/g GR) [90]. Using the LC/MS method, lysine was the most prominent FAA (62.43 mg/100 g) ahead of proline (58.76 mg/100 g) [91,92]. Proline is thought to protect membranes and proteins from stress conditions and also act as an antioxidant. Cysteine is involved in the synthesis of glutathione, an effective cellular antioxidant [92].
2.3. Lipids
The lipid´s fraction appears in variable concentrations—3–8% in fresh RJ and 7–19% in dry RJ [2,10]. The majority of lipids (80–90%) are free fatty acids, with few being esterified. Phenols (4–10%), waxes (5–6%), steroids (3–4%), and phospholipids (0.4–0.8%) have also been reported [7,15,59,62]. Around 80–90% of the fatty acid fraction has a fairly unusual structure in nature, consisting of mono- and dihydroxy- acids and dicarboxylic acids with 8 and 10 carbon atoms in the chain. Most of the biological properties are associated with these lipids [2,10]. 10-HDA constitutes about 70% of total RJ lipids and more than 50% of free fatty acids. The precursor of 10-HDA, 10-hydroxydecanoic acid (HDAA), constitutes 17% of free fatty acids. Together, 10-HDA and 10-HDAA make up 60–80% of the fatty acids identified [27,59]. HDA and HDAA are specific components of RJ [62]. 10-HDA is known for having many pharmacological and health effects [7]. Other dominant fatty acids are 8-hydroxy octanoic acid, 3-hydroxydecanoic acid, 3,10-dihydroxydecanoic acid, and 9-hydroxy-2-decenoic acid [59].
RJ contains sterols in minor amounts, originating from plant sources. 24-methylene cholesterol constitutes 49–58% of RJ sterols [15,60]. Two 4-desmethylsterols, β-sitosterol and Δ5-avenasterol, are the next major sterols, whereas cholesterol, desmosterol, campesterol, stigmasterol and Δ7-avenasterol are found at lower concentrations, less than 0.1 mg/g of total lipids. [59,70].
2.4. Vitamins
Pantothenic acid (vitamin B5) and niacin (B3 or PP) are the most abundant vitamins in RJ: 52.8 and 42.42 mg/100 g, respectively. RJ also contains small quantities of various B-complex vitamins (B1, B2, B6, B8, B9, and B12), vitamin C, D, E, and A [18,25,49,93,94].
Pantothenic acid is recognized for being a lifespan-extending agent and for its ability to reduce stress levels and reverse hair aging. Therefore, RJ has been used as a treatment for hair. Biotin promotes keratin production [18,25].
2.5. Minerals
Ash content constitute 0.8–3% of RJ fresh matter and 2–5% of RJ dry matter [9,10]. It contains not only different minerals—K+, P3−, S2−, Na+, Ca2+, Al3+, Mg2+, Zn2+, Fe2+, Cu+, and Mn2+—but also it contains trace elements such as Ni, Cr, Sn, W, Sb, Bi and Ti [15,20,25]. The main element is potassium (2462–3120 mg/kg), followed by phosphorus (1940–2350 mg/kg), sulfur (1420–1154 mg/kg), calcium (145–113 mg/kg), magnesium (264–312 mg/kg) and sodium (106–142 mg/kg) [95]. The functions of potassium in the body are varied. It regulates fluid balance, controls the electrical activity of the heart and other muscles, reduces blood pressure, decreases the risk of stroke, and preserves bone mineral density [18]. Zn, Fe, Cu, Al, and Mn are the most abundant trace elements in RJ. Trace elements play a key role in the biomedical activities associated with RJ, and these elements have a multitude of known and unknown biological functions [7,95,96].
Depending on the botanical origin and the type of soil, trace and mineral concentrations widely vary between different samples of honey and pollen [97]. However, mineral and trace concentrations from different RJ samples do not show significant variability despite having different botanical and geographical origins. This fact highlights the homeostatic adjustment of trace and mineral element concentrations, produced in the endocrine glands of nurse bees. RJ shows the same homeostatic adjustment as mammalian and human breast milk [95,96].
2.6. Phenolic and Volatile Compounds
There are few references to volatile compounds (VC) in RJ, being the least studied and identified components of it [13,65,98,99,100]. However, the mixture of these various VC directly affects the taste and quality odor of the RJ [98,99]. There are great differences between the results obtained by different authors in the composition of the volatile fraction. This is because VC can be influenced by different factors, such as honeybee species, harvesting time, geographical origin, storage methods, or processing technologies [98].
Isidorov et al. (2009) determined by headspace solid-phase microextraction/gas chromatography-mass spectrometry (HS-SPME/GC–MS) a list of 25 different compounds in the volatile fraction of RJ from two Apis mellifera carnica colonies in Poland. The most abundant compounds were carbonyls (37–43%), with 2-heptanone at the head representing about 20% of the total. The remaining carbonyl compounds identified were, in decreasing order: acetone > 2-nonanone > benzaldehyde > 2-butanone > 2-pentanone and hexanal [13,101]. It has been reported the repellent effect of 2-heptanone, which can be directed against the destructive Varroa mite, an ectoparasite of the honeybee Apis mellifera L. It can also increase the repellent action of octanoic acid, 7% of the total volatile fraction. This compound can interfere with the process of cellular invasion by the mite [13,65]. In addition, the volatile phenolic compounds phenol, o-guiacol, and methyl salicylate are another significant group (10–20%). These compounds, together with benzoic acid (2.5–3.6%), possess antibacterial properties. The main constituents of VC (47%) are protective components whose activities are directed against both parasites and microorganisms [13]. Many types of polyphenolic compounds, with high antioxidant activity, are present in RJ [15,25]. Ferulic acid is the only phenolic acid found, comprising more than 68% of the total amount of polyphenols. In lower percentages, compounds belonging to flavanons, flavones, flavonols, and isoflavonoids have been found [102].
Also using HS-SPME/GC–MS technique, Zhao et al. (2016) determined 40 VC in 10 different RJ samples. Esters were the most abundant VC accounting for 25%, followed by aldehydes (17.5%), ketones (15%), acids (10%) and alcohols (10%). There was a significant variation of different classes of compounds in each RJ sample type. However, 2-nonanone and acetic acid were both detected in all samples, whereas toluene, benzaldehyde, octanoic acid, 2-pentanone, benzoic acid, and methyl ester were found in eight to nine samples. Acetic acid, benzoic acid methyl ester, hexanoic acid and octanoic acid contribute much more to RJ flavor and can be used for differentiating different RJ [98].
3. Royal Jelly Authenticity and Adulteration
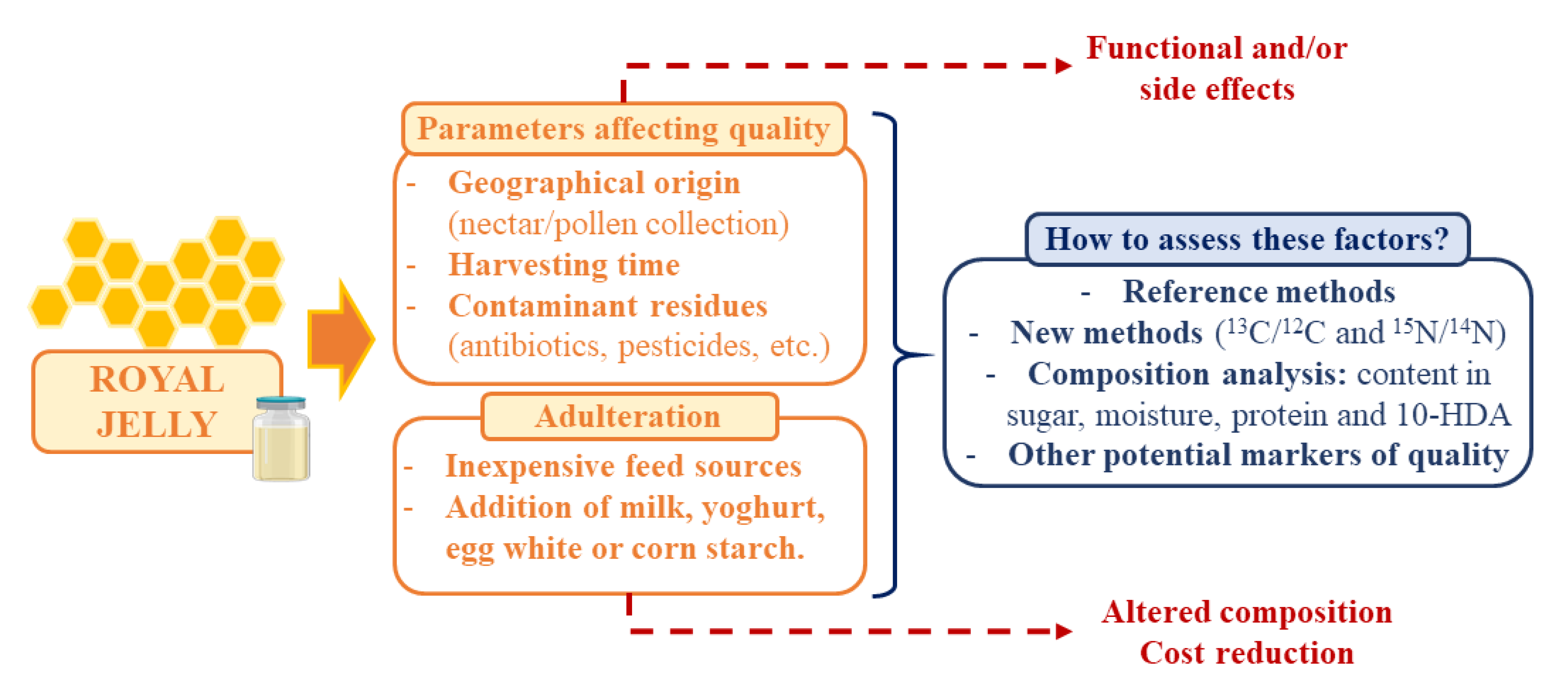
These days, RJ is considered as an attractive functional food but also an expensive product that can be submitted to adulteration as means to reduce production costs. In addition, other parameters can affect the final quality of RJ, such as the influence of geographical origin, harvesting time, or the presence of contaminant residues [7,103]. Furthermore, the use of reference methods to analyze the composition of RJ and the assessment of these factors is essential to guarantee the quality of the product and its expected physicochemical and organoleptic properties (Figure 2).
