A Novel Nutraceuticals Mixture Improves Liver Steatosis by Preventing Oxidative Stress and Mitochondrial Dysfunction in a NAFLD Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
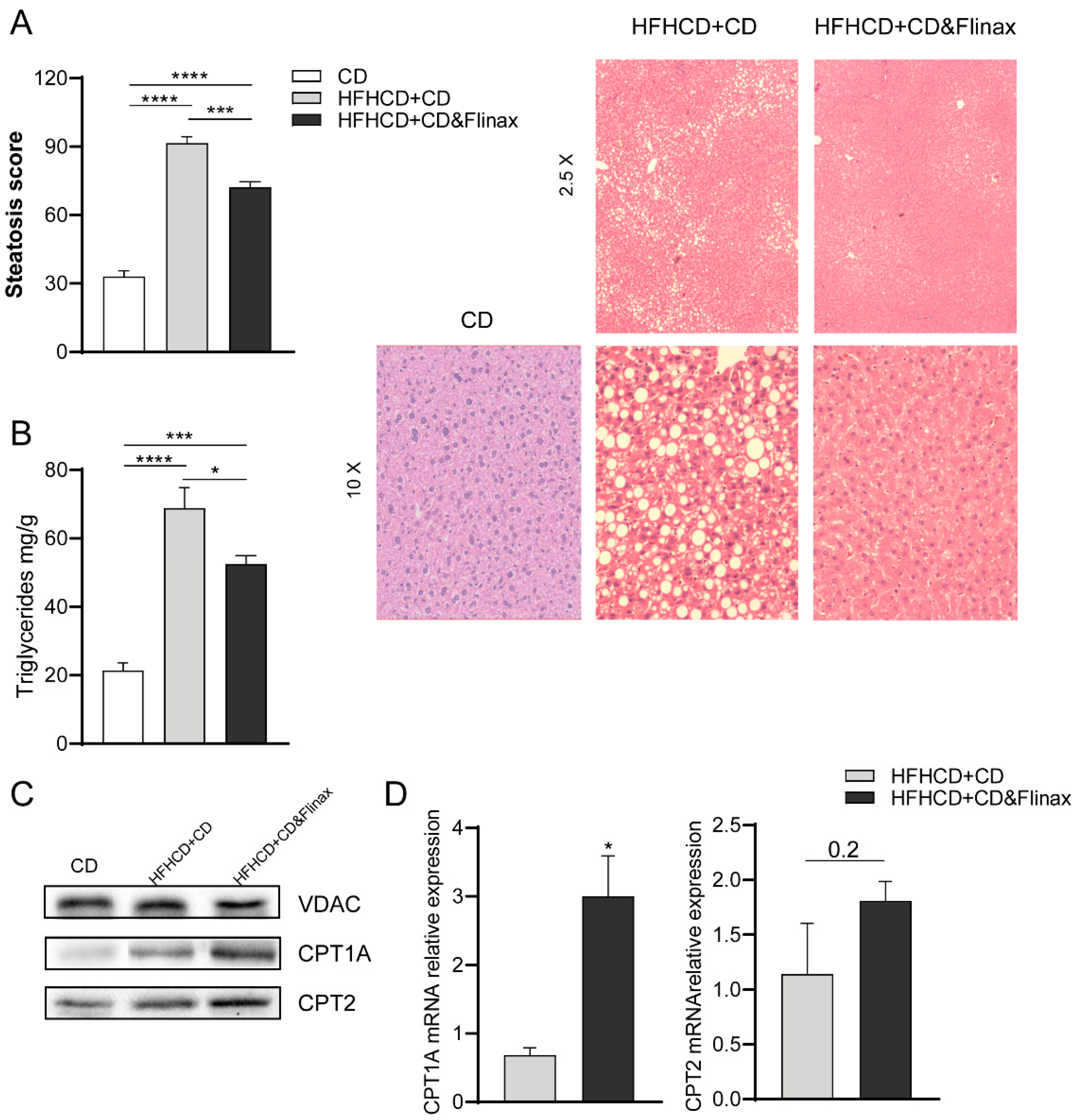
2.2. Histology
2.3. Triglycerides Analysis
2.4. Isolation of Mitochondria
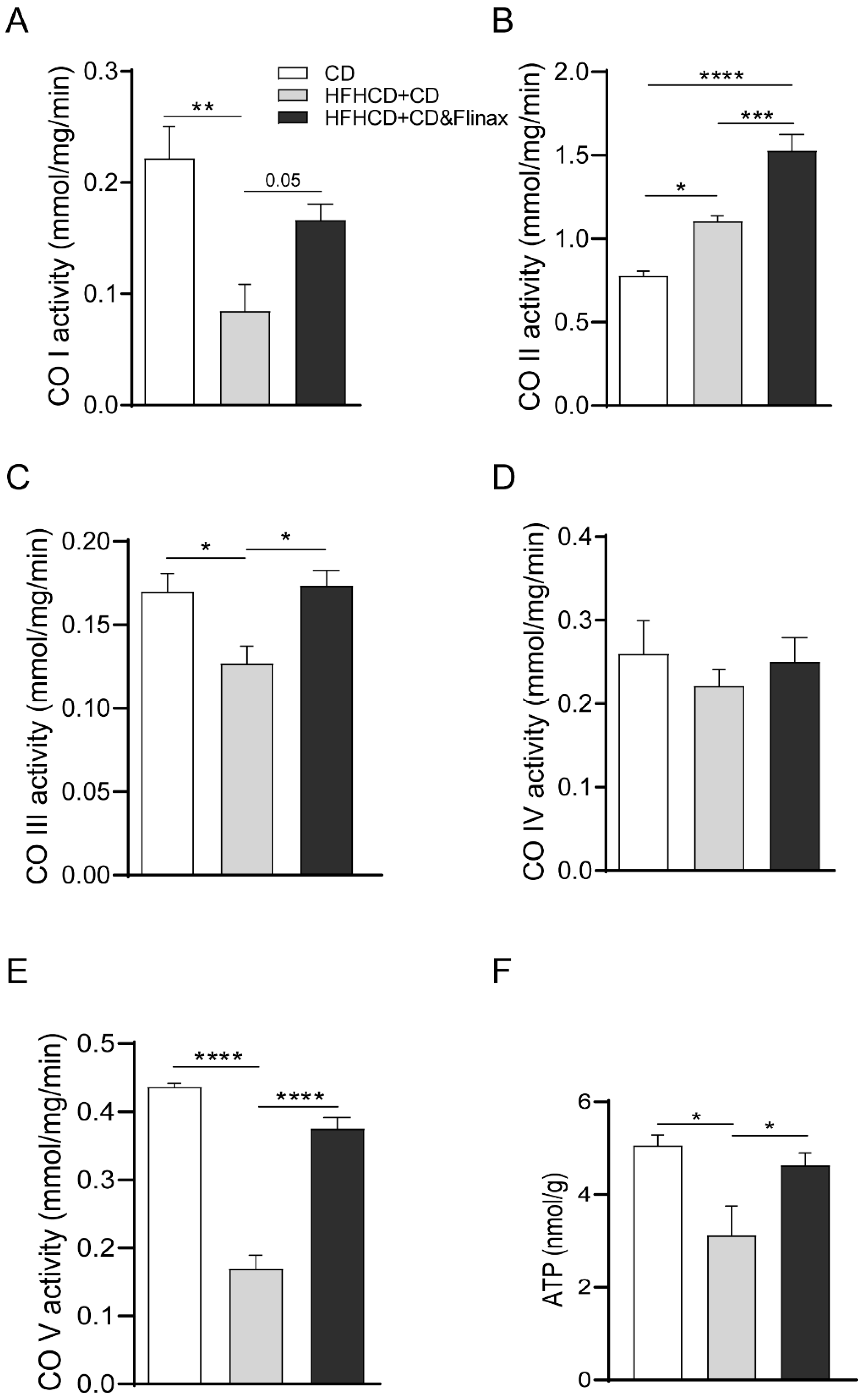
2.5. Mitochondrial Complexes Activity
2.5.1. Complex I Assay
2.5.2. Complex II Assay
2.5.3. Complex III Assay
2.5.4. Complex IV Assay
2.5.5. Complex V (ATP-Synthase) Assay
2.6. Western Blot Analysis
2.7. Gene Expression Analysis by RT-PCR
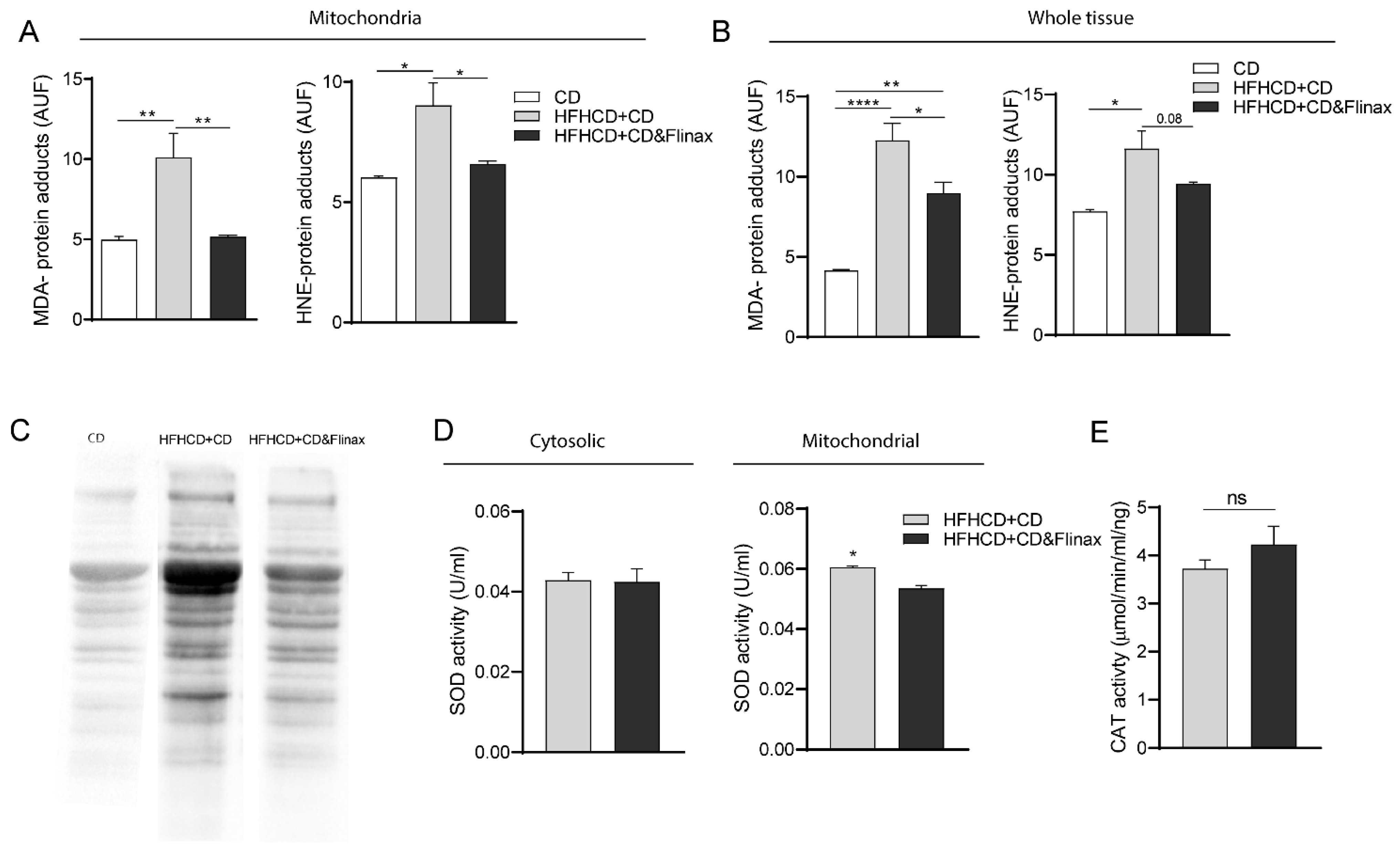
2.8. Liver Tissue and Mitochondrial HNE and MDA Adducts
2.9. SOD and Catalase Activity
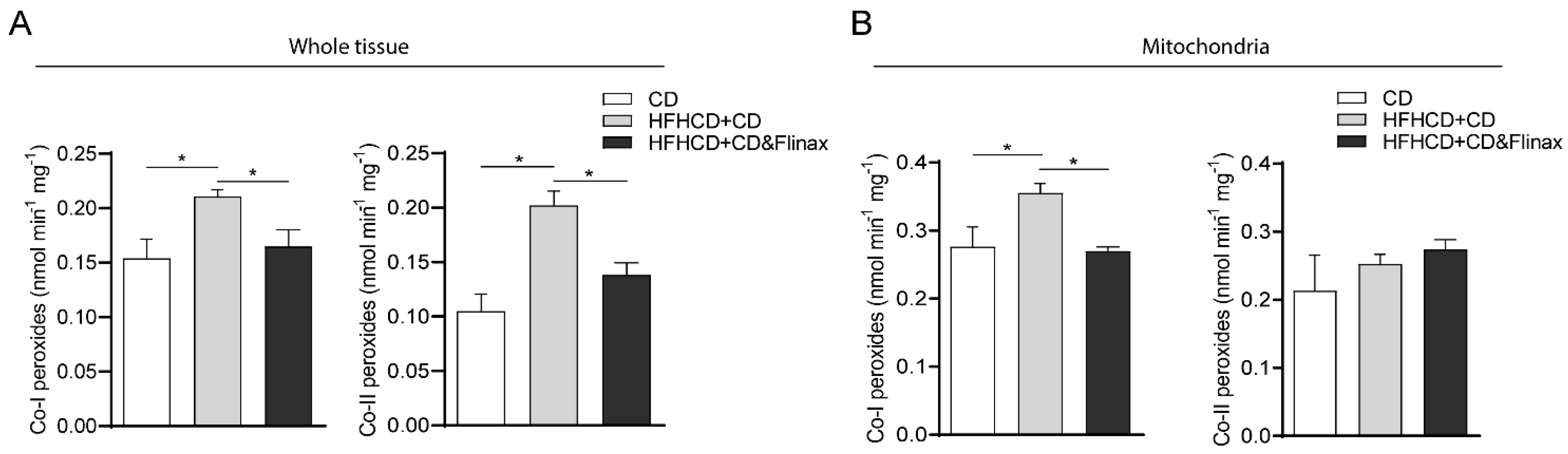
2.10. Measurement of Mitochondrial H2O2 Production
2.11. Western Blot Analysis of Hepatic Oxidized Proteins
2.12. Statistical Analysis
3. Results
3.1. Flinax Reduced Hepatic Steatosis and Oxidative Stress
3.2. Flinax Enhances the Mitochondrial Respiratory Chain Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. 2016, 11, 451–496. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Mato, J.M.; Lu, S.C. Nonalcoholic fatty liver disease: Update on pathogenesis, diagnosis, treatment and the role of S-adenosylmethionine. Exp. Biol. Med. 2015, 240, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 377–386. [Google Scholar] [CrossRef]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Skeans, M.A.; Harper, A.M.; Wainright, J.L.; Snyder, J.J.; Israni, A.K.; Kasiske, B.L. OPTN/SRTR 2016 Annual Data Report: Liver. