Dietary Acrylamide Intake and the Risk of Hematological Malignancies: The Japan Public Health Center-Based Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
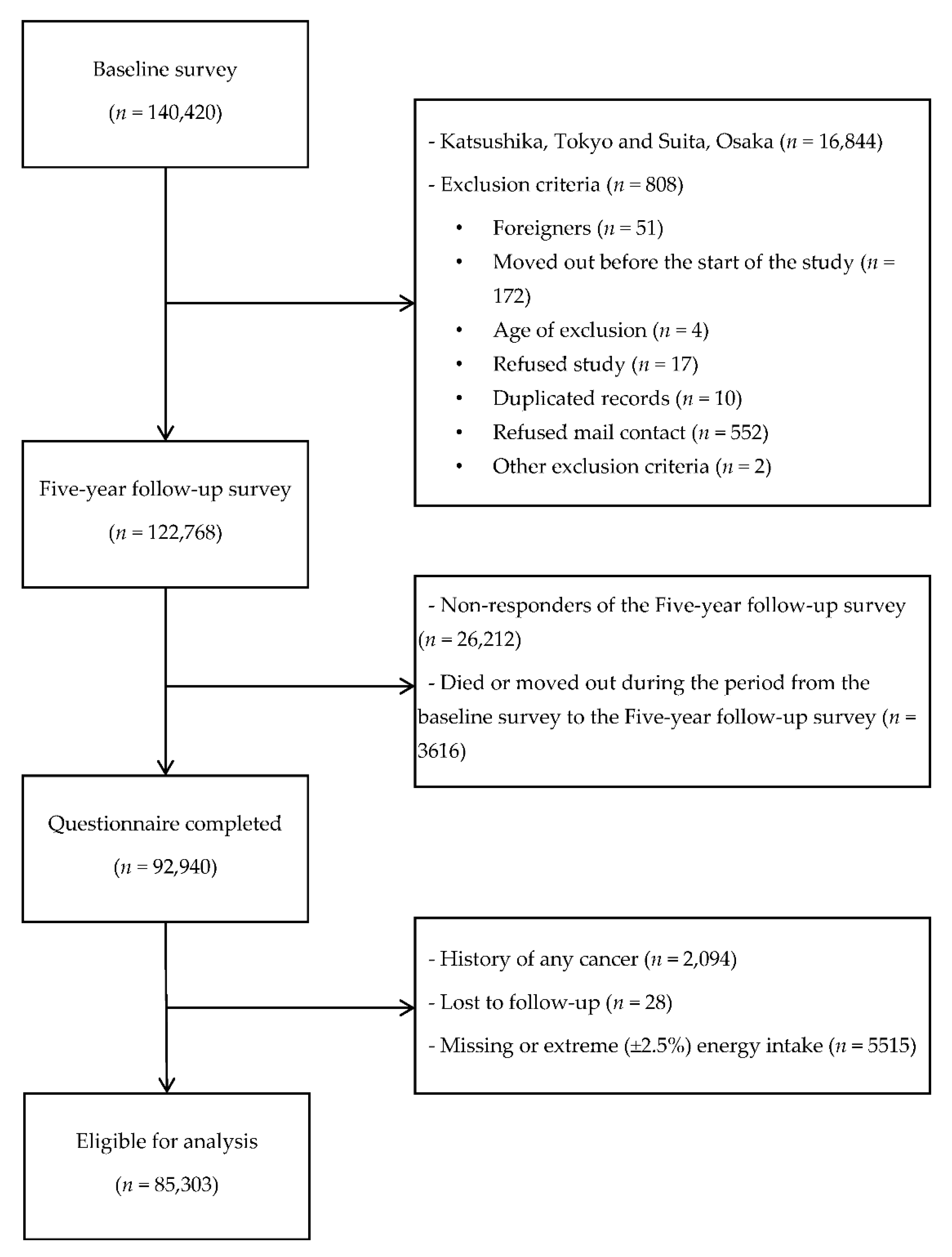
2.1. Study Cohort and Participants
2.2. Assessment of Acrylamide Intake
2.3. Follow-Up and Identification of Cancer Cases
2.4. Statistical Analysis
3. Results
3.1. Analysis of Characteristics
3.2. Association between Dietary Acrylamide Intake and Hematological Malignancy Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Some industrial chemicals. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 1994, 60, 560. [Google Scholar]
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Gierisch, J.M.; Coeytaux, R.R.; Urrutia, R.P.; Havrilesky, L.J.; Moorman, P.G.; Lowery, W.J.; Dinan, M.; McBroom, A.J.; Hasselblad, V.; Sanders, G.D. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: A systematic review. Cancer Epidemiol. Prev. Biomark. 2013, 22, 1931–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogervorst, J.G.; Baars, B.-J.; Schouten, L.J.; Konings, E.J.; Goldbohm, R.A.; van den Brandt, P.A. The carcinogenicity of dietary acrylamide intake: A comparative discussion of epidemiological and experimental animal research. Crit. Rev. Toxicol. 2010, 40, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Semla, M.; Goc, Z.; Martiniakova, M.; Omelka, R.; Formicki, G. Acrylamide: A common food toxin related to physiological functions and health. Physiol. Res. 2017, 66, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Riboldi, B.P.; Vinhas, Á.M.; Moreira, J.D. Risks of dietary acrylamide exposure: A systematic review. Food Chem. 2014, 157, 310–322. [Google Scholar] [CrossRef]
- Virk-Baker, M.K.; Nagy, T.R.; Barnes, S.; Groopman, J. Dietary acrylamide and human cancer: A systematic review of literature. Nutr. Cancer 2014, 66, 774–790. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Sobue, T.; Kitamura, T.; Kitamura, Y.; Ishihara, J.; Kotemori, A.; Zha, L.; Ikeda, S.; Sawada, N.; Iwasaki, M. Dietary Acrylamide Intake and Risk of Esophageal, Gastric, and Colorectal Cancer: The Japan Public Health Center-Based Prospective Study. Cancer Epidemiol. Prev. Biomark. 2019, 28, 1461–1468. [Google Scholar] [CrossRef]
- Kotemori, A.; Ishihara, J.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S.; Group, J.S. Dietary acrylamide intake and risk of breast cancer: The Japan Public Health Center-based Prospective Study. Cancer Sci. 2018, 109, 843–853. [Google Scholar] [CrossRef]
- Kotemori, A.; Ishihara, J.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S.; Group, J.S. Dietary acrylamide intake and the risk of endometrial or ovarian cancers in Japanese women. Cancer Sci. 2018, 109, 3316–3325. [Google Scholar] [CrossRef]