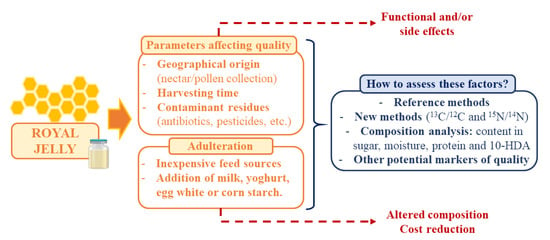
Figure 2.
Summary of the main parameters affecting RJ quality, adulteration techniques and measures to assess these factors and guarantee the final quality of the product.
Naturally, RJ is produced by the transformation of nectar and pollen collected by bees [12]. In large production systems, the most common sugar sources for bee-feeding are sugar syrups or honey and yeast powder or pollen as protein sources [103]. So, the use of inexpensive feed sources is a tempting option for reducing costs, which has been previously reported. The studies on this area are focused on establishing new methods for evaluating adulteration and authenticity, such as the 13C/12C and 15N/14N stable isotope ratios mass spectrometer [104]. The most common criteria for assessing RJ quality and authenticity is sugar, moisture, protein, and 10-HDA [12]. In addition, other studies have highlighted certain amino acids, amines, carbohydrates, and vitamins as potential markers [103]. Among all of them, 10-HDA is the main marker for RJ quality and authenticity [10]. However, the measure of 10-HDA content cannot be considered as a good freshness criterion [105]. Proteins, despite their importance and quantity, are also not good indicators of freshness [106]. Moreover, adulteration has been performed by adding milk, yoghurt, egg white, or corn starch [7,106]. For instance, RJ has been adulterated with powdered milk, since the taste characteristics are similar to pure RJ [107]. Melamine is a triazine ring with three amino groups commonly used for plastics or resin production that has been associated to possible incidence of nephrolithiasis and related deaths [7,108]. This compound has been also found in RJ by hydrophilic interaction chromatography/tandem mass spectrometry. It was added to apparently increase the protein content of RJ over its real value [108].
Alternatively, bees’ diets are linked to the environment, so RJ geographical origin will determine its final composition. A recent study showed that environment (e.g., coastal or inland areas) could modify RJ composition. Furthermore, the distinct composition in the sugar content was apparently correlated with radical free radical scavenging activity [19]. In addition, geographical origin can be inferred from pollen analysis [7]. Likewise, harvesting time will also affect RJ characteristics. A recent study proposed that VC could be used for distinguishing RJ harvested in flowering seasons and discriminating different pollen and nectar plants [98].
At last, exposure to different substances can modify RJ production and characteristics. Veterinary drugs are one common class of residues produced as a result of their use for prevention of specific diseases. Chloramphenicol, nitroimidazole, sulphonamides, fluoroquinolone, or tetracycline are a few examples of the antibiotics that can be retained in RJ [5,109,110]. Another important source of contaminants is pesticides. The repeated use of these products on the environment has been demonstrated to have functional and behavioral effects on bees, but they also entail other side effects. In a recent study, carbendazim treatment during larval stage caused the down regulation of the expression of MRJP [111]. Other authors evaluated the effects of exposing colonies to a multi-pesticide (nine compounds) pollen treatment and found that RJ production was reduced together with some target compounds such as MRJP or 10-HDA [112]. Similarly, neonicotinoid insecticides are widely used in the world and due to their translocation ability, they can pass throughout the plants to pollen or nectar and reach bees [113]. Several studies have demonstrated the presence of these residues in RJ by performing ultra- or high-performance liquid chromatography coupled to mass spectrometry [113,114,115]. Overall, the adulteration and authenticity of RJ needs to be further studied to set the optimal composition standards and guarantee the quality and authenticity of the product to avoid food fraud and the loss of its essential characteristics and properties.
4. Biological and Health Promoting Properties
RJ is one of the most popular functional foods. This product was commercialized, principally, for its dietetic and cosmetic activity. However, RJ has been demonstrated to have many pharmacological activities which have the potential to prevent or combat numerous diseases [7]. Recently, diverse works have been performed on RJ’s bioactivities, giving a broader vision of how RJ can contribute to the development of drugs and human health.
4.1. Anti-Lipidemic Activity: Lipid Metabolism
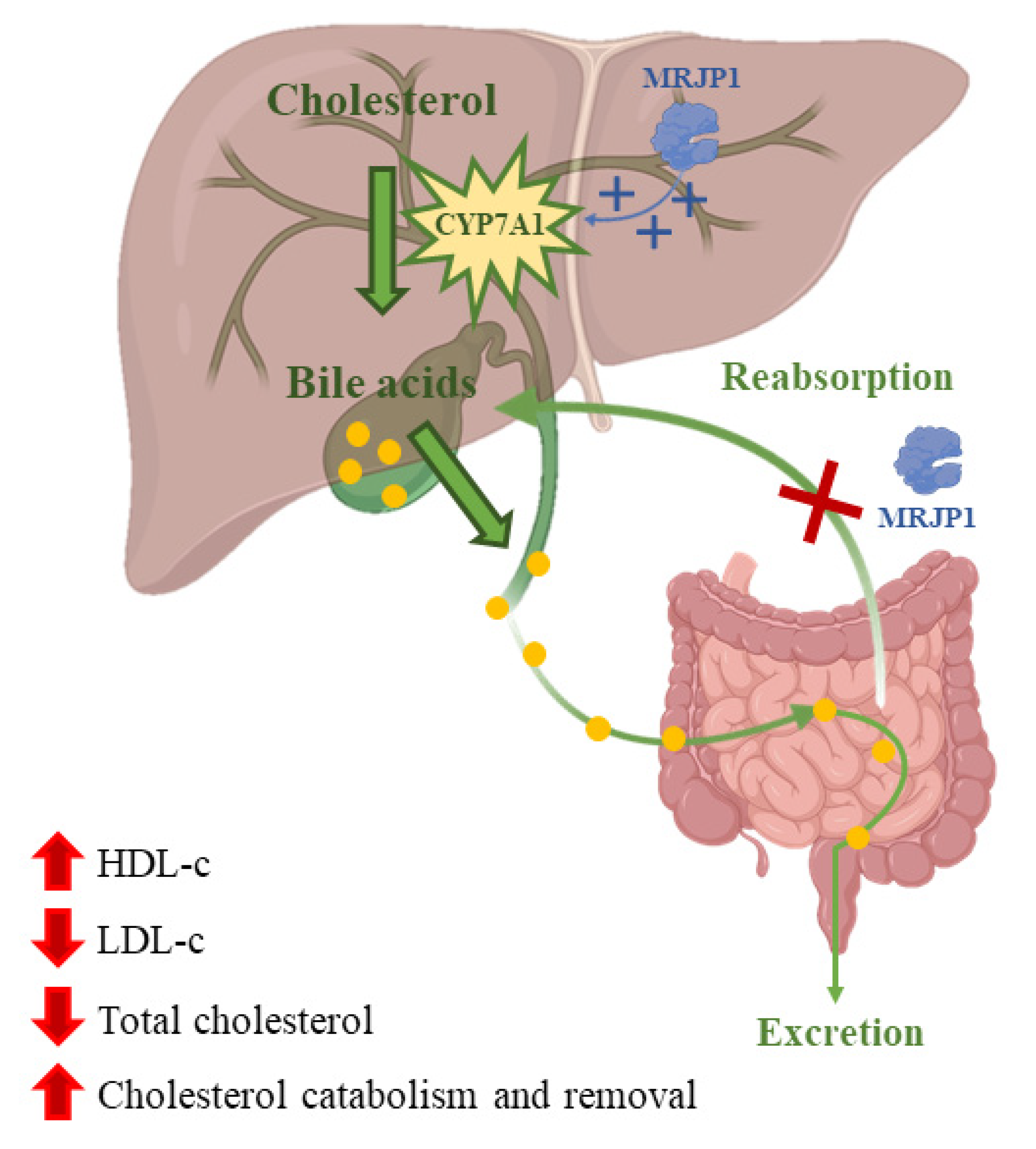
Nowadays, dyslipidemia is a high-risk factor for cardiovascular diseases that gets worse due to bad eating habits. Atherosclerotic cardiovascular disease is produced by low levels of high-density lipoprotein cholesterol (HDL-C) and high levels of triglycerides and low-density lipoprotein cholesterol (LDL-C) in the plasma [116]. Many studies have been done on how RJ affects lipids concentration in blood. A meta-analysis of those studies showed that treatments with RJ could reduce total cholesterol in the blood and increase HDL-C levels [117]. These changes in the concentration of blood lipids could be caused by the inhibition of the absorption of cholesterol in the jejunum by the MRJP. Moreover, MRJP1 can also block the reabsorption of bile acids [33]. Furthermore, RJ could produce the upregulation of cholesterol 7-α-hydroxylase (CYP7A1) that would increase the activity of hepatic receptors for the synthesis of very low-density lipoproteins, which are precursors of LDL-C (Figure 3) [118]. RJ has been suggested as a hypocholesterolemic agent since total cholesterol and LDL-C levels were reduced with an intake treatment of nine capsules (350 mg/capsule) of RJ or placebo/day, respectively, for three months [119]. Another study found that dietary RJ was able to suppress high fat diet-induced accumulation of white adipose tissue and liver and to increase brown adipose tissue thermogenic capacity in mice without modifying food and energy levels [120]. However, previous works have stated that the effects of RJ on lipid profile are contradictory [121].
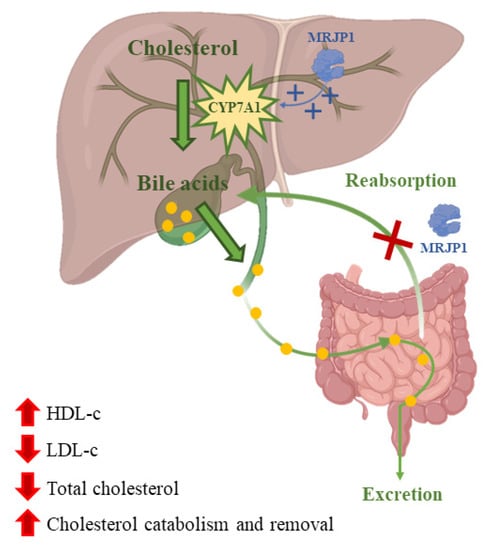
Figure 3.
Cholesterol is converted into bile acids catalyzed by the enzyme cholesterol 7-α-hydroxylase (CYP7A1). Bile acids are then excreted or reabsorbed at the jejunum. MRJP1 upregulates the expression of CYP7A1 increasing bile acids synthesis and their excretion. In addition, MRJP1 can inhibit cholesterol and bile acid reabsorption.
