Soluble Fiber Inulin Consumption Limits Alterations of the Gut Microbiota and Hepatic Fatty Acid Metabolism Caused by High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Diets
2.2. Determination of Blood Glucose Concentration
2.3. Quantification of Serum Lipids and Lipoproteins
2.4. Lipid Extraction and Determination of Fatty Acid Profiles in Livers
2.5. Gene Expression
2.6. Microbiota Analysis by 16S rRNA Gene Sequencing Using Illumina Technology
2.7. Statistical Analyses
3. Results
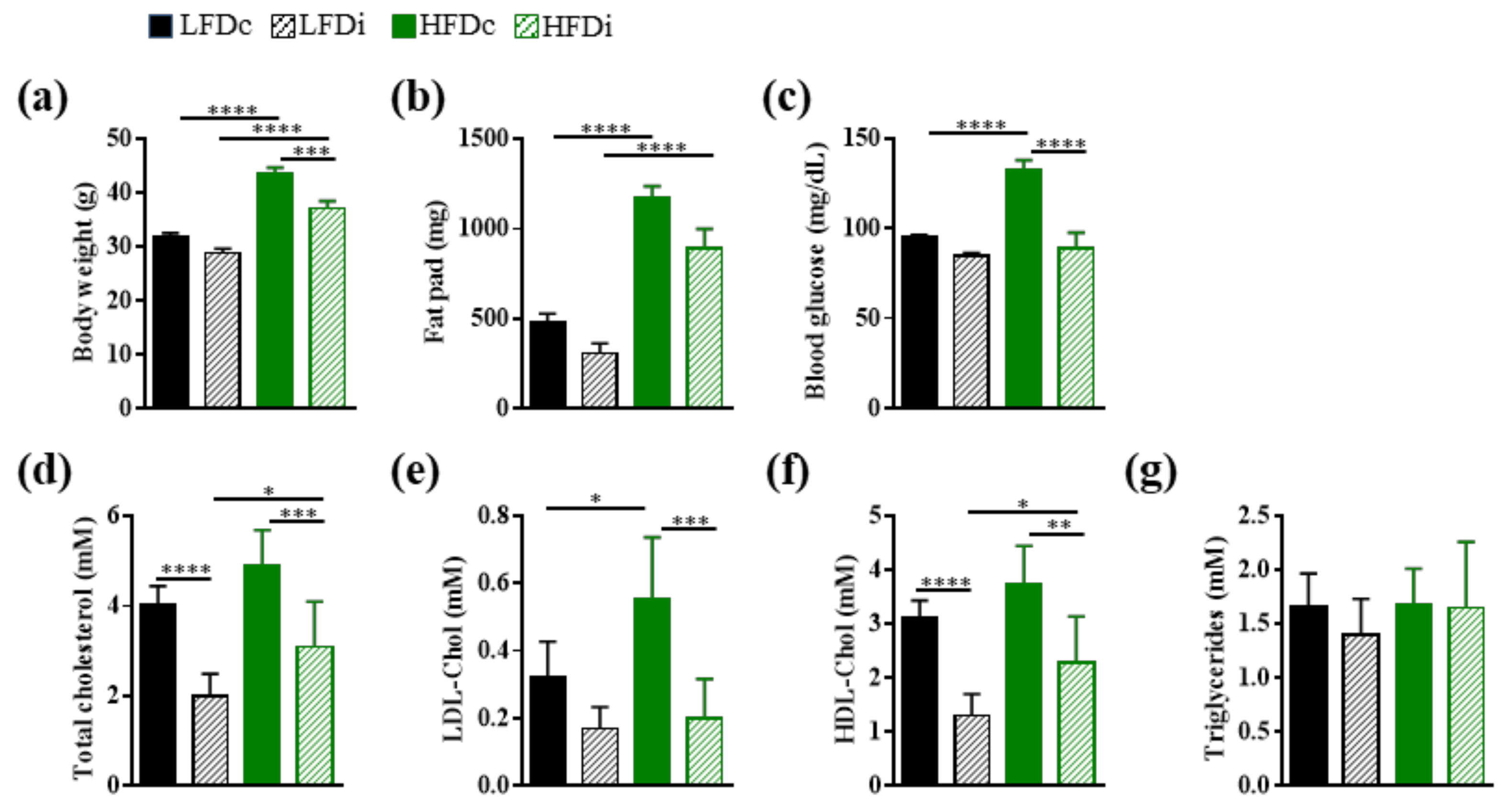
3.1. Enrichment of Diet with Inulin Impacts the Metabolic Parameters
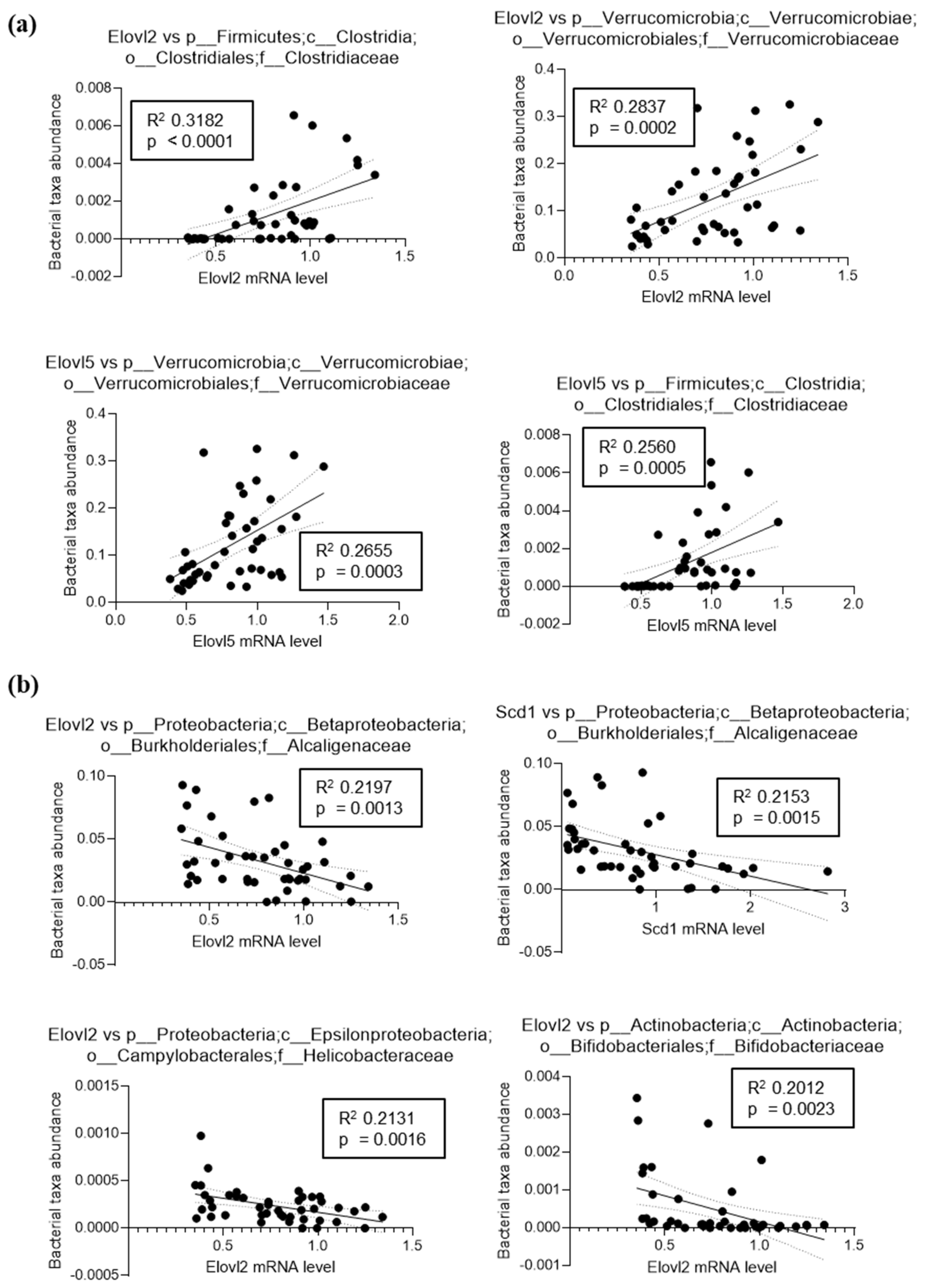
3.2. Inulin Supplementation Induces Specific Changes in the Gut Microbiota of Mice Fed LFD or HFD
3.3. Inulin Supplementation Modulates Hepatic Fatty Acid Profile
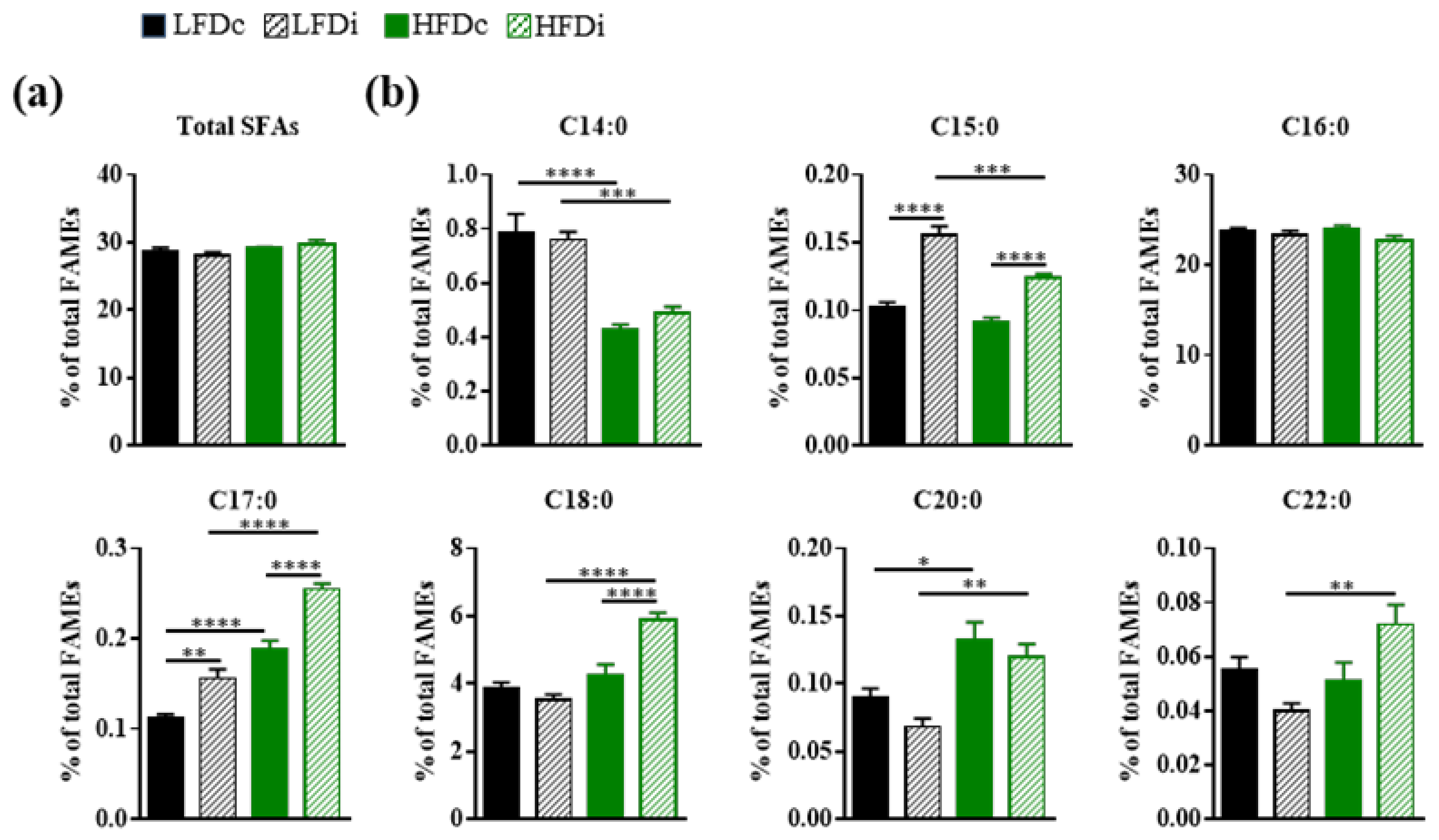
3.3.1. Saturated Fatty Acids
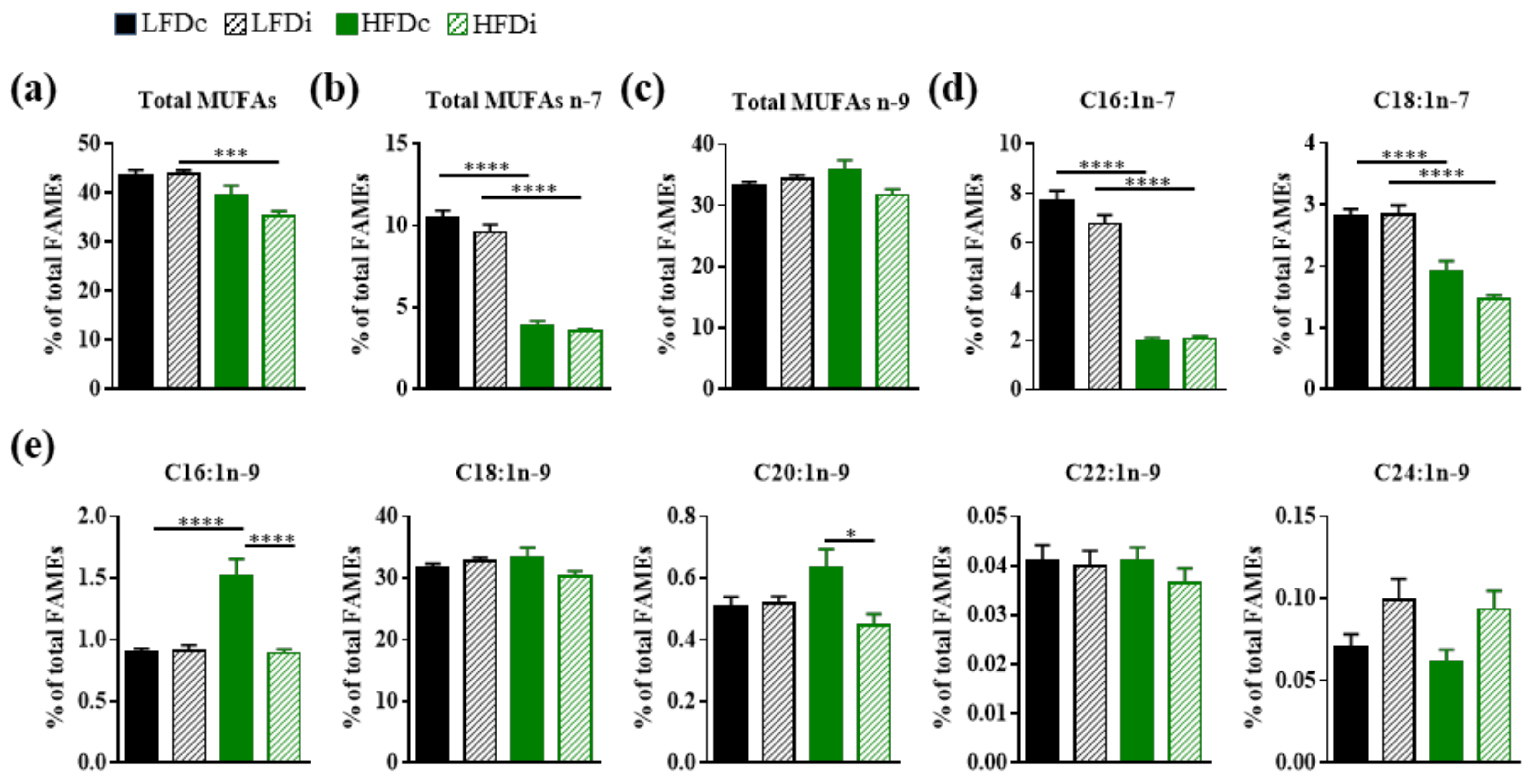
3.3.2. Monounsaturated Fatty Acids
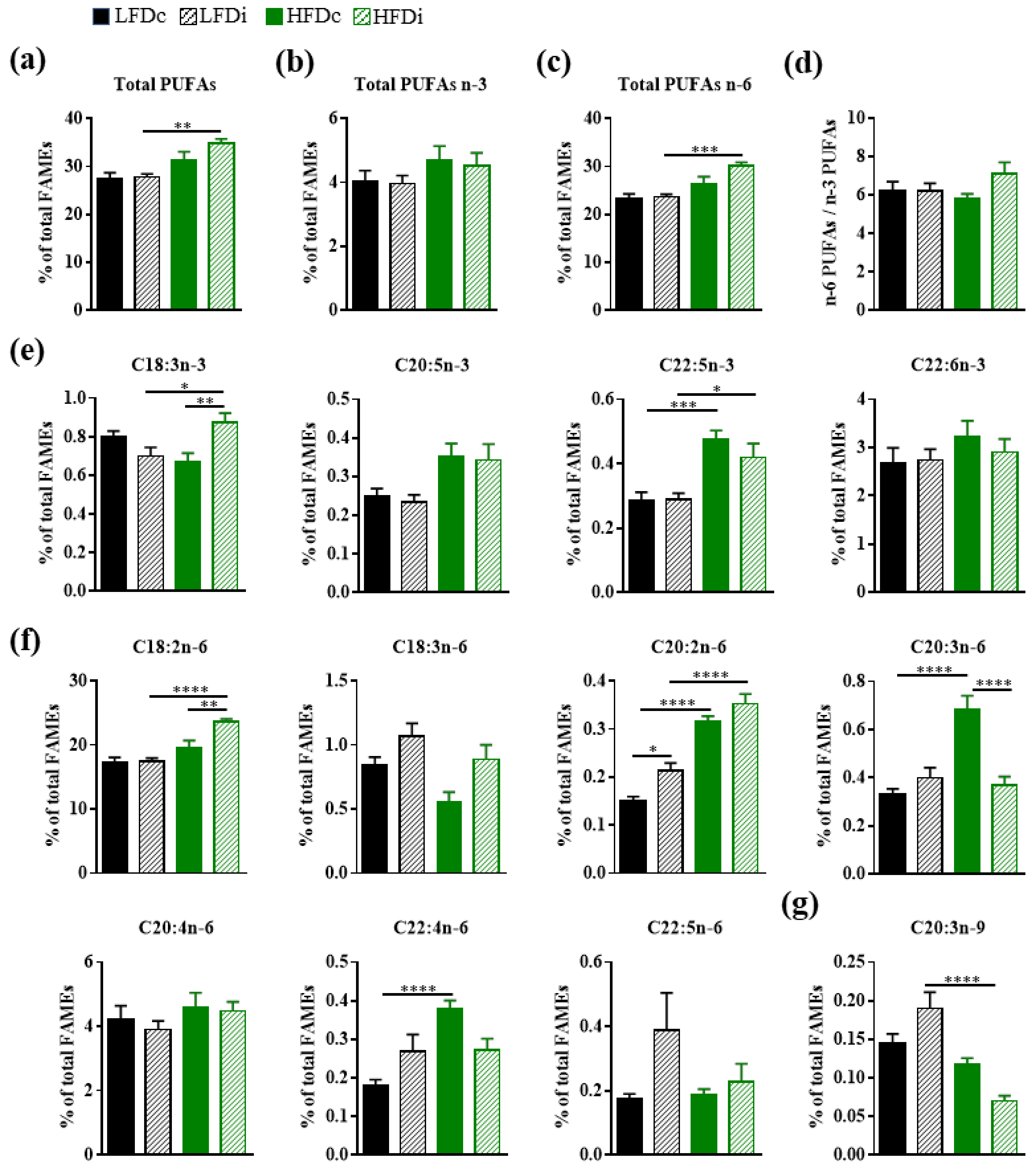
3.3.3. Polyunsaturated Fatty Acids
3.4. Fatty Acid Elongase and Desaturase Expressions in the Liver Are Modulated by the Fat and Fiber Content of the Diet
3.5. The Hepatic Activity of Enzymes Involved in the Biosynthesis of Fatty Acids Is Modulated by the Fat and Fiber Content of the Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [Green Version]
- Moszak, M.; Szulinska, M.; Bogdanski, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [Green Version]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Marcos, J.A.; Perez-Jimenez, F.; Camargo, A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J. Nutr. Biochem. 2019, 70, 1–27. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahren, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53 (Suppl. 3), S215–S219. [Google Scholar] [CrossRef] [Green Version]
- Alcock, J.; Lin, H.C. Fatty acids from diet and microbiota regulate energy metabolism. F1000Res 2015, 4, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53.e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef]