- Liu, R.; Zha, L.; Sobue, T.; Kitamura, T.; Ishihara, J.; Kotemori, A.; Ikeda, S.; Sawada, N.; Iwasaki, M.; Tsugane, S. Dietary Acrylamide Intake and Risk of Lung Cancer: The Japan Public Health Center Based Prospective Study. Nutrients 2020, 12, 2417. [Google Scholar] [CrossRef]
- Zha, L.; Sobue, T.; Kitamura, T.; Kitamura, Y.; Ishihara, J.; Kotemori, A.; Liu, R.; Ikeda, S.; Sawada, N.; Iwasaki, M. Dietary acrylamide intake and the risk of liver cancer: The Japan public health center-based prospective study. Nutrients 2020, 12, 2503. [Google Scholar] [CrossRef] [PubMed]
- Kito, K.; Ishihara, J.; Kotemori, A.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. Dietary Acrylamide Intake and the Risk of Pancreatic Cancer: The Japan Public Health Center-Based Prospective Study. Nutrients 2020, 12, 3584. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, T.; Kontto, J.; Jestoi, M.; Valsta, L.; Peltonen, K.; Pietinen, P.; Virtanen, S.; Sinkko, H.; Kronberg-Kippilä, C.; Albanes, D. Dietary acrylamide intake and the risk of cancer among Finnish male smokers. Cancer Causes Control 2010, 21, 2223–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongers, M.L.; Hogervorst, J.G.; Schouten, L.J.; Goldbohm, R.A.; Schouten, H.C.; van den Brandt, P.A. Dietary acrylamide intake and the risk of lymphatic malignancies: The Netherlands Cohort Study on diet and cancer. PLoS ONE 2012, 7, e38016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirtane, K.; Lee, S.J. Racial and ethnic disparities in hematologic malignancies. Blood 2017, 130, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Abreu, D.; Bordoni, A.; Zucca, E. Epidemiology of hematological malignancies. Ann. Oncol. 2007, 18, i3–i8. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Piñeros, M.; Ferlay, J.; Soerjomataram, I.; Monnereau, A.; Bray, F. Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet Haematol. 2018, 5, e14–e24. [Google Scholar] [CrossRef]
- Chihara, D.; Ito, H.; Matsuda, T.; Shibata, A.; Katsumi, A.; Nakamura, S.; Tomotaka, S.; Morton, L.M.; Weisenburger, D.D.; Matsuo, K. Differences in incidence and trends of haematological malignancies in J apan and the U nited S tates. Br. J. Haematol. 2014, 164, 536–545. [Google Scholar] [CrossRef]
- Mucci, L.A.; Wilson, K.M. Acrylamide intake through diet and human cancer risk. J. Agric. Food Chem. 2008, 56, 6013–6019. [Google Scholar] [CrossRef]
- Tsugane, S.; Sawada, N. The JPHC study: Design and some findings on the typical Japanese diet. Jpn. J. Clin. Oncol. 2014, 44, 777–782. [Google Scholar] [CrossRef]