4.2. Antioxidant Activity: Oxidative Stress
Oxidative stress produced by the liberation of reactive oxygen species (ROS) in the body is related to some pathological processes [122]. In several studies, the multiple benefits of the antioxidant activity of specific compounds against these pathologies have been demonstrated. At the beginning of this century, it was demonstrated that RJ has antioxidant activity. Moreover, it was discovered that this activity came from its proteins (MRJP 1–9) and peptides [94,123]. Since then, numerous studies have been carried out on how the antioxidant capacity of RJ can help treating different pathologies. For instance, a recent study evaluated the antioxidant capacity of RJ by 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity and found that it ranged from 102 to 354 mg/mL and that there was no apparent link between antioxidant activity and geographical origin or phytochemicals [19].
Recently, the in vivo hepatoprotective activity of RJ against nonalcoholic fatty liver disease (NAFLD) was tested. NAFLD is the liver disease with greatest incidence in the world, with a prevalence of 25% in the population [124]. Factors such as age, postmenopausal status, and obesity increase the risk [125]. Moreover, NAFLD can lead to necrotic inflammation, fibrosis, and cirrhosis, and increases the risk of suffering other illnesses such hepatocellular carcinoma or cardiovascular diseases [126]. The pathogenesis of NAFLD is closely related to oxidative stress in hepatic cells [127]. Mice previously ovariectomized were treated with different doses of RJ (150, 300 and 450 mg/kg) intragastrical. In the control, an improvement in the oxidative stress level caused by an increment of the lipid peroxidation in the liver and the rise of nitric oxidase and malondialdehyde levels was observed. The mice treated with RJ had reduced oxidative stress thanks to an increase in hepatic antioxidant enzymes. Furthermore, RJ possesses compounds with antioxidant activity that could help to control the oxidative stress exerting hepatoprotective activity in ovariectomized mice [128]. Alternatively, another experiment was performed to observe the antioxidant activity of RJ. Rats with induced nephrotoxicity by cadmium administration were pretreated with different doses of RJ. The administration of cadmium produced a kidney dysfunction caused by an increase in ROS and reactive nitrogen species (RNS) resulting in cellular damage in the control group. Groups that were pretreated with RJ where able to restore the oxidative stress, decreasing the NO and lipid peroxidation to normal levels [129].
4.3. Antiproliferative Activity
Several strategies can combat cancer, but most of them also have some detrimental effect on the health of patients. One strategy to combat the growth of tumor cells without so many negative effects is to decrease their replication by natural antiproliferative compounds. RJ was demonstrated to have antitumor activity against Lewis lung carcinoma and colorectal adenocarcinoma cells [130,131]. To determine if RJ compounds have antiproliferative activity against cancer cells, six different types of RJ were tested against SH-SY5Y human neuroblastoma cells in vitro. For the study, the hydrophilic and the lipophilic compounds of the RJ were separated and administrated to the cell cultures in different concentrations (250, 750 and 1250 μg/mL). The results were similar for the six types of RJ; all of them presented a high antiproliferative effect in the cell cultures treated with the lipophilic extract. This antiproliferative activity could be linked to the 10-HDA that in other studies presented differentiating and neurotrophic activity on murine neuronal cells [132]. The connection between 10-HDA and RJ has been proposed previously, e.g., in 1960, it was found that it inhibited the development of transplantable AKR leukemia and different lines of ascitic tumors in mice [133]. 10-HDA has also shown an antiproliferative effect against fibroblast-like synoviocytes of rheumatoid arthritis (RA) patients by PI3K–AKT pathway. 10-HDA acts as HADACIs (histone deacetylase inhibitors), which have been considered as drugs with strong anti-inflammatory activity, related to the down-regulation of PI3K and the phosphorylation of AKT [134]. Another study assessed the inhibition of vascular endothelial growth factor (VEGF) induced angiogenesis by avoiding the proliferation of human umbilical vein endothelial cells [57].
4.4. Antimicrobial Activity
The search for new antibiotics to counteract the high level of antibiotic resistance is a priority to control different pathogens. In this search, antimicrobial peptides (AMPs) appear to be a natural alternative to conventional antibiotics [135]. The positive charge of these compounds allow them to interact with cell membranes that are negatively charged [136]. The antimicrobial capacity of AMPs comes from its ability to disrupt bacterial cells, causing the death of the bacteria. There are four theories (barrel-stave model, carpet-like model, toroidal pore model and aggregate channel model) of disruption that can explain this antibacterial activity but the exact mechanism of action is still unknown [137]. Compounds of RJ like jelleins, royalisin, MRJPs, royalactin, and apisimin could be classified as AMPs [20]. Moreover, there are other compounds of RJ with antimicrobial activity out of the AMPs, such as 10-HDA, which exhibits growth-inhibitory activity against different bacteria [138].
Since the first time that RJ was studied for its antibacterial activity [139], several studies have been performed (Table 2). These studies demonstrated the high antibacterial activity against Gram-positive bacteria and, in a minor way, against Gram-negative bacteria of RJ and its principal compounds. Alternatively, in a recent study, the RJ was tested as an anti-biofilm agent. Formation of biofilms is a big problem in alimentary industries for the contamination of food and drinks and the possibility to infect consumers. RJ was tested against Listeria monocytogenes, which is usually present in food biofilms, and produced 2549 infections in 2018 in Europe [140,141]. In this study, L. monocytogenes cultures were treated with different concentrations (0.33–41.67 mg/mL). The results show that the minimum inhibitory concentration (MIC)90 was 23.85 mg/mL. This result confirms the possibility of using RJ as an antibiofilm agent to reduce the risk of listeriosis contamination [142].

Table 2.
Antimicrobial activity of RJ compounds in terms of minimal inhibitory concentration (MIC) (µg/mL). Based on [1,48,51,138,143,144,145,146,147].
4.5. Neuroprotective Effect
The aging of the population in developed countries is increasing the number of neurodegenerative disorders. Alzheimer’s disease (AD) and Parkinson´s disease are two of the most important neurodegenerative diseases in developed countries due to their prevalence in the old population and their specific symptoms [148,149]. Therefore, a search for new therapies is required to mitigate the repercussions in the population. RJ has demonstrated its neuroprotective effects [150,151,152,153]. Recently, a study about the capacity of RJ to reduce cadmium-induced neuronal damage in vivo was carried out. Cadmium exposition produces impaired neurodevelopment associated with AD [154]. The study was completed in mice divided into three control groups (control, cadmium control and RJ control) and a RJ-cadmium group. The RJ-cadmium group was treated with 85 mg/kg of RJ orally administrated and with 6.5 mg/kg CdCl2 by intraperitoneal injection. The results show neuroprotective activity. This protection could be produced by the RJ antioxidant activity, reducing the lipid peroxidation and NO levels. Moreover, RJ administration restored the activity of antioxidant enzymes (GSH-Px1, GSH-R, SOD2, and CAT) that were depleted by the Cd-intoxication. Furthermore, the treatment was able to upregulate the Nrf2 that regulates the gene expression of antioxidant enzymes [155]. Additionally, TNF-α and IL-1β levels were decreased, reducing brain inflammation. In addition, anti-apoptotic activity by the downregulation of Bax and caspase 3 and upregulation of Bcl-2 was found, reducing the neuronal damage [156].
One predisposing factor in woman of old age is menopause. Menopausal hormonal alteration produces an increase in the risk of suffering neurodegenerative diseases such as AD and diseases related to autonomic nervous system dysfunction [157,158]. Recently, an in vivo study was performed to figure out if RJ could possess beneficial properties against the adverse neurological effects of menopause. The study was performed in ovariectomized rabbits fed with a high-cholesterol diet. After the ovariectomization, 400 mg/kg of RJ was orally administrated. The results of the study show beneficial neurological effects against postmenopausal neurological disorders. This neuroprotective effect could be produced by RJ components such as 10-hydroxy succinic acid, trans-2-ylenic acid, and 10-hydroxy-2-olenoic acid, which have estrogen-like effects. Moreover, RJ improved the estradiol and progesterone levels that can attenuate mild cognitive impairments [159]. In previous sections the capacity of RJ to reduce cholesterol levels was displayed. Therefore, this reduction in blood lipids could also produce neuroprotective effects by means of the reduction in amyloid-beta concentration in the brain, which undergoes upregulation with high cholesterol levels. Furthermore, RJ reduces the expression of β-site APP cleaving enzyme, which is related to high levels of amyloid-beta [153,160]. Additionally, the presence of sterols and some fatty acids in RJ with estrogenic activity can activate the estrogen receptor β in the brain due to their capacity to cross the blood–brain barrier due to their low polarity [60]. RJ also can improve the cholinergic system and antioxidant capacities, producing an enhancement of the autonomic nervous system [153].
4.6. Anti-Inflammatory Activity
Inflammation is one of the first responses of the body against infection or injury and produces the beginning of the immunological process. Inflammation is a normal process of the innate immune response, but if it is not well regulated it can produce tissue damage, and is derived in some pathological disorders [161]. RJ has shown anti-inflammatory activity in different diseases related to an abnormal inflammation [11,41,61,162]. To understand better the mechanism of this activity, in a recent study, murine microglial cell line BV-2 was cultivated with RJ and later cultivated with lipopolysaccharides that stimulate the inflammatory response [163]. The results show that cells treated with RJ presented a better response to the inflammation. This protective effect could be caused by the inhibition of the transcription of TNF-α, IL-1β and IL-6 cytokines that are pro-inflammatory.
Furthermore, RJ could inhibit the expression of the pro-inflammatory protein COX-2. Moreover, in this study, RJ also presented antioxidant effects, reducing NO and ROS levels in the cells. In addition, RJ presented an inhibitory effect on the production of inflammatory mediators through JNK, p38 and NF-kB pathways [163]. Alternatively, a recent study performed in asymptomatic overweight adults showed anti-inflammatory benefits in the patients. The patients were treated with 333 mg per day by oral ingestion of capsules for 8 weeks. The RJ administration produced a decrease in the inflammatory marker CRP and an increase in anti-inflammatory adiponectin, and IL-6 cytokine levels were reduced. Furthermore, RJ increased the expression of adiponectin receptor 1 as well as the adiponectin that could be linked to the increase in the peroxisome proliferator-activated receptor-α, AMP-activated protein kinase (pAMPK), and peroxisome proliferator-activated receptor gamma coactivator 1-α [164,165].