- Chassaing, B.; Miles-Brown, J.; Pellizzon, M.; Ulman, E.; Ricci, M.; Zhang, L.; Patterson, A.D.; Vijay-Kumar, M.; Gewirtz, A.T. Lack of soluble fiber drives diet-induced adiposity in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G528–G541. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Han, K.H.; Kobayashi, Y.; Nakamura, Y.; Shimada, K.; Aritsuka, T.; Ohba, K.; Morita, T.; Fukushima, M. Comparison of the effects of longer chain inulins with different degrees of polymerization on colonic fermentation in a mixed culture of Swine fecal bacteria. J. Nutr. Sci. Vitaminol. 2014, 60, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Zhao, A.; Wang, Q.; Yang, X.; Ren, D. Supplementation of Inulin with Various Degree of Polymerization Ameliorates Liver Injury and Gut Microbiota Dysbiosis in High Fat-Fed Obese Mice. J. Agric. Food Chem. 2020, 68, 779–787. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, Y.T.; Zhu, L.M.; Liu, Z.Y.; Ye, C.Q.; Qin, S. Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci. Rep. 2020, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Pompei, A.; Cordisco, L.; Raimondi, S.; Amaretti, A.; Pagnoni, U.M.; Matteuzzi, D.; Rossi, M. In vitro comparison of the prebiotic effects of two inulin-type fructans. Anaerobe 2008, 14, 280–286. [Google Scholar] [CrossRef]
- Zhu, L.; Qin, S.; Zhai, S.; Gao, Y.; Li, L. Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The effects of inulin on gut microbial composition: A systematic review of evidence from human studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.W.; Tunsjo, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzetto, L.; Costabile, G.; Della Pepa, G.; Ciciola, P.; Vetrani, C.; Vitale, M.; Rivellese, A.A.; Annuzzi, G. Dietary Fibre as a Unifying Remedy for the Whole Spectrum of Obesity-Associated Cardiovascular Risk. Nutrients 2018, 10, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, T.; He, F.; Zhang, X.; Zhu, L.; Wang, Z.; Lu, H.; Wang, T.; Li, Y.; Yang, S.; Wang, H. Inulin Exerts Beneficial Effects on Non-Alcoholic Fatty Liver Disease via Modulating gut Microbiome and Suppressing the Lipopolysaccharide-Toll-Like Receptor 4-Mpsi-Nuclear Factor-kappaB-Nod-Like Receptor Protein 3 Pathway via gut-Liver Axis in Mice. Front. Pharmacol. 