- Tsugane, S.; Kobayashi, M.; Sasaki, S. Validity of the self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I: Comparison with dietary records for main nutrients. J. Epidemiol. 2003, 13, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugane, S.; Sasaki, S.; Kobayashi, M.; Tsubono, Y.; Akabane, M. Validity and reproducibility of the self-administered food frequency questionnaire in the JPHC Study Cohort I: Study design, conduct and participant profiles. J. Epidemiol. 2003, 13, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, J.; Sobue, T.; Yamamoto, S.; Yoshimi, I.; Sasaki, S.; Kobayashi, M.; Takahashi, T.; Iitoi, Y.; Akabane, M.; Tsugane, S. Validity and reproducibility of a self-administered food frequency questionnaire in the JPHC Study Cohort II: Study design, participant profile and results in comparison with Cohort I. J. Epidemiol. 2003, 13, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, J.; Inoue, M.; Kobayashi, M.; Tanaka, S.; Yamamoto, S.; Iso, H.; Tsugane, S. Impact of the revision of a nutrient database on the validity of a self-administered food frequency questionnaire (FFQ). J. Epidemiol. 2006, 16, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Kagawa, Y. Standard tables of food composition in Japan. Stand. Tables Food Compos. Japan Fifth Revis. Enlarg. Ed. 2008, 28–36. [Google Scholar]
- Kotemori, A.; Ishihara, J.; Nakadate, M.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. Validity of a self-administered food frequency questionnaire for the estimation of acrylamide intake in the Japanese population: The JPHC FFQ Validation Study. J. Epidemiol. 2018, 28, 482–487. [Google Scholar] [CrossRef] [Green Version]
- FAO; WHO. Health Implications of Acrylamide in Food; FAO: Rome, Italy; WHO: Geneve, Switzerland, 2002. [Google Scholar]
- Takatsuki, S.; Nemoto, S.; Sasaki, K.; Maitani, T. Production of acrylamide in agricultural products by cooking. Shokuhin Eiseigaku Zasshi. J. Food Hyg. Soc. Jpn. 2004, 45, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Mizukami, Y.; Kohata, K.; Yamaguchi, Y.; Hayashi, N.; Sawai, Y.; Chuda, Y.; Ono, H.; Yada, H.; Yoshida, M. Analysis of acrylamide in green tea by gas chromatography—mass spectrometry. J. Agric. Food Chem. 2006, 54, 7370–7377. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture, Forestry and Fisheries. Risk Profile Sheet Relating to the Food Safety for Acrylamide; Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2015.
- Food Safety Commission of Japan. Acrylamide in Foods Generated through Heating (Contaminants); Safety Commission of Japan: Tokyo, Japan. Food Safety 2016, 4, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food Safety Commission of Japan. Information Clearing Sheet for Acrylamide. Available online: https://www.fsc.go.jp/fsciis/attachedFile/download?retrievalId=kai20111222sfc&fileId=520 (accessed on 30 November 2020)[in Japanese].
- National Institute of Health Sciences. Acrylamide Analysis in Food. Available online: http://www.mhlw.go.jp/topics/2002/11/tp1101-1a.html (accessed on 30 November 2020)[in Japanese].