4.7. Additional Effects
Aging affects the muscles and bones of the old population. Therefore, new treatments and functional food development are very important to improve the health and quality of life of elderly people. RJ presented an improvement in the bone quality of ovariectomized rats by a reduction in collagen crosslink (pyridinoline and deoxypyridinoline) and stimulation of the expression of collagen-modifying enzymes. These effects produced posttranslational modifications in type I collagen [166]. To find the compounds involved and the mechanism of this activity, a study in vitro with 10-hydroxy-2-decenoic acid (10H2DA) was recently performed. In the study, ovariectomized mice were orally treated with RJ (1 g/kg of body weight) and 10H2DA (40 mg/kg of body weight). The results show a suppression of osteoclastogenesis decreasing bone resorption [167]. This effect could be caused by the inhibition of NF-kB signaling through the FFAR4 receptor [168]. Alternatively, RJ demonstrated positives effects in the function and conservation of the skeletal muscle [169,170,171,172]. To improve this effect, RJ treated with proteases was tested against denervation-induced skeletal muscle atrophy. In this study, mice were treated with protease-treated RJ (pRJ) orally for 3 weeks. Afterwards, a fragment of the sciatic nerve was cut and excised. After six days, a section of muscle was collected. The results show that the treatment improved the decrement of myofiber size after denervation. Moreover, pRJ produced an increment in the expression of regeneration-related genes (IGF-1 and IGFR) [173].
5. Royal Jelly Health Applications
5.1. Nutraceutical Industry
RJ has been historically employed as a health enhancer and is still very relevant in China due to traditional medicine and apitherapy. The first chemical analysis of RJ was carried out in 1852 by the American Reverend Langstroth; nevertheless, his analyses did not guarantee reliable information. During the 1850s, Langstroth proposed the commercialization of RJ as a solution in areas where honey was not produced, and its use as a functional product was enhanced from 1860 due to the discovery of its properties [20]. From then on, RJ has been produced in large scale for commercial purposes and marketed as capsules, tablets, in ampoules, etc. China produces 90% of the global production (4000 tons of RJ per year) and mainly exports to Japan, Europe, and the United States. The increase in the production of RJ during the last 4 decades is mainly due to the development and optimization of production techniques and the development of genetic selection of Italian bees so that they are capable of producing up to a 10 times greater amount of RJ than the bees not selected [15]. RJ industry applications and some of their benefits to promote human health are described below and collected in Table 3.

Table 3.
Royal Jelly industry applications and their benefits to promote human health.
5.1.1. Functional Food
Reliance on foods with health benefits has been encouraged by socioeconomic changes and in the lifestyle of the population. Therefore, national authorities, the scientific community, and the food industry seek new formulations to facilitate these changes in an efficient way. Functional foods, together with a balanced diet, can offer improved health and the prevention of some illness [186]. In the case of incorporating RJ into food products, they would compensate or enhance the nutritional contribution of the diet that is acquired. It is an excellent remedy that can increase the contribution of nutrients of great importance for maintaining health. Currently, there are research works focused on new functional foods based on RJ, their properties, and the pharmacological effects on the human body [186].
It has been suggested that the inoculation of different quantities of RJ in skimmed milk has the potential to manage human diseases such as hyperglycemia (type 2 diabetes), hypertension, and several types of cancer, including breast and skin [174]. The study evaluated the beneficial properties of Lactobacillus acidophilus fermented milk with RJ. The biological parameters checked were total microorganisms, pH, antioxidant activity and inhibitory activities of angiotensin 1-converting enzyme, α-amylase, and two cancer cell lines growth, SW480 (colorectal) and MV3 (skin). Antioxidant activities increased after four hours of fermentation in skimmed milk fortified with RJ, and the accumulation of bioactive peptides with inhibitory activity of angiotensin 1-converting enzyme and with positive effects in the treatment of high blood pressure after one day was observed [174]. Rosenthal and colleagues evaluated the antibacterial effect of milk supplemented with RJ and found that this additive inhibits the growth of various Gram-positive and Gram-negative bacteria and mesophilic and thermophilic dairy starters in milk [175]. In addition, heat pasteurization did not inactivate the antibacterial activity of this additive. RJ, with a wide range of antimicrobial effects, would prevent the conversion of milk into fermented products.
5.1.2. Supplements and Other Formulations
Different bioactive compounds previously described and mainly proteins, peptides, fatty acids, and phenolics give RJ multiple physiologic activities and medical applications. The beneficial effects as a result of the ingestion of RJ through capsules, tablets, or other preparations are based on experimental studies in which groups of patients were treated daily with different doses of this additive, whereas a control group was treated with a placebo.
RJ has been reported for its hypolipidemic beneficial effect, maintaining body weight and body fat [119]. The improvement of the dehydroepiandrosterone sulphate hormone concentration and the decrease in the serum total cholesterol and LDL-C levels after the administration of RJ in doses of 3.15 g/day for 12 weeks have been shown. Thus, RJ reduces the risk of cardiovascular disease without hepatic or renal damage. A recent study determining the effects of RJ on postmenopausal people (up to 60 years) indicated that women who received 1 g of RJ capsules for eight weeks had their menopausal symptoms significantly reduced [176]. The effect of the commercial dietary supplement (Memo®) on mental state evaluation in patients with moderate cognitive decline was also tested [177]. This product comprises 0.75 g of lyophilized RJ, 0.12 g of plant extracts Ginkgo biloba, and 0.15 g of Panax ginseng, and it was observed that ingesting one capsule once a day for 1 month on an empty stomach could be beneficial in dealing the cognitive decline typical of the aging process and of the early stages of the AD. However, more studies in this research field are needed to corroborate this benefit [177].
RJ has also demonstrated a positive effect against the premenstrual syndrome. Two months of consumption of one capsule daily (1 g RJ per capsule), starting on the first day of menstruation and continuing the same treatment over two consecutive menstrual cycles, alleviated premenstrual syndrome [178]. The nephroprotective effect of RJ was also tested. Cisplatin is one of the most effective antineoplastic drugs; nevertheless, the kidney damage it causes has limited its clinical use. In this sense, the role of RJ in the protection of cisplatin-induced acute nephrotoxicity in people with cancer was assessed, and RJ was found that to be involved in the nephroprotective effect against cisplatin toxicity [179]. In another experiment, the effect of JR in patients with dry eye affection was checked. A total of 43 Japanese volunteers with dry eye symptoms were subjected to six tablets of RJ daily (0.12 g/tablet) for two months. After that, the tear secretion volume, the meibum grade, and subjective dry eye symptoms, among others, were analyzed. The results indicate that the tear volume significantly increased after intervention in these patients [180].
The conclusion of another study revealed the improvement of mental health, erythropoiesis, and glucose tolerance after the intake of RJ. A total of 31 healthy adults were administered with three grams of this additive in 100 mL liquid/day for 6 months [181]. Doctors analyzed the effects and compared them with the control group. The initial results confirm modifications in anthropometric measurements and biochemical parameters from the beginning to 6 months after administration. Finally, the regular consumption of RJ demonstrated positive results on sperm and its mobility in infertility treatments [182].
5.2. Cosmetic Industry
RJ has been used since ancient times as an embellishing agent, including by historical figures such as Cleopatra. Nowadays, it is still very important in the cosmetic industry due to the rich content of bioactive compounds that confer diverse beauty and health benefits, on which care professionals focus their attention. Despite the previously described anti-inflammatory, antidiabetic, anti-lipidemic, antioxidant and antimicrobial properties, RJ is considered a natural anti-aging nutraceutical which leads to improved body composition and fertility enhancement [20].
Fatemeh Seyyedi and co-workers studied the therapeutic effects of vaginal cream of RJ on vaginal atrophy of postmenopausal women [183]. Participants were split into three groups. Women from the first group were administered with RJ vaginal cream 15%, the second group with vaginal Premarin commercial product, and the third group with placebo (lubricant), for three months. The results indicate that RJ has estrogen-like effects, since their cream was more effective than Premarin cream and lubricant in the improvement of quality of life in postmenopausal women. In the same way, the effectiveness of vaginal RJ in the treatment of sexual and urinary problems of postmenopausal women was confirmed in another experiment, leading to the conclusion that the effect is related to its estrogenic properties [185]. Another study revealed that 10-HDA may be useful in treating the dysfunction of the skin barrier. The activity of its synthetic counterpart, Hydroxydecine®, was evaluated and shown to be effective in restoring the skin barrier, reducing inflammation and hydrating dry skin [184].
6. Final Remarks and Conclusions
Royal jelly is a complex mixture of substances which is commonly utilized by the nutraceutical and cosmetic industry. Its composition is mainly formed by water, proteins, carbohydrates, lipids and, in a minor proportion, trace minerals, vitamins, and phenols. Among the proteins, MRJP and FAA are essential components of RJ, whereas, regarding lipids, 10-had is the most important substance, since it is a unique active compound. Phenolic and volatile compounds can be also important for their biological properties and their potential use as markers to differentiate RJ of different origins or harvesting times. RJ composition is highly variable, and so new analytical techniques are essential to study and address the authenticity and quality of the product. In respect to its biological properties, research focuses on anti-lipidemic, antioxidant, antimicrobial, anti-inflammatory and other effects, such as antiaging or estrogenic properties. However, further study of its mechanism of action is essential. RJ is consumed worldwide in different ways, its main use being as a functional food, although supplements have also been commercialized. The cosmetic industry has produced creams with estrogenic-like effects, but future lines of research are aimed at investigating RJ causing agents of antiaging effects. Due to this rapid expansion and increase in demand for RJ, the need to regulate the RJ market, currently dominated by China, becomes evident. These measures should not only be aimed at establishing check measures on the authenticity and origin of the RJ, but should also be aimed at preserving the environment and bees. Future research should focus on the full understanding of the routes of RJ substances to develop new applications and products for the nutraceutical and cosmetic, but also the pharmaceutical industry. In addition, it is essential to develop the LC-MS methodology, among other techniques, to study the entire composition of RJ.