2020, 11, 558525. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Caparros-Martin, J.A.; Matthews, V.B.; Koch, H.; O’Gara, F.; Croft, K.D.; Ward, N.C. Isoquercetin and inulin synergistically modulate the gut microbiome to prevent development of the metabolic syndrome in mice fed a high fat diet. Sci. Rep. 2018, 8, 10100. [Google Scholar] [CrossRef] [Green Version]
- Brooks, L.; Viardot, A.; Tsakmaki, A.; Stolarczyk, E.; Howard, J.K.; Cani, P.D.; Everard, A.; Sleeth, M.L.; Psichas, A.; Anastasovskaj, J.; et al. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol. Metab. 2017, 6, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Muccioli, G.G.; Deldicque, L.; Bindels, L.B.; Pachikian, B.D.; Sohet, F.M.; et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARgamma-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 712–722. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Nyman, M.; Fak, F. Designing future prebiotic fiber to target metabolic syndrome. Nutrition 2014, 30, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Dewulf, E.M.; Neyrinck, A.M.; Bindels, L.B.; Cani, P.D.; Mahillon, J.; de Vos, W.M.; Thissen, J.P.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; et al. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin. Nutr. 2015, 34, 501–507. [Google Scholar] [CrossRef]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469.e455. [Google Scholar] [CrossRef]
- Albouery, M.; Buteau, B.; Gregoire, S.; Cherbuy, C.; Pais de Barros, J.P.; Martine, L.; Chain, F.; Cabaret, S.; Berdeaux, O.; Bron, A.M.; et al. Age-Related Changes in the Gut Microbiota Modify Brain Lipid Composition. Front. Cell. Infect. Microbiol. 2019, 9, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindt, A.; Liebisch, G.; Clavel, T.; Haller, D.; Hormannsperger, G.; Yoon, H.; Kolmeder, D.; Sigruener, A.; Krautbauer, S.; Seeliger, C.; et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat. Commun. 2018, 9, 3760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremmyda, L.S.; Tvrzicka, E.