- Ugai, T.; Matsuo, K.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Sasazuki, S.; Inoue, M.; Tsugane, S. Japan Public Health Center-based Prospective Study Group. Smoking and subsequent risk of leukemia in Japan: The Japan Public Health Center-based Prospective Study. J. Epidemiol. 2017, 27, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Ugai, T.; Matsuo, K.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Sasazuki, S.; Inoue, M.; Kanda, Y.; Tsugane, S. Smoking and alcohol and subsequent risk of myelodysplastic syndromes in Japan: The Japan Public Health Centre-based Prospective Study. Br. J. Haematol. 2017, 178, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Schettgen, T.; Rossbach, B.; Kütting, B.; Letzel, S.; Drexler, H.; Angerer, J. Determination of haemoglobin adducts of acrylamide and glycidamide in smoking and non-smoking persons of the general population. Int. J. Hyg. Environ. Health 2004, 207, 531–539. [Google Scholar] [CrossRef]
- Marshall, S.W. Power for tests of interaction: Effect of raising the Type I error rate. Epidemiol. Perspect. Innov. 2007, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvin, S. Statistical Analysis of Epidemiologic Data; Back to cited text; Oxford University Press: New York, NY, USA, 1996; pp. 213–214. [Google Scholar]
- Pelucchi, C.; La Vecchia, C.; Bosetti, C.; Boyle, P.; Boffetta, P. Exposure to acrylamide and human cancer—A review and meta-analysis of epidemiologic studies. Ann. Oncol. 2011, 22, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
| Histologic Subtype | ICD-O-3 | N |
|---|---|---|
| Malignant lymphomas, all a,b | 959–972, 974–975 | 326 |
| Diffuse large-cell lymphoma c | 9675, 9680, 9684 | 123 |
| Follicular lymphoma c | 9690–9698 | 29 |
| T-cell lymphoma or Mucosis fungoides | 9700–9709, 9714 | 18 |
| Extranodal marginal B-cell lymphoma or MALT | 9699 | 18 |
| Mantle cell lymphoma | 9673 | 4 |
| Walden-ström’s macroglobulinemia and immunocytoma | 9671, 9761 | 3 |
| Burkitt’s lymphoma | 9687 | 2 |
| Lymphoblastic lymphoma | 9727–9728 | 1 |
| Malignant lymphoma NOS c | 9590–9596 | 80 |
| Hodgkin lymphoma | 9650–9669 | 10 |
| Multiple myeloma (MM) b | 973, 976 | 126 |
| Leukemia, all a,b | 980–994 | 224 |
| Acute lymphoblastic leukemia | 9832–9835 | 11 |
| Acute myeloid leukemia c | 9840, 9861, 9866–9867, 9873–9875, 9891, 9896 | 63 |
| Chronic myeloid leukemia | 9863, 9875 | 18 |
| Other specific subtypes d | ||
| Adult T-cell leukemia/lymphoma c | 9827 | 77 |
| Myelodysplastic syndromes (MDS) c | 9980, 9982–9983, 9985, 9987–9989 | 53 |
| Chronic lymphocytic leukemia | 9670, 9823 | 7 |
| Characteristics | Tertile of Energy-Adjusted Acrylamide Intake | ||||||
|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | p-Value d | ||||
| Number of Participants | 28,435 | 28,434 | 28,434 | ||||
| Men, % | 57.6 | 44.3 | 38.8 | ||||
| Dietary variables | |||||||
| Acrylamide intake | |||||||
| Range, μg/d | 0.00–4.84 | 4.85–7.67 | 7.68–66.68 | ||||
| Median, a μg/d | 3.58 | (1.47) | 6.09 | (1.37) | 10.11 | (3.66) | |