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2018, 18 (Suppl. 1), 172–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.J.; She, Z.G.; Li, H. Time to step-up the fight against NAFLD. Hepatology 2018, 67, 2068–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, R.M.; Oranu, A.; Khungar, V. Nonalcoholic Fatty Liver Disease: Pathophysiology and Management. Gastroenterol. Clin. N. Am. 2016, 45, 639–652. [Google Scholar] [CrossRef] [Green Version]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef]
- Shenkin, A. The key role of micronutrients. Clin. Nutr. 2006, 25, 1–13. [Google Scholar] [CrossRef]
- Garcia, O.P.; Long, K.Z.; Rosado, J.L. Impact of micronutrient deficiencies on obesity. Nutr. Rev. 2009, 67, 559–572. [Google Scholar] [CrossRef]
- Pickett-Blakely, O.; Young, K.; Carr, R.M. Micronutrients in Nonalcoholic Fatty Liver Disease Pathogenesis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzawa, N.; Takamura, T.; Kurita, S.; Misu, H.; Ota, T.; Ando, H.; Yokoyama, M.; Honda, M.; Zen, Y.; Nakanuma, Y.; et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 2007, 46, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Sangineto, M.; Grabherr, F.; Adolph, T.E.; Grander, C.; Reider, S.; Jaschke, N.; Mayr, L.; Schwärzler, J.; Dallio, M.; Moschen, A.R.; et al. Dimethyl fumarate ameliorates hepatic inflammation in alcohol related liver disease. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 1610–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serviddio, G.; Bellanti, F.; Romano, A.; Tamborra, R.; Rollo, T.; Altomare, E.; Vendemiale, G. Bioenergetics in aging: Mitochondrial proton leak in aging rat liver, kidney and heart. Redox Rep. Commun. Free Radic. Res. 2007, 12, 91–95. [Google Scholar] [CrossRef]
- Barrientos, A. In vivo and in organello assessment of OXPHOS activities. Methods 2002, 26, 307–316. [Google Scholar] [CrossRef]
- Bellanti, F.; Villani, R.; Tamborra, R.; Blonda, M.; Iannelli, G.; Di Bello, G.; Facciorusso, A.; Poli, G.; Iuliano, L.; Avolio, C.; et al. Synergistic interaction of fatty acids and oxysterols impairs mitochondrial function and limits liver adaptation during nafld progression. Redox Biol. 2018, 15, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Romano, A.D.; Giudetti, A.M.; Rollo, T.; Blonda, M.; Tamborra, R.; Vendemiale, G.; Serviddio, G. Many faces of mitochondrial uncoupling during age: Damage or defense? J. Gerontol. Ser. Abiological Sci. Med. Sci. 2013, 68, 892–902. [Google Scholar] [CrossRef] [Green Version]
- Massarenti, P.; Biasi, F.; De Francesco, A.; Pauletto, D.; Rocca, G.; Silli, B.; Vizio, B.; Serviddio, G.; Leonarouzzi, G.; Poli, G.; et al. 4-Hydroxynonenal is markedly higher in patients on a standard long-term home parenteral nutrition. Free Radic. Res. 2004, 38, 73–80. [Google Scholar] [CrossRef]
- Serviddio, G.; Sastre, J. Measurement of mitochondrial membrane potential and proton leak. Methods Mol. Biol. 2010, 594, 107–121. [Google Scholar] [PubMed]