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease: A review. part 2: Fatty acid physiological roles and applications in human health and disease. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2011, 155, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride—Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Albouery, M.; Buteau, B.; Gregoire, S.; Martine, L.; Gambert, S.; Bron, A.M.; Acar, N.; Chassaing, B.; Bringer, M.A. Impact of a high-fat diet on the fatty acid composition of the retina. Exp. Eye Res. 2020, 196, 108059. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Bretin, A.; Dube, P.E.; Maue, A.C.; Dauriat, C.J.G.; Barnich, N.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifiers Directly Impact Adherent-Invasive E. coli Gene Expression to Drive Chronic Intestinal Inflammation. Cell Rep. 2020, 33, 108229. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gomez-Valades, A.G.; Matamoros, S.; Ramirez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [Green Version]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Chassaing, B.; Zhang, L.; San Yeoh, B.; Xiao, X.; Kumar, M.; Baker, M.T.; Cai, J.; Walker, R.; Borkowski, K.; et al. Microbiota-Dependent Hepatic Lipogenesis Mediated by Stearoyl CoA Desaturase 1 (SCD1) Promotes Metabolic Syndrome in TLR5-Deficient Mice. Cell Metab. 2015, 22, 983–996. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Botolin, D.; Xu, J.; Christian, B.; Mitchell, E.; Jayaprakasam, B.; Nair, M.G.; Peters, J.M.; Busik, J.V.; Olson, L.K.; et al. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J. Lipid Res. 2006, 47, 2028–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva-Santi, L.G.; Antunes, M.M.; Caparroz-Assef, S.M.; Carbonera, F.; Masi, L.N.; Curi, R.; Visentainer, J.V.; Bazotte, R.B. Liver Fatty Acid Composition and Inflammation in Mice Fed with High-Carbohydrate Diet or High-Fat Diet. Nutrients 2016, 8, 682. [Google Scholar] [CrossRef] [Green Version]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- De Carvalho, C.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [Green Version]