| Median, a μg/kg body weight·d | 0.06 | (0.03) | 0.11 | (0.03) | 0.18 | (0.08) | <0.001 |
| Total energy intake, a kcal/d | 1902.6 | (864.4) | 1916.1 | (824.3) | 1904.3 | (824.8) | 0.015 |
| Coffee, a g/d | 17.8 | (67.6) | 83.3 | (139.6) | 210.1 | (325.1) | <0.001 |
| Green tea, a g/d | 223.0 | (372.2) | 405.9 | (513.2) | 575.6 | (848.2) | <0.001 |
| Vegetables, a g/d | 156.5 | (129.5) | 197.0 | (144.9) | 202.0 | (153.6) | <0.001 |
| Fruit, a g/d | 140.3 | (170.1) | 185.7 | (185.8) | 184.5 | (186.8) | <0.001 |
| Meat, a g/d | 48.4 | (48.8) | 50.5 | (43.7) | 50.2 | (42.4) | <0.001 |
| Fish, a g/d | 75.3 | (62.2) | 80.3 | (58.0) | 75.1 | (55.8) | <0.001 |
| Biscuits, a g/d | 0.3 | (1.4) | 1.4 | (2.3) | 2.0 | (5.8) | <0.001 |
| Potato, a g/d | 7.5 | (11.0) | 13.8 | (18.4) | 15.1 | (21.4) | <0.001 |
| Fiber, a g/d | 10.6 | (5.7) | 12.6 | (6.0) | 12.9 | (6.3) | <0.001 |
| Carbohydrates, a g/d | 254.3 | (58.7) | 261.0 | (49.4) | 265.0 | (49.0) | <0.001 |
| Niacin, a mg/d | 16.6 | (5.9) | 18.1 | (5.3) | 19.2 | (5.3) | <0.001 |
| Saturated fatty acid, a g/d | 14.4 | (8.1) | 15.5 | (6.9) | 16.4 | (6.9) | <0.001 |
| Mono unsaturated fat, a g/d | 16.8 | (8.0) | 18.3 | (7.0) | 19.1 | (6.9) | <0.001 |
| Poly unsaturated fat, a g/d | 11.5 | (4.7) | 12.5 | (4.3) | 12.6 | (4.3) | <0.001 |
| Nondietary variables | |||||||
| Age at Five-year follow-up study, a y | 58.0 | (11.0) | 57.0 | (13.0) | 55.0 | (13.0) | <0.001 |
| Body mass index, b,c kg/m2 | 23.7 | (3.1) | 23.6 | (3.0) | 23.4 | (3.0) | <0.001 |
| Smoking status, % | |||||||
| Never smoker | 58.0 | 64.9 | 64.0 | <0.001 | |||
| Former smoker | 10.6 | 8.3 | 6.8 | ||||
| Current smoker | 25.0 | 21.0 | 23.5 | ||||
| Missing | 6.4 | 5.8 | 5.6 | ||||
| Number of cigarettes/d, a,c only for current smokers | 20.0 | (9.0) | 20.0 | (10.0) | 20.0 | (15.0) | <0.001 |
| Alcohol intake, % | |||||||
| Nondrinker | 45.6 | 54.9 | 59.8 | <0.001 | |||
| <150 g/week | 17.8 | 20.7 | 22.7 | ||||
| ≥150 g/week | 34.5 | 20.4 | 15.3 | ||||
| Missing | 2.1 | 2.1 | 2.2 | ||||
| Physical activity (METs), % | |||||||
| Quartile 1 | 22.0 | 22.4 | 23.4 | <0.001 | |||
| Quartile 2 | 18.1 | 19.9 | 20.2 | ||||
| Quartile 3 | 19.1 | 22.4 | 23.5 | ||||
| Quartile 4 | 18.3 | 18.3 | 18.1 | ||||
| Missing | 22.6 | 16.9 | 14.8 | ||||
| Diabetes, % yes | 8.3 | 6.7 | 5.4 | <0.001 | |||
| Occupation, % | |||||||
| Professional or office worker | 15.9 | 16.2 | 17.0 | <0.001 | |||
| Sales clerk or other | 26.6 | 27.1 | 30.1 | ||||
| Farmer | 18.7 | 15.0 | 12.6 | ||||
| Manual laborer | 10.6 | 14.9 | 15.5 | ||||
| Unemployed | 9.4 | 8.2 | 6.9 | ||||
| Missing | 18.7 | 18.7 | 18.0 | ||||
| Quartile of Energy-Adjusted Acrylamide Intake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 μg/d | Tertile 1 (Lowest) | Tertile 2 | Tertile 3 (Highest) | p for Interaction d | ||||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | p for Trend | ||
| Number of subjects | 85,303 | 28,435 | 28,434 | 28,434 | ||||||
| Person-years | 1,267,766 | 417,242 | 424,878 | 425,646 | ||||||
| Number of malignant lymphoma | 326 | 117 | 119 | 90 | ||||||