- Sangineto, M.; Villani, R.; Cavallone, F.; Romano, A.; Loizzi, D.; Serviddio, G. Lipid Metabolism in Development and Progression of Hepatocellular Carcinoma. Cancers 2020, 12, 1419. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Sangineto, M.; Romeo, M.; Villani, R.; Romano, A.D.; Loguercio, C.; Serviddio, G.; Federico, A. Immunity as Cornerstone of Non-Alcoholic Fatty Liver Disease: The Contribution of Oxidative Stress in the Disease Progression. Int. J. Mol. Sci. 2021, 22, 436. [Google Scholar] [CrossRef]
- Azzimato, V.; Jager, J.; Chen, P.; Morgantini, C.; Levi, L.; Barreby, E.; Sulen, A.; Oses, C.; Willerbrords, J.; Xu, C.; et al. Liver macrophages inhibit the endogenous antioxidant response in obesity-associated insulin resistance. Sci. Transl. Med. 2020, 12, eaaw9709. [Google Scholar] [CrossRef]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2020. [Google Scholar] [CrossRef]
- Argo, C.K.; Patrie, J.T.; Lackner, C.; Henry, T.D.; de Lange, E.E.; Weltman, A.L.; Shah, N.L.; Al-Osaimi, A.M.; Pramoonjago, P.; Jayakumar, S.; et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: A double-blind, randomized, placebo-controlled trial. J. Hepatol. 2015, 62, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Cocco, T.; Di, M.; Papa, P.; Lorusso, M. Arachidonic acid interaction with the mitochondrial electron transport chain promotes reactive oxygen species generation. Free Radic. Biol. Med. 1999, 27, 51–59. [Google Scholar] [CrossRef]
- Gille, L.; Nohl, H. The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Arch. Biochem. Biophys. 2001, 388, 34–38. [Google Scholar] [CrossRef]