- Echeverria, F.; Valenzuela, R.; Bustamante, A.; Alvarez, D.; Ortiz, M.; Espinosa, A.; Illesca, P.; Gonzalez-Manan, D.; Videla, L.A. High-fat diet induces mouse liver steatosis with a concomitant decline in energy metabolism: Attenuation by eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation and the additive effects upon EPA and HT co-administration. Food Funct. 2019, 10, 6170–6183. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Song, Z.H.; Ying, M.D.; Zhu, H.; He, Q.J.; Yang, B.; Cao, J. N-myristoylation: From cell biology to translational medicine. Acta Pharmacol. Sin. 2020, 41, 1005–1015. [Google Scholar] [CrossRef]
- De Souza, C.O.; Vannice, G.K.; Rosa Neto, J.C.; Calder, P.C. Is Palmitoleic Acid a Plausible Nonpharmacological Strategy to Prevent or Control Chronic Metabolic and Inflammatory Disorders? Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Hayashi, T.; Kashima, K.; Kurotani, K.; Shirouchi, B.; Mizoue, T.; Sato, M. Alteration of Serum Phospholipid n-6 Polyunsaturated Fatty Acid Compositions in Nonalcoholic Fatty Liver Disease in the Japanese Population: A Cross-Sectional Study. Lipids 2020, 55, 599–614. [Google Scholar] [CrossRef]
- Matsuda, M.; Kawamoto, T.; Tamura, R. Predictive value of serum dihomo-gamma-linolenic acid level and estimated Delta-5 desaturase activity in patients with hepatic steatosis. Obes. Res. Clin. Pract. 2017, 11, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Qing, K.; Chen, Y. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and-resistant mice. Obes. Res. 2004, 12, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Barrera, C.; Espinosa, A.; Llanos, P.; Orellana, P.; Videla, L.A. Reduction in the desaturation capacity of the liver in mice subjected to high fat diet: Relation to LCPUFA depletion in liver and extrahepatic tissues. Prostaglandins Leukot. Essent. Fat. Acids 2015, 98, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, A.L.; Kolberg, L.W.; Thomas, W.; Slavin, J.L. Gastrointestinal tolerance of chicory inulin products. J. Am. Diet. Assoc. 2010, 110, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Carabin, I.G.; Flamm, W.G. Evaluation of safety of inulin and oligofructose as dietary fiber. Regul. Toxicol. Pharmacol. 1999, 30, 268–282. [Google Scholar] [CrossRef]