Author Contributions
Conceptualization, M.A.P. and J.S.-G.; methodology, N.C., M.C., B.N.-E., P.O.; formal analysis, N.C., M.C., B.N.-E., P.O.; investigation, N.C., M.C., B.N.-E., P.O.; writing—original draft preparation, N.C., M.C., B.N.-E., P.O., J.S.-G. and M.A.P.; writing—review and editing, M.A.P. and J.S.-G.; supervision, M.A.P. and J.S.-G.; project administration, M.A.P. and J.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results was supported by FEDER under the program Interreg V-A Spain-Portugal (POPTEC) 2014–2020 ref. 0377_IBERPHENOL_6_E and ref. 0181_NANOEATERS_01_E; by Xunta de Galicia supporting with the Axudas Conecta Peme the IN852A 2018/58 NeuroFood Project and the program EXCELENCIA-ED431F 2020/12; by EcoChestnut Project (Erasmus+ KA202); by Ibero-American Program on Science and Technology (CYTED—AQUA-CIBUS, P317RT0003) and by the Bio Based Industries Joint Undertaking (JU) under grant agreement No 888003 UP4HEALTH Project (H2020-BBI-JTI-2019) that supports the work of P. Otero, the JU receives support from the European Union’s Horizon 2020 research and innovation program and the Bio Based Industries Consortium.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The research leading to these results was supported by MICINN supporting the Ramón & Cajal grant for M.A. Prieto (RYC-2017-22891) and by University of Vigo for the predoctoral grant for M. Carpena (Uvigo-00VI 131H 6410211).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| RJ | Royal Jelly |
| 10-HDA | 10-hydroxy-2-decenoic acid |
| MRJP | Major Royal Jelly Proteins |
| FAA | Free Amino Acids |
| HDAA | 10-hydroxydecanoic acid |
| VC | Volatile Compounds |
| HDL-C | High-Density Lipoprotein Cholesterol |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| ROS | Reactive Oxygen Species |
| NAFLD | Nonalcoholic Fatty Liver Disease |
| RNS | Reactive Nitrogen Species |
| AMPs | Antimicrobial Peptides Appear |
| AD | Alzheimer’s Disease |
| HS-SPME/GC–MS | Headspace Solid-Phase Microextraction/Gas Chromatography-Mass Spectrometry |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| RA | Rheumatoid Arthritis |
| VEGF | Vascular Endothelial Growth Factor |
| pRJ | protease-treated RJ |
References
- Fontana, R.; Mendes, M.A.; De Souza, B.M.; Konno, K.; César, L.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Buttstedt, A.; Mureşan, C.I.; Lilie, H.; Hause, G.; Ihling, C.H.; Schulze, S.H.; Pietzsch, M.; Moritz, R.F.A. How Honeybees Defy Gravity with Royal Jelly to Raise Queens. Curr. Biol. 2018, 28, 1095–1100.e3. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef]
- Hu, F.L.; Bíliková, K.; Casabianca, H.; Daniele, G.; Salmen Espindola, F.; Feng, M.; Guan, C.; Han, B.; Krištof Kraková, T.; Li, J.K.; et al. Standard methods for Apis mellifera royal jelly research. J. Apic. Res. 2019, 58, 1–68. [Google Scholar] [CrossRef]
- Scarselli, R.; Donadio, E.; Giuffrida, M.G.; Fortunato, D.; Conti, A.; Balestreri, E.; Felicioli, R.; Pinzauti, M.; Sabatini, A.G.; Felicioli, A. Towards royal jelly proteome. Proteomics 2005, 5, 769–776. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Argüelles, S. Bee Products: Royal Jelly and Propolis; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128124918. [Google Scholar]
- Garcia-Amoedo, L.H.; De Almeida-Muradian, L.B. Physicochemical composition of pure and adulterated royal jelly. Quim. Nova 2007, 30, 257–259. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; de Almeida-Muradian, L.B. Quality and standardisation of Royal Jelly. J. ApiProd. ApiMed. Sci. 2009, 1, 1–6. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wytrychowski, M.; Chenavas, S.; Daniele, G.; Casabianca, H.; Batteau, M.; Guibert, S.; Brion, B. Physicochemical characterisation of French royal jelly: Comparison with commercial royal jellies and royal jellies produced through artificial bee-feeding. J. Food Compos. Anal. 2013, 29, 126–133. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Czyzewska, U.; Isidorova, A.G.; Bakier, S. Gas chromatographic and mass spectrometric characterization of the organic acids extracted from some preparations containing lyophilized royal jelly. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3776–3780. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Grzech, I. Gas chromatographic-mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 885–886, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef]
- Altaye, S.Z.; Meng, L.; Li, J. Molecular insights into the enhanced performance of royal jelly secretion by a stock of honeybee (Apis mellifera ligustica) selected for increasing royal jelly production. Apidologie 2019, 50, 436–453. [Google Scholar] [CrossRef]
- Kanelis, D.; Tananaki, C.; Liolios, V.; Dimou, M.; Goras, G.; Rodopoulou, M.A.; Karazafiris, E.; Thrasyvoulou, A. A suggestion for royal jelly specifications. Arhiv za Higijenu Rada i Toksikologiju 2015, 66, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kamyab, S.; Gharachorloo, M.; Honarvar, M.; Ghavami, M. Quantitative analysis of bioactive compounds present in Iranian royal jelly. J. Apic. Res. 2020, 59, 42–52. [Google Scholar] [CrossRef]
- Mokaya, H.O.; Njeru, L.K.; Lattorff, H.M.G. African honeybee royal jelly: Phytochemical contents, free radical scavenging activity, and physicochemical properties. Food Biosci. 2020, 37, 100733. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Premratanachai, P.; Chanchao, C. Review of the anticancer activities of bee products. Asian Pac. J. Trop. Biomed. 2014, 4, 337–344. [Google Scholar] [CrossRef]
- Ahmed, W.M.S.; Khalaf, A.A.; Moselhy, W.A.; Safwat, G.M. Royal jelly attenuates azathioprine induced toxicity in rats. Environ. Toxicol. Pharmacol. 2014, 37, 431–437. [Google Scholar] [CrossRef]
- Ibrahim, A.A.E.-M. Immunomodulatory effects of royal jelly on aorta CD3, CD68 and eNOS expression in hypercholesterolaemic rats. J. Basic Appl. Zool. 2014, 67, 140–148. [Google Scholar] [CrossRef]
- Cihan, Y.B.; Ozturk, A.; Gokalp, S.S. Protective role of royal jelly against radiation-induced oxidative stress in rats. UHOD—Uluslararasi Hematoloji-Onkoloji Dergisi 2013, 23, 79–87. [Google Scholar] [CrossRef]
- Kunugi, H.; Ali, A.M. Royal jelly and its components promote healthy aging and longevity: From animal models to humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Ferioli, F.; Armaforte, E.; Caboni, M.F. Comparison of the lipid content, fatty acid profile and sterol composition in local Italian and commercial royal jelly samples. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 875–884. [Google Scholar] [CrossRef]
- Wongchai, V.; Ratanavalachai, T. Seasonal variation of chemical composition of royal jelly produced in Thailand. Thammasat Int. J. Sci. Technol. 2002, 7, 1–8. [Google Scholar]
- Kimura, M.; Kimura, Y.; Tsumura, K.; Okihara, K.; Sugimoto, H.; Yamada, H.; Yonekura, M. 350-kDa royal jelly glycoprotein (Apisin), which stimulates proliferation of human monocytes, bears the β1-3galactosylated N-glycan: Analysis of the N-glycosylation site. Biosci. Biotechnol. Biochem. 2003, 67, 2055–2058. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Rakwal, R.; Nam, H.W.; Shibato, J.; Agrawal, G.K.; Kim, Y.S.; Ogawa, Y.; Yoshida, Y.; Kouzuma, Y.; Masuo, Y.; et al. Comprehensive royal jelly (RJ) proteomics using one- and two-dimensional proteomics platforms reveals novel RJ proteins and potential phospho/glycoproteins. J. Proteome Res. 2008, 7, 3194–3229. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.C.; Morgan, S.L.; Spencley, A.L.; Mariano, N.; Chang, E.Y.; Shankar, G.; Luo, Y.; Li, T.H.; Huh, D.; Huynh, S.K.; et al. Honey bee Royalactin unlocks conserved pluripotency pathway in mammals. Nat. Commun. 2018, 9, 5078. [Google Scholar] [CrossRef]
- Šimúth, J.; Bíliková, K.; Kováčová, E.; Kuzmová, Z.; Schroder, W. Immunochemical Approach to Detection of Adulteration in Honey: Physiologically Active Royal Jelly Protein Stimulating TNF-α Release Is a Regular Component of Honey. J. Agric. Food Chem. 2004, 52, 2154–2158. [Google Scholar] [CrossRef]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef]
- Fan, P.; Han, B.; Feng, M.; Fang, Y.; Zhang, L.; Hu, H.; Hao, Y.; Qi, Y.; Zhang, X.; Li, J. Functional and Proteomic Investigations Reveal Major Royal Jelly Protein 1 Associated with Anti-hypertension Activity in Mouse Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Kamakura, M.; Suenobu, N.; Fukushima, M. Fifty-seven-kDa protein in royal jelly enhances proliferation of primary cultured rat hepatocytes and increases albumin production in the absence of serum. Biochem. Biophys. Res. Commun. 2001, 282, 865–874. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, B.Y.; Park, H.G.; Deng, Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Major royal jelly protein 2 acts as an antimicrobial agent and antioxidant in royal jelly. J. Asia. Pac. Entomol. 2019, 22, 684–689. [Google Scholar] [CrossRef]
- Bíliková, K.; Mirgorodskaya, E.; Bukovská, G.; Gobom, J.; Lehrach, H.; Simúth, J. Towards functional proteomics of minority component of honeybee royal jelly: The effect of post-translational modifications on the antimicrobial activity of apalbumin2. Proteomics 2009, 9, 2131–2138. [Google Scholar] [CrossRef]
- Mostafa, R.E.; El-Marasy, S.A.; Abdel Jaleel, G.A.; Bakeer, R.M. Protective effect of royal jelly against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats. Heliyon 2020, 6, e03330. [Google Scholar] [CrossRef] [PubMed]
- Abu-Serie, M.M.; Habashy, N.H. Two purified proteins from royal jelly with in vitro dual anti-hepatic damage potency: Major royal jelly protein 2 and its novel isoform X1. Int. J. Biol. Macromol. 2019, 128, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shao, Q.; Zhang, M.; Lu, C.; Fleming, J.; Su, S. Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model. BMC Complement. Altern. Med. 2019, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef]