| Crude rate (per 100,000) | 25.7 | 28.0 | 28.0 | 21.1 | ||||||
| Gender-, age-and area-adjusted model a | 0.98 | (0.95–1.01) | 1.00 | (Reference) | 1.07 | (0.83–1.39) | 0.89 | (0.67–1.19) | 0.47 | |
| Multivariable model 1 b | 0.98 | (0.94–1.01) | 1.00 | (Reference) | 1.07 | (0.82–1.40) | 0.87 | (0.64–1.18) | 0.40 | 0.38 |
| Multivariable model 1 (excluding cases <3 years) | 0.98 | (0.94–1.01) | 1.00 | (Reference) | 1.09 | (0.82–1.45) | 0.86 | (0.62–1.19) | 0.39 | |
| By smoking status | ||||||||||
| Never smoker | ||||||||||
| Number of subjects | 53,136 | 18,137 | 18,102 | 16,897 | ||||||
| Person-years | 817,851 | 252,824 | 284,192 | 280,835 | ||||||
| Number of malignant lymphoma | 176 | 67 | 65 | 44 | ||||||
| Crude rate (per 100,000) | 21.5 | 26.5 | 22.9 | 15.7 | ||||||
| Multivariable model 1 | 0.99 | (0.95–1.04) | 1.00 | (Reference) | 0.93 | (0.65–1.33) | 0.72 | (0.48–1.10) | 0.14 | |
| Ever smoker c | ||||||||||
| Number of subjects | 27,082 | 8475 | 8663 | 9944 | ||||||
| Person-years | 382,535 | 140,840 | 118,384 | 123,311 | ||||||
| Number of malignant lymphoma | 129 | 41 | 47 | 41 | ||||||
| Crude rate (per 100,000) | 33.7 | 29.1 | 39.7 | 33.2 | ||||||
| Multivariable model 1 | 0.96 | (0.92–1.01) | 1.00 | (Reference) | 1.40 | (0.91–2.18) | 1.19 | (0.73–1.93) | 0.48 | |
| Quartile of Energy-Adjusted Acrylamide Intake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 μg/d | Tertile 1 (Lowest) | Tertile 2 | Tertile 3 (Highest) | p for Interaction d | ||||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | p for Trend | ||
| Number of subjects | 85,303 | 28,435 | 28,434 | 28,434 | ||||||
| Person-years | 1,267,766 | 417,242 | 424,878 | 425,646 | ||||||
| Number of multiple myeloma | 126 | 49 | 48 | 29 | ||||||
| Crude rate (per 100,000) | 9.9 | 11.7 | 11.3 | 6.8 | ||||||
| Gender-, age- and area-adjusted model a | 0.98 | (0.93–1.03) | 1.00 | (Reference) | 0.99 | (0.66–1.48) | 0.65 | (0.41–1.04) | 0.09 | |
| Multivariable model 1 b | 0.98 | (0.93–1.03) | 1.00 | (Reference) | 0.99 | (0.65–1.50) | 0.64 | (0.38–1.05) | 0.09 | 0.24 |
| Multivariable model 1 (excluding cases <3 years) | 0.98 | (0.93–1.04) | 1.00 | (Reference) | 1.00 | (0.63–1.58) | 0.66 | (0.38–1.14) | 0.15 | |
| By smoking status | ||||||||||
| Never smoker | ||||||||||
| Number of subjects | 53,136 | 18,137 | 18,102 | 16,897 | ||||||
| Person-years | 817,851 | 252,824 | 284,192 | 280,835 | ||||||
| Number of multiple myeloma | 76 | 28 | 29 | 19 | ||||||
| Crude rate (per 100,000) | 9.3 | 11.1 | 10.2 | 6.8 | ||||||
| Multivariable model 1 | 0.99 | (0.93–1.05) | 1.00 | (Reference) | 1.00 | (0.58–1.73) | 0.70 | (0.37–1.34) | 0.29 | |
| Ever smoker c | ||||||||||
| Number of subjects | 27,082 | 8475 | 8663 | 9944 | ||||||
| Person-years | 382,535 | 140,840 | 118,384 | 123,311 | ||||||
| Number of multiple myeloma | 38 | 17 | 12 | 9 | ||||||
| Crude rate (per 100,000) | 9.9 | 12.1 | 10.1 | 7.3 | ||||||
| Multivariable model 1 | 0.97 | (0.88–1.07) | 1.00 | (Reference) | 0.72 | (0.33–1.57) | 0.52 | (0.21–1.27) | 0.15 | |