- Coussement, P.A. Inulin and oligofructose: Safe intakes and legal status. J. Nutr. 1999, 129, 1412S–1417S. [Google Scholar] [CrossRef]
- Van Loo, J.; Coussement, P.; de Leenheer, L.; Hoebregs, H.; Smits, G. On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit. Rev. Food Sci. Nutr. 1995, 35, 525–552. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Ward, L.C.; Brown, L. Inulin oligofructose attenuates metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Br. J. Nutr. 2016, 116, 1502–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asto, E.; Mendez, I.; Rodriguez-Prado, M.; Cune, J.; Espadaler, J.; Farran-Codina, A. Effect of the Degree of Polymerization of Fructans on Ex Vivo Fermented Human Gut Microbiome. Nutrients 2019, 11, 1293. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.L.; Timm, D.A.; Slavin, J.L. Fructooligosaccharides exhibit more rapid fermentation than long-chain inulin in an in vitro fermentation system. Nutr. Res. 2008, 28, 329–334. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Daubioul, C.; Neyrinck, A.; Lasa, M.; Taper, H.S. Inulin and oligofructose modulate lipid metabolism in animals: Review of biochemical events and future prospects. Br. J. Nutr. 2002, 87 (Suppl. 2), S255–S259. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, Y.; Luo, X.; Wu, Q.; He, J.; Li, S.; Barba, F.J. Modulation of lipid metabolism and colonic microbial diversity of high-fat-diet C57BL/6 mice by inulin with different chain lengths. Food Res. Int. 2019, 123, 355–363. [Google Scholar] [CrossRef]
- Reis, S.A.; Conceicao, L.L.; Rosa, D.D.; Dias, M.M.; Peluzio Mdo, C. Mechanisms used by inulin-type fructans to improve the lipid profile. Nutr. Hosp. 2014, 31, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T. Altered Gut Microbiota: A Link Between Diet and the Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 321–328. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Zhang, Y.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut bacteria Akkermansia is associated with reduced risk of obesity: Evidence from the American Gut Project. Nutr. Metab. 