- Tamura, S.; Kono, T.; Harada, C.; Yamaguchi, K.; Moriyama, T. Estimation and characterisation of major royal jelly proteins obtained from the honeybee Apis merifera. Food Chem. 2009, 114, 1491–1497. [Google Scholar] [CrossRef]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, K.S.; Jung, B.; Choi, Y.S.; Kim, H.K.; Yoon, H.J.; Gui, Z.Z.; Lee, J.; Jin, B.R. Honeybee (Apis cerana) major royal jelly protein 4 exhibits antimicrobial activity. J. Asia-Pac. Entomol. 2019, 22, 175–182. [Google Scholar] [CrossRef]
- Santos, K.S.; Delazari Dos Santos, L.; Anita Mendes, M.; Monson De Souza, B.; Malaspina, O.; Palma, M.S. Profiling the proteome complement of the secretion from hypopharyngeal gland of Africanized nurse-honeybees (Apis mellifera L.). Insect Biochem. Mol. Biol. 2005, 35, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Y.; Li, R.; Feng, M.; Han, B.; Zhou, T.; Li, J. Towards posttranslational modification proteome of royal jelly. J. Proteom. 2012, 75, 5327–5341. [Google Scholar] [CrossRef]
- Bachanová, K.; Klauduny, J.; Kopernický, J.; Simúth, J. Identification of honeybee peptide active against Paenibacillus larvae larvae through bacterial growth-inhibition assay on polyacrylamide gel. Apidologie 2002, 33, 259–269. [Google Scholar] [CrossRef]
- Bílikova, K.; Huang, S.C.; Lin, I.P.; Šimuth, J.; Peng, C.C. Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190–196. [Google Scholar] [CrossRef]
- Bărnuţiu, L.I.; Mărghitaş, L.A.; Dezmirean, D.S.; Mihai, C.M.; Bobiş, O. Chemical Composition and Antimicrobial Activity of Royal Jelly—Review. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 67–72. [Google Scholar]
- Bíliková, K.; Hanes, J.; Nordhoff, E.; Saenger, W.; Klaudiny, J.; Šimúth, J. Apisimin, a new serine-valine-rich peptide from honeybee (Apis mellifera L.) royal jelly: Purification and molecular characterization. FEBS Lett. 2002, 528, 125–129. [Google Scholar] [CrossRef]
- Han, B.; Fang, Y.; Feng, M.; Lu, X.; Huo, X.; Meng, L.; Wu, B.; Li, J. In-depth phosphoproteomic analysis of royal jelly derived from western and eastern honeybee species. J. Proteom. Res. 2014, 13, 5928–5943. [Google Scholar] [CrossRef]
- Alreshoodi, F.M.; Sultanbawa, Y. Antimicrobial activity of royal jelly. Antiinfect. Agents 2015, 13, 50–59. [Google Scholar] [CrossRef]
- Garcia, M.C.; Finola, M.S.; Marioli, J.M. Bioassay directed identification of Royal Jelly’s active compounds against the growth of bacteria capable of infecting cutaneous wounds. Adv. Microbiol 2013, 3, 138–144. [Google Scholar] [CrossRef]
- Yang, X.; Yang, D.; Wei, Z.; Wang, J.; Li, C.; Hui, Y.; Lei, K.; Chen, X.; Shen, N.; Jin, L.; et al. 10-Hydroxy-2-decenoic acid from Royal jelly: A potential medicine for RA. J. Ethno-Pharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef]
- Vucevic, D.; Melliou, E.; Vasilijic, S.; Gasic, S.; Ivanovski, P.; Chinou, I.; Colic, M. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int. Immunopharmacol. 2007, 7, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, D.; Rajkovic, I.; Chinou, I.; Colic, M. Dose-dependent immunomodulatory effects of 10-hydroxy-2-decenoic acid on human monocyte-derived dendritic cells. J. Funct. Foods 2013, 5, 838–846. [Google Scholar] [CrossRef]
- Izuta, H.; Chikaraishi, Y.; Shimazawa, M.; Mishima, S.; Hara, H. 10- Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid. Based Complement. Altern. Med 2009, 6, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.; Chinou, I.; Karshikoff, A.; et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef]
- Li, X.; Huang, C.; Xue, Y. Contribution of Lipids in Honeybee (Apis mellifera) royal jelly to health. J. Med. Food 2013, 16, 96–102. [Google Scholar] [CrossRef]
- Suzuki, K.M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid. Based Complement. Altern. Med. 2008, 5, 295–302. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, K.; Zhang, Y.Z.; Zheng, Y.F.; Hu, F.L. In Vitro Anti-Inflammatory Effects of Three Fatty Acids from Royal Jelly. Mediat. Inflamm. 2016, 2016, 3583684. [Google Scholar] [CrossRef]
- Terada, Y.; Narukawa, M.; Watanabe, T. Specific hydroxy fatty acids in Royal Jelly activate TRPA1. J. Agric. Food Chem. 2011, 59, 2627–2635. [Google Scholar] [CrossRef]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-Decenoic Acid, the Major Lipid Component of Royal Jelly, Extends the Lifespan of Caenorhabditis Elegans Through Dietary Restriction and Target of Rapamycin Signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Hwang, E.; Lee, K.; Han, S.-M.; Cho, Y.; Kim, S. Royal Jelly Protects Against Ultraviolet B–Induced Photoaging in Human Skin Fibroblasts via Enhancing Collagen Production. J. Med. Food 2011, 14, 899–906. [Google Scholar] [CrossRef]
- Nazzi, F.; Bortolomeazzi, R.; Della Vedova, G.; Del Piccolo, F.; Annoscia, D.; Milani, N. Octanoic acid confers to royal jelly varroa-repellent properties. Naturwissenschaften 2009, 96, 309–314. [Google Scholar] [CrossRef]
- Broberg, A.; Jacobsson, K.; Ström, K.; Schnürer, J. Metabolite Profiles of Lactic Acid Bacteria in Grass Silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Dzopalic, T.; Vucevic, D.; Tomic, S.; Djokic, J.; Chinou, I.; Colic, M. 3,10-Dihydroxy-decanoic acid, isolated from royal jelly, stimulates Th1 polarising capability of human monocyte-derived dendritic cells. Food Chem. 2011, 126, 1211–1217. [Google Scholar] [CrossRef]
- De Paula, R.; Rabalski, I.; Messia, M.C.; Abdel-Aal, E.S.M.; Marconi, E. Effect of processing on phenolic acids composition and radical scavenging capacity of barley pasta. Food Res. Int. 2017, 102, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Y. Isolation and characterization of proteins and lipids from honeybee (Apis mellifera L.) queen larvae and royal jelly. Food Res. Int. 2013, 54, 330–337. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Chung-Eun, H. Chapter 36—Vitamin Metabolism. In Essentials of Medical Biochemistry; Bhagavan, N.V., Chung-Eun, H., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 683–699. ISBN 9780124166875. [Google Scholar]
- Awasthi, S.; Awasthi, A. Role of vitamin a in child health and nutrition. Clin. Epidemiol. Glob. Health 2020, 8, 1039–1042. [Google Scholar] [CrossRef]
- Bates, C.J. Pantothenic Acid. In Encyclopedia of Human Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1–5. [Google Scholar]
- Bhagavan, N.V.; Chung-Eun, H. Chapter 25—Nucleotide Metabolism. In Essentials of Medical Biochemistry; Bhagavan, N.V., Chung-Eun, H., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 465–487. ISBN 9780124166875. [Google Scholar]
- Popescu, O.; Mărghitas, L.; Bobis, O.; Stanciu, O.; Bonta, V.; Moise, A.; Dezmirean, D. Sugar profile and total proteins content of fresh royal jelly. Bull. UASVM Anim. Sci. Biotechnol. 2009, 66, 265–269. [Google Scholar]
- Daniele, G.; Casabianca, H. Sugar composition of French royal jelly for comparison with commercial and artificial sugar samples. Food Chem. 2012, 134, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Sesta, G. Determination of sugars in royal jelly by HPLC. Apidologie 2006, 37, 84–90. [Google Scholar] [CrossRef]
- Schmitzová, J.; Klaudiny, J.; Albert, Š.; Schröder, W.; Schreckengost, W.; Hanes, J.; Júdová, J.; Šimúth, J. A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell. Mol. Life Sci. C. 1998, 54, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Albert, Š.; Klaudiny, J. The MRJP/YELLOW protein family of Apis mellifera: Identification of new members in the EST library. J. Insect Physiol. 2004, 50, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef]
- Schönleben, S.; Sickmann, A.; Mueller, M.J.; Reinders, J. Proteome analysis of Apis mellifera royal jelly. Anal. Bioanal. Chem. 2007, 389, 1087–1093. [Google Scholar] [CrossRef]
- Tamura, S.; Amano, S.; Kono, T.; Kondoh, J.; Yamaguchi, K.; Kobayashi, S.; Ayabe, T.; Moriyama, T. Molecular characteristics and physiological functions of major royal jelly protein 1 oligomer. Proteomics 2009, 9, 5534–5543. [Google Scholar] [CrossRef]
- Cruz, G.C.N.; Garcia, L.; Silva, A.J.; Barbosa, J.A.R.G.; Ricart, C.A.O.; Freitas, S.M.; Sousa, M.V. Calcium effect and pH-dependence on self-association and structural stability of the Apis mellifera major royal jelly protein 1. Apidologie 2011, 42, 252–269. [Google Scholar] [CrossRef]
- Kimura, Y.; Washino, N.; Yonekura, M. N-Linked Sugar Chains of 350-kDa Royal Jelly Glycoprotein. Biosci. Biotechnol. Biochem. 1995, 59, 507–509. [Google Scholar] [CrossRef]
- Šimúth, J. Some properties of the main protein of honeybee (Apis mellifera) royal jelly. Apidologie 2001, 32, 69–80. [Google Scholar] [CrossRef]
- Tian, W.; Li, M.; Guo, H.; Peng, W.; Xue, X.; Hu, Y.; Liu, Y.; Zhao, Y.; Fang, X.; Wang, K.; et al. Architecture of the native major royal jelly protein 1 oligomer. Nat. Commun. 2018, 9, 3373. [Google Scholar] [CrossRef]
- Kolayli, S.; Keskin, M. Natural Bee Products and Their Apitherapeutic Applications, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 66, ISBN 9780128179079. [Google Scholar]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef]
- Liming, W.; Jinhui, Z.; Xiaofeng, X.; Yi, L.; Jing, Z. Fast determination of 26 amino acids and their content changes in royal jelly during storage using ultra-performance liquid chromatography. J. Food Compos. Anal. 2009, 22, 242–249. [Google Scholar] [CrossRef]
- Boselli, E.; Caboni, M.; Sabatini, A.; Marcazzan, G.; Giovanni, L. Determination and changes of free amino acids in royal jelly during storage. Apidologie 2003, 34, 129–137. [Google Scholar] [CrossRef]