| Quartile of Energy-Adjusted Acrylamide Intake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 μg/d | Tertile 1 (Lowest) | Tertile 2 | Tertile 3 (Highest) | p for Interaction d | ||||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | p for Trend | ||
| Number of subjects | 85,303 | 28,435 | 28,434 | 28,434 | ||||||
| Person-years | 1,267,766 | 417,242 | 424,878 | 425,646 | ||||||
| Number of leukemia | 224 | 82 | 74 | 68 | ||||||
| Crude rate (per 100,000) | 17.7 | 19.7 | 17.4 | 16.0 | ||||||
| Gender-, age- and area-adjusted model a | 1.00 | (0.97–1.04) | 1.00 | (Reference) | 0.92 | (0.67–1.26) | 0.87 | (0.62–1.21) | 0.39 | |
| Multivariable model 1 b | 1.02 | (0.98–1.05) | 1.00 | (Reference) | 1.01 | (0.73–1.40) | 1.01 | (0.71–1.45) | 0.94 | 0.13 |
| Multivariable model 1 (excluding cases <3 years) | 1.02 | (0.99–1.06) | 1.00 | (Reference) | 1.04 | (0.73–1.49) | 1.03 | (0.70–1.52) | 0.87 | |
| By smoking status | ||||||||||
| Never smoker | ||||||||||
| Number of subjects | 53,136 | 18,137 | 18,102 | 16,897 | ||||||
| Person-years | 817,851 | 252,824 | 284,192 | 280,835 | ||||||
| Number of leukemia | 128 | 48 | 40 | 40 | ||||||
| Crude rate (per 100,000) | 15.7 | 19.0 | 14.1 | 14.2 | ||||||
| Multivariable model 1 | 1.01 | (0.97–1.06) | 1.00 | (Reference) | 0.82 | (0.53–1.27) | 0.86 | (0.53–1.37) | 0.51 | |
| Ever smoker c | ||||||||||
| Number of subjects | 27,082 | 8475 | 8663 | 9944 | ||||||
| Person-years | 382,535 | 140,840 | 118,384 | 123,311 | ||||||
| Number of leukemia | 69 | 23 | 24 | 22 | ||||||
| Crude rate (per 100,000) | 18.0 | 16.3 | 20.3 | 17.8 | ||||||
| Multivariable model 1 | 1.03 | (0.97–1.09) | 1.00 | (Reference) | 1.42 | (0.78–2.59) | 1.33 | (0.69–2.59) | 0.38 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, L.; Liu, R.; Sobue, T.; Kitamura, T.; Ishihara, J.; Kotemori, A.; Ikeda, S.; Sawada, N.; Iwasaki, M.; Tsugane, S.; et al. Dietary Acrylamide Intake and the Risk of Hematological Malignancies: The Japan Public Health Center-Based Prospective Study. Nutrients 2021, 13, 590. https://doi.org/10.3390/nu13020590
Zha L, Liu R, Sobue T, Kitamura T, Ishihara J, Kotemori A, Ikeda S, Sawada N, Iwasaki M, Tsugane S, et al. Dietary Acrylamide Intake and the Risk of Hematological Malignancies: The Japan Public Health Center-Based Prospective Study. Nutrients. 2021; 13(2):590. https://doi.org/10.3390/nu13020590
Chicago/Turabian StyleZha, Ling, Rong Liu, Tomotaka Sobue, Tetsuhisa Kitamura, Junko Ishihara, Ayaka Kotemori, Sayaka Ikeda, Norie Sawada, Motoki Iwasaki, Shoichiro Tsugane, and et al. 2021. "Dietary Acrylamide Intake and the Risk of Hematological Malignancies: The Japan Public Health Center-Based Prospective Study" Nutrients 13, no. 2: 590. https://doi.org/10.3390/nu13020590
APA StyleZha, L., Liu, R., Sobue, T., Kitamura, T., Ishihara, J., Kotemori, A., Ikeda, S., Sawada, N., Iwasaki, M., Tsugane, S., & for the JPHC Study Group. (2021). Dietary Acrylamide Intake and the Risk of Hematological Malignancies: The Japan Public Health Center-Based Prospective Study. Nutrients, 13(2), 590. https://doi.org/10.3390/nu13020590