2020, 17, 90. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Miles, J.P.; Zou, J.; Kumar, M.V.; Pellizzon, M.; Ulman, E.; Ricci, M.; Gewirtz, A.T.; Chassaing, B. Supplementation of Low- and High-fat Diets with Fermentable Fiber Exacerbates Severity of DSS-induced Acute Colitis. Inflamm. Bowel Dis. 2017, 23, 1133–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Aguilera Olvera, R.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e622. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Diets | LFDc 1 D12450J 5 | LFDi 2 D13081108 5 | HFDc 3 D12492 5 | HFDi 4 D13081106 5 |
|---|---|---|---|---|
| Protein source | Casein | Casein | Casein | Casein |
| Fiber source | Cellulose | Inulin | Cellulose | Inulin |
| Protein (kcal%) | 20 | 20 | 20 | 20 |
| Carbohydrates (kcal%) | 70 | 65 | 20 | 20 |
| Fat (kcal%) | 10 | 10 | 60 | 60 |
| kcal/gm | 3.8 | 3.5 | 5.24 | 4.6 |
| Ingredients (g) | ||||
| Casein | 200 | 200 | 200 | 200 |
| L-Cystine | 3 | 3 | 3 | 3 |
| Corn Starch | 506.2 | 456.2 | 0 | 0 |
| Maltodextrin 10 | 125 | 125 | 125 | 75 |
| Sucrose | 63.8 | 63.8 | 68.8 | 68.8 |
| Cellulose | 50 | 0 | 50 | 0 |
| Inulin | 0 | 200 | 0 | 200 |
| Soybean Oil | 25 | 25 | 25 | 25 |
| Lard | 20 | 20 | 245 | 245 |
| Mineral Mix, S10026 | 10 | 10 | 10 | 10 |
| DiCalcium Phosphate | 13 | 13 | 13 | 13 |
| Calcium Carbonate | 5.5 | 5.5 | 5.5 | 5.5 |
| Potassium Citrate, 1 H2O | 16.5 | 16.5 | 16.5 | 16.5 |
| Vitamin Mix, V10001 | 15 | 15 | 10 | 10 |
| Choline Bitartrate | 2 | 2 | 2 | 2 |
| Fatty Acids 1 | Low-Fat Diet | High-Fat Diet |
|---|---|---|
| C10:0 | 0.0 | 0.0 |
| C12:0 | 0.0 | 0.1 |
| C14:0 | 0.5 | 1.1 |
| C15:0 | 0.0 | 0.1 |
| C16:0 | 14.9 | 19.6 |
| C16:1 | 0.7 | 1.3 |
| C17:0 | 0.2 | 0.4 |
| C18:0 | 7.1 | 10.6 |
| C18:1 | 28.8 | 34.0 |
| C18:2n-6 | 41.9 | 28.7 |
| C18:3n-3 | 5.0 | 2.0 |
| C20:0 | 0.0 | 0.2 |
| C20:1 | 0.2 | 0.6 |
| C20:3n-6 | 0.0 | 0.1 |
| C20:4n-6 | 0.2 | 0.3 |
| C20:5n-3 | 0.0 | 0.0 |
| C22:0 | 0.0 | 0.0 |
| C22:1 | 0.0 | 0.0 |
| C22:4n-6 | 0.0 | 0.0 |
| C22:5n-3 | 0.0 | 0.1 |
| C22:5n-6 | 0.0 | 0.0 |
| C22:6n-3 | 0.0 | 0.0 |