- Kanbur, M.; Eraslan, G.; Beyaz, L.; Silici, S.; Liman, B.C.; Altinordulu, Ş.; Atasever, A. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp. Toxicol. Pathol. 2009, 61, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Silici, S.; Ekmekcioglu, O.; Eraslan, G.; Demirtas, A. Antioxidative Effect of Royal Jelly in Cisplatin-induced Testes Damage. Urology 2009, 74, 545–551. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical Composition of Royal Jelly. In Bee Products—Chemical and Biological Properties; Springer: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar]
- Nagai, T.; Inoue, R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Stocker, A.; Schramel, P.; Kettrup, A.; Bengsch, E. Trace and mineral elements in royal jelly and homeostatic effects. J. Trace Elem. Med. Biol. 2005, 19, 183–189. [Google Scholar] [CrossRef]
- Balkanska, R.; Mladenova, E.; Karadjova, I. Quantification of selected trace and mineral elements in royal jelly from Bulgaria by ICP-OES and etaas. J. Apic. Sci. 2017, 61, 223–232. [Google Scholar] [CrossRef]
- Rodriguez-Otero, J.L.; Paseiro, P.; Simal, J.; Cepeda, A. Mineral content of the honeys produced in Galicia (North-west Spain). Food Chem. 1994, 49, 169–171. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Li, Z.G.; Tian, W.L.; Fang, X.M.; Su, S.K.; Peng, W.J. Differential volatile organic compounds in royal jelly associated with different nectar plants. J. Integr. Agric. 2016, 15, 1157–1165. [Google Scholar] [CrossRef]
- Qi, D.; Ma, C.; Wang, W.; Zhang, L.; Hao, J.; Li, J. Gas chromatography-mass spectrometry analysis reveals the differences in volatile components of royal jelly from different honeybee stocks. LWT 2020, 124, 109143. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Machado, A.M.; Aazza, S.; Lyoussi, B.; Miguel, M.G.; Mateus, M.C.; Figueiredo, A.C. Chemical Characterization and Biological Properties of Royal Jelly Samples From the Mediterranean Area. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Miguel, M.; El-Guendouz, S. Volatile Compounds of Royal Jelly. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J., Ed.; Springer: Cham, Switzerland, 2017; pp. 191–197. [Google Scholar]
- López-Gutiérrez, N.; del Aguilera-Luiz, M.M.; Romero-González, R.; Vidal, J.L.M.; Garrido Frenich, A. Fast analysis of polyphenols in royal jelly products using automated TurboFlowTM-liquid chromatography-Orbitrap high resolution mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 973, 17–28. [Google Scholar] [CrossRef]
- Virgiliou, C.; Kanelis, D.; Pina, A.; Gika, H.; Tananaki, C.; Zotou, A.; Theodoridis, G. A targeted approach for studying the effect of sugar bee feeding on the metabolic profile of Royal Jelly. J. Chromatogr. A 2020, 1616, 460783. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Wytrychowski, M.; Batteau, M.; Guibert, S.; Casabianca, H. Stable isotope ratio measurements of royal jelly samples for controlling production procedures: Impact of sugar feeding. Rapid Commun. Mass Spectrom. 2011, 25, 1929–1932. [Google Scholar] [CrossRef] [PubMed]
- Antinelli, J.F.; Zeggane, S.; Davico, R.; Rognone, C.; Faucon, J.P.; Lizzani, L. Evaluation of (E)-10-hydroxydec-2-enoic acid as a freshness parameter for royal jelly. Food Chem. 2003, 80, 85–89. [Google Scholar] [CrossRef]
- Morgado Schmidt, E.; da Silva Cunha, I.B.; Nogueira Eberlin, M.; C H F Sawaya, A. Characterization of Royal Jelly by Electrospray Ionization Mass Spectrometry Fingerprinting. Mass Spectrom. Purif. Tech. 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Abdelnur, P.V.; Abe, S.; Cunha, I.B.S.; Lima-Pallone, J.A.; Godoy, H.T.; Eberlin, M.N.; Catharino, R.R. Metabolic fingerprinting of royal jelly: Characterization and proof of authenticity. Qual. Assur. Saf. Crop. Foods 2011, 3, 185–190. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Xue, X.; Zhang, J.; Chen, F.; Li, Y.; Wu, L.; Li, C.; Mi, J. Hydrophilic interaction chromatography/tandem mass spectrometry for the determination of melamine in royal jelly and royal jelly lyophilized powder. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 4164–4170. [Google Scholar] [CrossRef] [PubMed]
- Reybroeck, W. Residues of antibiotics and chemotherapeutics in honey. J. Apic. Res. 2018, 57, 97–112. [Google Scholar] [CrossRef]
- Calvarese, S.; Forti, A.F.; Scortichini, G.; Diletti, G. Chloramphenicol in royal jelly: Analytical aspects and occurrence in Italian imports. Apidologie 2006, 37, 673–678. [Google Scholar] [CrossRef][Green Version]
- Wang, K.; Chen, H.; Lin, Z.-G.; Niu, Q.-S.; Wang, Z.; Gao, F.; Ji, T. Carbendazim exposure during the larval stage suppresses major royal jelly protein expression in nurse bees (Apis mellifera). Chemosphere 2020, 266, 129011. [Google Scholar] [CrossRef] [PubMed]
- Milone, J.P.; Chakrabarti, P.; Sagili, R.R.; Tarpy, D.R. Colony-level pesticide exposure affects honey bee (Apis mellifera L.) royal jelly production and nutritional composition. Chemosphere 2021, 263, 128183. [Google Scholar] [CrossRef] [PubMed]
- Giroud, B.; Bruckner, S.; Straub, L.; Neumann, P.; Williams, G.R.; Vulliet, E. Trace-level determination of two neonicotinoid insecticide residues in honey bee royal jelly using ultra-sound assisted salting-out liquid liquid extraction followed by ultra-high-performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2019, 151, 104249. [Google Scholar] [CrossRef]
- Hou, J.; Xie, W.; Hong, D.; Zhang, W.; Li, F.; Qian, Y.; Han, C. Simultaneous determination of ten neonicotinoid insecticides and two metabolites in honey and Royal-jelly by solid−phase extraction and liquid chromatography−tandem mass spectrometry. Food Chem. 2019, 270, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Ares, A.M.; Arribas, M.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Development and validation of UHPLC–MS/MS methods for determination of neonicotinoid insecticides in royal jelly-based products. J. Food Compos. Anal. 2018, 70, 105–113. [Google Scholar] [CrossRef]
- Yan, A.T.; Yan, R.T.; Tan, M.; Hackam, D.G.; Leblanc, K.L.; Kertland, H.; Tsang, J.L.; Jaffer, S.; Kates, M.L.; Leiter, L.A.; et al. Contemporary Management of Dyslipidemia in High-Risk Patients: Targets Still Not Met. Am. J. Med. 2006, 119, 676–683. [Google Scholar] [CrossRef]
- Hadi, A.; Najafgholizadeh, A.; Aydenlu, E.S.; Shafiei, Z.; Pirivand, F.; Golpour, S.; Pourmasoumi, M. Royal jelly is an effective and relatively safe alternative approach to blood lipid modulation: A meta-analysis. J. Funct. Foods 2018, 41, 202–209. [Google Scholar] [CrossRef]
- Kamakura, M.; Moriyama, T.; Sakaki, T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J. Pharm. Pharmacol. 2006, 58, 1683–1689. [Google Scholar] [CrossRef]
- Chiu, H.F.; Chen, B.K.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharm. Biol. 2017, 55, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Kaede, R.; Nagaya, K.; Aoyama, J.; Saito, M.; Okamatsu-Ogura, Y.; Kimura, K.; Terao, A. Royal jelly ameliorates diet-induced obesity and glucose intolerance by promoting brown adipose tissue thermogenesis in mice. Obes. Res. Clin. Pract. 2018, 12, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, D.; Fujiwara, N.; Suzuki, K. Antioxidants: Benefits and risks for long-term health. Maturitas 2010, 67, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The Emergence of the Metabolic Syndrome with Menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Roden, M. Non-alcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2020, 101122. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Vinciguerra, M.; Oben, J.; Tarquini, R.; De Cosmo, S. Non-alcoholic fatty liver disease: The role of nuclear receptors and circadian rhythmicity. Liver Int. 2014, 34, 1133–1152. [Google Scholar] [CrossRef]
- You, M.M.; Liu, Y.C.; Chen, Y.F.; Pan, Y.M.; Miao, Z.N.; Shi, Y.Z.; Si, J.J.; Chen, M.L.; Hu, F.L. Royal jelly attenuates nonalcoholic fatty liver disease by inhibiting oxidative stress and regulating the expression of circadian genes in ovariectomized rats. J. Food Biochem. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Filipič, B.; Gradišnik, L.; Rihar, K.; Šooš, E.; Pereyra, A.; Potokar, J. The influence of royal jelly and human interferon-alpha (HuIFN-αN3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro. Arhiv Higijenu Rada Toksikologiju 2015, 66, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y. Antitumor and antimetastatic actions of various natural products. Stud. Nat. Prod. Chem. 2008, 34, 35–76. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed. Res. 2007, 28, 261–266. [Google Scholar] [CrossRef]
- Townsend, G.F.; Morgan, J.F.; Tolnai, S.; Hazlett, B.; Morton, H.J.; Shuel, R.W. Studies on the in Vitro Antitumor Activity of Fatty Acids 10-Hydroxy-2-decenoic Acid from Royal Jelly. Cancer Res. 1960, 20, 503–510. [Google Scholar] [PubMed]
- Wang, J.; Zhang, W.; Zou, H.; Lin, Y.; Lin, K.; Zhou, Z.; Qiang, J.; Lin, J.; Chuka, C.M.; Ge, R.; et al. 10-Hydroxy-2-decenoic acid inhibiting the proliferation of fibroblast-like synoviocytes by PI3K-AKT pathway. Int. Immunopharmacol. 2015, 28, 97–104. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms: My perspective. Adv. Exp. Med. Biol. 2019, 1117, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.K.; Srivastava, S.; Singh, M.; Ghosh, J.K. Inducing toxicity by introducing a leucine-zipper-like motif in frog antimicrobial peptide, magainin 2. Biochem. J. 2011, 436, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Šedivá, M.; Laho, M.; Kohútová, L.; Mojžišová, A.; Majtán, J.; Klaudiny, J. 10-HDA, A Major Fatty Acid of Royal Jelly, Exhibits pH Dependent Growth-Inhibitory Activity Against Different Strains of Paenibacillus larvae. Molecules 2018, 23, 3236. [Google Scholar] [CrossRef] [PubMed]
- McCleskey, C.S.; Melampy, R.M. Bactericidal Properties of Royal Jelly of the Honeybee*. J. Econ. Entomol. 1939, 32, 581–587. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef]