- Loskovich, M.V.; Grivennikova, V.G.; Cecchini, G.; Vinogradov, A.D. Inhibitory effect of palmitate on the mitochondrial NADH:ubiquinone oxidoreductase (complex I) as related to the active-de-active enzyme transition. Biochem. J. 2005, 387 Pt 3, 677–683. [Google Scholar] [CrossRef]
- Schonfeld, P.; Reiser, G. Rotenone-like action of the branched-chain phytanic acid induces oxidative stress in mitochondria. J. Biol. Chem. 2006, 281, 7136–7142. [Google Scholar] [CrossRef] [Green Version]
- Schonfeld, P.; Wojtczak, L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim. Biophys. Acta 2007, 1767, 1032–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mito chondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.M.; Caldeira da Silva, C.C.; Cerqueira, F.M.; Kowaltowski, A.J. Mild mitochondrial uncoupling as a therapeutic strategy. Curr. Drug Targets 2011, 12, 783–789. [Google Scholar] [CrossRef] [PubMed]


| Vitamin D3 | 25 mcg |
| Vitamin E | 60 mg |
| Olive dry extract (Olea Europaea) titrated in 20% hydroxytyrosol | 15 mg |
| Cinnamon dry extract (Cinnamomum cassia Presi cortex) titrated in 1% flavonoids | 14 mg |
| Fish oil 56% DHA/EPA | 830 mg |
| S.No. | Gene | Forward Primer | Reverse Primer |
|---|---|---|---|
| 1 | GAPDH | TCAAGGCTGAGAATGGGAAG | ATGGTGGTGAAGATGCCAGT |
| 2 | SCD1 | TGTTCGTCAGCACCTTCTTG | TCTTGTCGTAGGGGCGATAC |
| 3 | SREBP1 | ATCTGTGAGAAGGCCAGTG | GCGGGCCACAAGAAGTAGA |
| 5 | CPT1 | TTCAAGGTCTGGCTCTACCA | TCCCTCGTGCAAAATAGGTC |