| C24:0 | 0.0 | 0.0 |
| Total SFA | 22.7 | 32.0 |
| Total MUFA | 29.7 | 36.0 |
| Total PUFA | 47.1 | 31.2 |
| C18:2n-6/C18:3n-3 | 8.3 | 14.1 |
| PUFA n-6/PUFA n-3 | 8.4 | 13.7 |
| Genes | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| Scd1 | CAGGAGGGCAGGTTTCCAAG | CGTTCATTTCCGGAGGGAGG |
| Fads1 | CGCCAAACGCGCTACTTTAC | CCACAAAAGGATCCGTGGCA |
| Fads2 | CGTGGGCAAGTTCTTGAAGC | TCTGAGAGCTTTTGCCACGG |
| Elovl1 | CCTGAAGCACTTCGGATGGT | TCACTTGCCCGTCCTTCTTC |
| Elovl2 | GTGATGTCCGGGTAGCCAAG | GGACGCGTGGTGATAGACAT |
| Elovl3 | TACTTCTTTGGCTCTCGCCC | AGCTTACCCAGTACTCCTCCA |
| Elovl5 | TGATGAACTGGGTTCCCTGC | CAGCTGCCCTTGAGTGATGT |
| Elovl6 | AGAACACGTAGCGACTCCGA | TCAGATGCCGACCACCAAAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albouery, M.; Bretin, A.; Buteau, B.; Grégoire, S.; Martine, L.; Gambert, S.; Bron, A.M.; Acar, N.; Chassaing, B.; Bringer, M.-A. Soluble Fiber Inulin Consumption Limits Alterations of the Gut Microbiota and Hepatic Fatty Acid Metabolism Caused by High-Fat Diet. Nutrients 2021, 13, 1037. https://doi.org/10.3390/nu13031037
Albouery M, Bretin A, Buteau B, Grégoire S, Martine L, Gambert S, Bron AM, Acar N, Chassaing B, Bringer M-A. Soluble Fiber Inulin Consumption Limits Alterations of the Gut Microbiota and Hepatic Fatty Acid Metabolism Caused by High-Fat Diet. Nutrients. 2021; 13(3):1037. https://doi.org/10.3390/nu13031037
Chicago/Turabian StyleAlbouery, Mayssa, Alexis Bretin, Bénédicte Buteau, Stéphane Grégoire, Lucy Martine, Ségolène Gambert, Alain M. Bron, Niyazi Acar, Benoit Chassaing, and Marie-Agnès Bringer. 2021. "Soluble Fiber Inulin Consumption Limits Alterations of the Gut Microbiota and Hepatic Fatty Acid Metabolism Caused by High-Fat Diet" Nutrients 13, no. 3: 1037. https://doi.org/10.3390/nu13031037
APA StyleAlbouery, M., Bretin, A., Buteau, B., Grégoire, S., Martine, L., Gambert, S., Bron, A. M., Acar, N., Chassaing, B., & Bringer, M.-A. (2021). Soluble Fiber Inulin Consumption Limits Alterations of the Gut Microbiota and Hepatic Fatty Acid Metabolism Caused by High-Fat Diet. Nutrients, 13(3), 1037. https://doi.org/10.3390/nu13031037