- Food, E.; Authority, S. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar] [CrossRef]
- Altuntas, S.; Cinar, A.; Altuntas, V. Modelling of listeria monocytogenes growth and survival in presence of royal jelly, a promising anti-biofilm agent. J. Food Nutr. Res. 2020, 59, 7–15. [Google Scholar]
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef]
- Romanelli, A.; Moggio, L.; Montella, R.C.; Campiglia, P.; Iannaccone, M.; Capuano, F.; Pedone, C.; Capparelli, R. Peptides from Royal Jelly: Studies on the antimicrobial activity of jelleins, jelleins analogs and synergy with temporins. J. Pept. Sci. 2011, 17, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, D.; Li, M.; Jin, F.; Din, M.; Parnell, L.D.; Lai, C.Q. Mechanism of Action of Recombinant Acc-Royalisin from Royal Jelly of Asian Honeybee against Gram-Positive Bacteria. PLoS ONE 2012, 7, e47194. [Google Scholar] [CrossRef]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K.; Milk, M.; Company, I. A Potent Antibacterial in Royal Jelly. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef]
- Gunalp, R.; Ulusoy, M.; Celebier, I.; Keskin, N. Antifungal effect of royal jelly on Candida albicans. J. Biotechnol. 2018, 280, S70–S71. [Google Scholar] [CrossRef]
- Alzheimer’s Association 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Mohamed, A.A.R.; Galal, A.A.A.; Elewa, Y.H.A. Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochem. 2015, 117, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Minami, A.; Matsushita, H.; Ieno, D.; Matsuda, Y.; Horii, Y.; Ishii, A.; Takahashi, T.; Kanazawa, H.; Wakatsuki, A.; Suzuki, T. Improvement of neurological disorders in postmenopausal model rats by administration of royal jelly. Climacteric 2016, 19, 568–573. [Google Scholar] [CrossRef]
- Aslan, A.; Cemek, M.; Buyukokuroglu, M.E.; Altunbas, K.; Bas, O.; Yurumez, Y. Royal jelly can diminish secondary neuronal damage after experimental spinal cord injury in rabbits. Food Chem. Toxicol. 2012, 50, 2554–2559. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, J.; Jin, P.; Yang, Q.; Zhu, K.; You, M.; Hu, F.; Chen, M. Royal jelly ameliorates behavioral deficits, cholinergic system deficiency, and autonomic nervous dysfunction in ovariectomized cholesterol-fed rabbits. Molecules 2019, 24, 1149. [Google Scholar] [CrossRef]
- Ashok, A.; Rai, N.K.; Tripathi, S.; Bandyopadhyay, S. Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol. Sci. 2015, 143, 64–80. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Almeer, R.S.; Kassab, R.B.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol. Biol. Rep. 2019, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Earnest, C.P.; Poirier, P.; Carnethon, M.R.; Blair, S.N.; Church, T.S. Autonomic function and change in insulin for exercising postmenopausal women. Maturitas 2010, 65, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wang, F.; Wei, C.; Zhou, A.; Jia, X.; Li, F.; Tang, M.; Chu, L.; Zhou, Y.; Zhou, C.; et al. The prevalence of dementia in urban and rural areas of China. Alzheimer’s Dement. 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Chin, J.; Kim, J.W.; Shin, M.H.; Ahn, S.; Lee, D.Y.; Seo, S.W.; Na, D.L. Menopausal hormone therapy and mild cognitive impairment: A randomized, placebo-controlled trial. Menopause 2018, 25, 870–876. [Google Scholar] [CrossRef]
- Jaya Prasanthi, R.P.; Schommer, E.; Thomasson, S.; Thompson, A.; Feist, G.; Ghribi, O. Regulation of β-amyloid levels in the brain of cholesterol-fed rabbit, a model system for sporadic Alzheimer’s disease. Mech. Ageing Dev. 2008, 129, 649–655. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2018, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Aslan, Z.; Aksoy, L. Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int. Braz. J. Urol. 2015, 41, 1008–1013. [Google Scholar] [CrossRef]
- You, M.M.; Chen, Y.F.; Pan, Y.M.; Liu, Y.C.; Tu, J.; Wang, K.; Hu, F.L. Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways. Mediat. Inflamm. 2018, 2018, 7834381. [Google Scholar] [CrossRef] [PubMed]
- Petelin, A.; Kenig, S.; Kopinč, R.; Deželak, M.; Černelič Bizjak, M.; Jenko Pražnikar, Z. Effects of royal jelly administration on lipid profile, satiety, inflammation, and antioxidant capacity in asymptomatic overweight adults. Evid. Based Complement. Altern. Med. 2019, 2019, 4969720. [Google Scholar] [CrossRef]
- Yoshida, M.; Hayashi, K.; Watadani, R.; Okano, Y.; Tanimura, K.; Kotoh, J.; Sasaki, D.; Matsumoto, K.; Maeda, A. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Sci. 2017, 79, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kaku, M.; Rocabado, J.M.R.; Kitami, M.; Ida, T.; Uoshima, K. Royal jelly affects collagen crosslinking in bone of ovariectomized rats. J. Funct. Foods 2014, 7, 398–406. [Google Scholar] [CrossRef]
- Møller, A.M.J.; Delaissé, J.M.; Olesen, J.B.; Madsen, J.S.; Canto, L.M.; Bechmann, T.; Rogatto, S.R.; Søe, K. Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Res. 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Hayashi, M.; Nagamatsu, K.; Ono, T.; Kamakura, M.; Iwata, T.; Nakashima, T. The key royal jelly component 10-hydroxy-2-decenoic acid protects against bone loss by inhibiting NF-κB signaling downstream of FFAR4. J. Biol. Chem. 2020, 295, 12224–12232. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Guo, H.; Guo, Y.; Ebihara, S.; Asada, M.; Ohrui, T.; Furukawa, K.; Ichinose, M.; Yanai, K.; Kudo, Y.; et al. Royal jelly prevents the progression of sarcopenia in aged mice in vivo and in vitro. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Wang, H.; Pei, Y.; Li, Y.; Wu, H.; Song, Y.; Guo, Q.; Guo, H.; Fukushima, S.; Tatefuji, T.; et al. Effects of protease-treated royal jelly on muscle strength in elderly nursing home residents: A randomized, double-blind, placebo-controlled, dose-response study. Sci. Rep. 2017, 7, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal jelly delays motor functional impairment during aging in genetically heterogeneous male mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hijikata, K.; Seike, K.; Nakano, S.; Banjo, M.; Sato, Y.; Takahashi, K.; Hatta, H. Effects of royal jelly administration on endurance training-induced mitochondrial adaptations in skeletal muscle. Nutrients 2018, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Protects, J.; Skeletal, A.D.; Atrophy, M. Daily Oral Administration of Protease-Treated Royal Jelly Protects Against Denervation-Induced Skeletal Muscle Atrophy. Nutrients 2020, 12, 3089. [Google Scholar]
- Alu’datt, M.H.; Rababah, T.; Obaidat, M.M.; Ereifej, K.; Alhamad, M.N.; Mhaidat, N.; Andrade, J.E.; Johargy, A.; Ayadi, W. Probiotics in Milk as Functional Food: Characterization and Nutraceutical Properties of Extracted Phenolics and Peptides from Fermented Skimmed Milk Inoculated with Royal Jelly. J. Food Saf. 2015, 35, 509–522. [Google Scholar] [CrossRef]
- Mendoza, M.R.; Olano, N.O.; Villamiel, M. Chemical Indicators of Heat Treatment in Fortified and Special Milks. J. Agric. Food Chem. 2005, 53, 2995–2999. [Google Scholar] [CrossRef]
- Sharif, S.N.; Darsareh, F. Effect of royal jelly on menopausal symptoms: A randomized placebo-controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 37, 47–50. [Google Scholar] [CrossRef]
- Yakoot, M.; Salem, A.; Helmy, S. Effect of Memo®, a natural formula combination, on Mini-Mental State Examination scores in patients with mild cognitive impairment. Clin. Interv. Aging 2013, 8, 975–981. [Google Scholar] [CrossRef]
- Taavoni, S.; Barkhordari, F.; Goushegir, A.; Haghani, H. Effect of Royal Jelly on premenstrual syndrome among Iranian medical sciences students: A randomized, triple-blind, placebo-controlled study. Complement. Ther. Med. 2014, 22, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Osama, H.; Abdullah, A.; Gamal, B.; Emad, D.; Sayed, D.; Hussein, E.; Mahfouz, E.; Tharwat, J.; Sayed, S.; Medhat, S.; et al. Effect of Honey and Royal Jelly against Cisplatin- Induced Nephrotoxicity in Patients with Cancer Effect of Honey and Royal Jelly against Cisplatin-Induced Nephrotoxicity in Patients. J. Am. Coll. Nutr. 2017, 36, 342–346. [Google Scholar] [CrossRef]
- Inoue, S.; Kawashima, M.; Hisamura, R.; Imada, T.; Izuta, Y.; Nakamura, S.; Ito, M.; Tsubota, K. Clinical Evaluation of a Royal Jelly Supplementation for the Restoration of Dry Eye: A Prospective Randomized Double Blind Placebo Controlled Study and an Experimental Mouse Model. PLoS ONE 2017, 12, e169069. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Ikeda, T.; Kajita, K.; Fujioka, K.; Mori, I.; Okada, H.; Uno, Y. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr. J. 2012, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahmadnia, H.; Sharifi, N.; Alizadeh, S.; Kamalati, A.; Safari, M.S. Wonderful Effects of Royal Jelly on Treatment of Male- Factor Related Infertility. Austin J. Reprod. Med. Infertil. 2015, 2, 1031. [Google Scholar]
- Salari, R.; Salari, R.; Medicine, C. Electronic Physician (ISSN: 2008-5842). Electron. Physician 2017, 9, 3592–3597. [Google Scholar] [CrossRef]
- Questel, E.; Hernandez-pigeon, H.; Galliano, M.F.; Caruana, A.; Ceruti, I.; Ambonati, M.; Mejean, C.; Damour, O.; Castex-rizzi, N.; Bessou-touya, S.; et al. Effects of Hydroxydecine ® (10-hydroxy-2-decenoic acid) on skin barrier structure and function. Eur. J. Dermatol. 2011, 2, 906–915. [Google Scholar] [CrossRef]
- Seyyedi, F.; Rafiean, M.; Miraj, S. Comparison of the effects of vaginal royal jelly and vaginal estrogen on quality of life, sexual and urinary function in postmenopausal women. J. Clin. Diagnostic Res. 2016, 10, QC01–QC05. [Google Scholar] [CrossRef]
- Fern, B.; Mart, J.M.; Chas-barbeito, C.; Bautista-casta, I. Food & Function Determinants of specific food consumption in the Canary Islands (Spain) †. Food Funct. 2011, 2, 627–632. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).