| 6 | CPT2 | GCCTCTCTTGGATGACAGC | CTGGTGTGCTTATTCTGCT |
| 7 | UCP2 | CTTTGAAGAACGGGACAC | TCCTGCTACCTCCCAGAA |
| 8 | UCP3 | ATGAGTTTTGCCTCCATTCG | AATCGGACCTTCACCACATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangineto, M.; Bukke, V.N.; Bellanti, F.; Tamborra, R.; Moola, A.; Duda, L.; Villani, R.; Romano, A.D.; Serviddio, G. A Novel Nutraceuticals Mixture Improves Liver Steatosis by Preventing Oxidative Stress and Mitochondrial Dysfunction in a NAFLD Model. Nutrients 2021, 13, 652. https://doi.org/10.3390/nu13020652
Sangineto M, Bukke VN, Bellanti F, Tamborra R, Moola A, Duda L, Villani R, Romano AD, Serviddio G. A Novel Nutraceuticals Mixture Improves Liver Steatosis by Preventing Oxidative Stress and Mitochondrial Dysfunction in a NAFLD Model. Nutrients. 2021; 13(2):652. https://doi.org/10.3390/nu13020652
Chicago/Turabian StyleSangineto, Moris, Vidyasagar Naik Bukke, Francesco Bellanti, Rosanna Tamborra, Archana Moola, Loren Duda, Rosanna Villani, Antonino Davide Romano, and Gaetano Serviddio. 2021. "A Novel Nutraceuticals Mixture Improves Liver Steatosis by Preventing Oxidative Stress and Mitochondrial Dysfunction in a NAFLD Model" Nutrients 13, no. 2: 652. https://doi.org/10.3390/nu13020652
APA StyleSangineto, M., Bukke, V. N., Bellanti, F., Tamborra, R., Moola, A., Duda, L., Villani, R., Romano, A. D., & Serviddio, G. (2021). A Novel Nutraceuticals Mixture Improves Liver Steatosis by Preventing Oxidative Stress and Mitochondrial Dysfunction in a NAFLD Model. Nutrients, 13(2), 652. https://doi.org/10.3390/nu13020652







